World Journal of Cardiovascular Diseases
Vol.4 No.5(2014), Article ID:46041,11 pages DOI:10.4236/wjcd.2014.45034
HIV Infection in Pregnancy and the Risk of Gestational Hypertension and Preeclampsia
Beatrice Landi1, Valeria Bezzeccheri2, Brunella Guerra3, Mariangela Piemontese1, Francesca Cervi3, Lucia Cecchi1, Eleonora Margarito3, Stefano R. Giannubilo1, Andrea Ciavattini1, Andrea L. Tranquilli1
1Dipartimento di Scienze Cliniche Specialistiche, Università Politecnica delle Marche, Ancona, Italy
2Dipartimento Materno Infantile, AOU OORR Ancona, Salesi Hospital, Ancona, Italy
3Dipartimento di Scienze Mediche e Chirurgiche, Università di Bologna, Bologna, Italy
Email: beatrice.landi@yahoo.it
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 4 April 2014; revised 7 May 2014; accepted 15 May 2014
ABSTRACT
The objective of this study was to evaluate the association between HIV infection and hypertensive disorders of pregnancy, comparing the rates of preeclampsia and gestational hypertension in a HIV-infected pregnant group and in a HIV-negative control pregnant group matched for age and parity. Furthermore, we aimed to compare the rates of hypertensive disorders in a subgroup of HIV-positive and HIV-negative African-American Black women. Patients and Methods: This was a prospective observational cohort study conducted at two University Departments of Obstetrics and Gynecology, Salesi Hospital, Ancona, and Sant’Orsola Hospital, Bologna. The HIV-infected patients’ group consisted of 126 pregnant women; 140 HIV-negative pregnant women matched for age and parity served as controls. Gestational hypertension and preeclampsia were diagnosed according to NHBPEP-ISSHP criteria. Categorical data were analyzed using the Fisher exact test. Statistical significance was set at a p value < 0.05. Results: Gestational hypertension and preeclampsia were diagnosed in 3 of 126 HIV-positive patients (2.38%) and in 14 of 140 HIV-negative patients (10%), with a relative risk of 0.24 (p = 0.0112). In the subgroup of African-American Black women, gestational hypertension and preeclampsia were diagnosed in 2 out of 43 HIV-positive (4.7%) and in 3 out of 18 HIV-negative patients (16.7%) with a relative risk of 0.28, not statistically significant (p = 0.1887). Conclusion: Pregnant women with HIV infection seem to be protected against gestational hypertension and preeclampsia and this protective effect remains also in a high risk population, such as African-American Black ethnic group. The effect is present independently from treatment received and virus copies. The lack of immune response present since the conception period should account for unopposed trophoblast invasion resulting in a better placentation.
Keywords:Human Immunodeficiency Virus, Preeclampsia, Gestational Hypertension, African Ethnic Group, Immune Tolerance

1. Introduction
Hypertensive disorders in pregnancy are a leading direct cause of maternal and perinatal morbidity and mortality in the developed [1] [2] and developing world [3] -[6] ; they are common obstetric complications [7] -[10] with a reported incidence of approximately 10% among pregnancies [11] [12] .
It is well documented that genetic [13] [14] and socio-demographic factors, such as ethnic group, have an influence on the incidence of preeclampsia and gestational hypertension [15] ; in African-American Black women the incidence of preeclampsia is much higher and it has a much more aggressive and rapid clinical course of presentation, leading to significant mortality [16] -[19] .
Preeclampsia is characterized by impaired placentation and it involves an enhanced maternal systemic inflammatory response [20] associated with diffuse endothelial cell activation, but the current understanding of the aetiology of preeclampsia still remains unclear [21] -[24] . It is believed that immunological factors may be involved in initiating the cascade of events that cause placental maladaptation. The role of the immune system as an etiological factor was first proposed by Need [25] and expanded upon by other authors [26] -[28] . According to this hypothesis, some authors postulated that the frequency of preeclampsia may be affected by immunosuppressive conditions, such as human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS).
Not long after AIDS was first described in 1981, pregnancies in HIV-positive women were studied and the possibility of mother-to-child transmission of the new syndrome was proposed [29] [30] ; concerted research efforts have brought about a dramatic decrease in such transmission, at least in the industrialized world, with interventions such as combination of antiretroviral prophylaxis and highly active antiretroviral therapy throughout pregnancy, elective cesarean delivery and avoidance of breast-feeding [31] [32] .
The risk of perinatal HIV transmission can be reduced substantially to <1% - 2% [33] -[37] , whereas evidence indicates that, in the absence of any interventions, 15% - 40% of infants born to HIV-infected mothers will become infected with HIV [33] [38] .
Current recommendations for treatment of HIV infection in pregnant women are the same as those for the initiation of treatment in non-pregnant individuals: treatment is recommended for all individuals with a CD4+ cell count of <200/mm3 or an AIDS-defining illness and should be considered for individuals with a CD4+ cell count of <350/mm3 [39] .
Standard treatment is highly active antiretroviral therapy (HAART) with >3 drugs [40] . For HIV-infected pregnant women who do not require therapy for their own health, antiretroviral drugs are recommended for the prevention of mother-to-child transmission; for women who are receiving antiretroviral drugs solely for prevention of perinatal transmission, delaying initiation of prophylaxis until after the first trimester can be considered.
The impact of the HIV infection on pregnancy complications has also been studied; some studies have evaluated maternal immune response to HIV infection in pregnancy and investigated whether its represents a risk factor for adverse outcome, however controversial results have been obtained [41] .
Data on the impact of HIV on the rate of preeclampsia are few and conflicting. There is no consensus as to whether HIV-infected women are at a lower, equal or higher risk of developing preeclampsia than the general population.
The objective of this study was to evaluate the association between HIV infection and hypertensive disorders of pregnancy, comparing the rates of preeclampsia and gestational hypertension in a HIV-infected pregnant group and in a HIV-negative control pregnant group matched for age and parity. Furthermore, we aim to compare rates of hypertensive disorders in a subgroup of HIV-positive and HIV-negative African-American Black women, a population in which HIV infection has high prevalence rates [42] -[46] . One more objective was to evaluate the possible impact of antiretroviral therapy and immunological status in pre-conceptional period, or at least at the beginning of pregnancy, through the CD4+ cell count, on the incidence of hypertensive disorders.
2. Patients and Methods
This was a prospective observational cohort study conducted at two University Departments of Obstetrics and Gynecology, Salesi Hospital, Ancona, and Sant’Orsola Hospital, Bologna, both tertiary hospitals with high risk obstetrical unit. The patients group comprised 126 HIV-infected pregnant women followed subsequently between 2004 and 2012 (excluding cases of spontaneous abortions, voluntary interruptions and therapeutic abortions). These patients were group matched for age and parity with 140 HIV-negative pregnant women followed between 2010 and 2012.
The inclusion criteria were known HIV status and singleton pregnancy; the exclusion criteria were preeclampsia or gestational hypertension in a previous pregnancy and chronic medical conditions, as chronic hypertension, diabetes, renal disease and connective-tissue disease. In addition, to rule out chronic hypertension, women whose high blood pressure had not returned to normal values after delivery were excluded from the study.
With the exclusion of the diagnosis of HIV, the inclusion and exclusion criteria of the controls were similar to those of the cases.
HIV-infected women were followed according to standard protocols. We recorded socio-demographic data and informations about clinical status, maternal antiretroviral therapy (ART), virological and immunological status, route of transmission, HIV status of the partner, and concomitant infectious diseases. Stage of infection was classified according to the 1993 Centers for Disease Control and Prevention (CDC) classification [47] . Data on pregnancy and delivery were recorded.
Gestational hypertension and preeclampsia were diagnosed according to NHBPEP-ISSHP criteria [8] [10] .
Statistical Analysis
Statistical analysis was carried out using GraphPad software. Data are expressed as media and standard deviation or n (%). Categorical data were analyzed using the Fisher exact test. Continuous data were analyzed using student’s T test for parametric and the Mann-Whitney-U test for nonparametric data. Statistical significance was set at a p value < 0.05.
3. Results
There were no statistically significant differences in parity, maternal age, BMI in patient profiles between both HIV-positive and HIV-negative groups. According to ethnic group, higher prevalence of African-American Black women was observed in the HIV-positive group.
The socio-demographic characteristics of the women are summarized in Table 1.
Gestational hypertension and preeclampsia were diagnosed in 3 of 126 HIV-positive patients (2.38%) and in 14 of 140 HIV-negative patients (10%), with a relative risk of 0.24 (p = 0.0112) (Figure 1).
Gestational hypertension and preeclampsia were diagnosed in 2 out of 43 HIV-positive African-American Black women (4.7%) and in only 1 out of 82 HIV-positive Caucasian white women (1.2%), with a relative risk of 0.17, although it is not statistically significant (p = 0.114).
In the subgroup of African-American Black women, 43 patients were HIV-infected and 18 were HIV-negative. Gestational hypertension and preeclampsia were diagnosed in 2 out of 43 HIV-positive (4.7%) and in 3 out of 18 HIV-negative patients (16.7%) with a relative risk of 0.28, although it is not statistically significant (p = 0.1887) (Figure 2).
There were no difference on rates of gestational hypertension and preeclampsia in women who started treatment during pregnancy (after the first trimester) and in women already treated before pregnancy (n.s.; p = 0.2575) (Figure 3).
Furthermore, all HIV-positive women who develop gestational hypertension or preeclampsia had a CD4+ cell count > 500/mm3 (class A) in pre-conceptional period; the results of CD4+ cell count were instead available in only 64 patients who do not develop gestational hypertension or preeclampsia; among these, 32 were in class A (CD4+ > 500/mm3), 27 in class B (200/mm3 < CD4+ > 499/mm3), and 5 in class C (CD4+ < 200/mm3) (data not shown).
4. Discussion
Our results suggest that the rate of gestational hypertension and preeclampsia is significantly lower in
Table 1. Socio-demographic characteristics of the women.
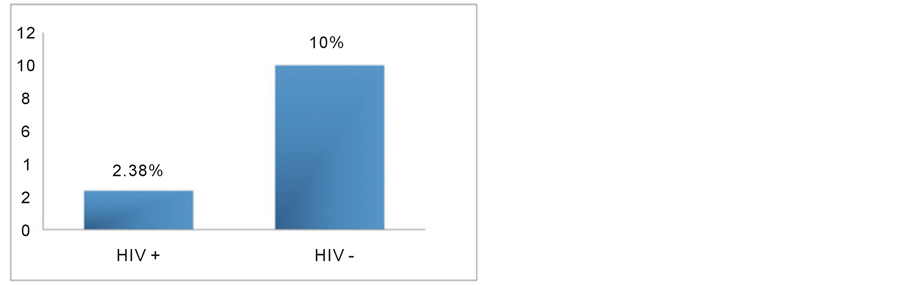
Figure 1. Rates of gestational hypertension and preeclampsia in HIV-positive and HIV-negative women (p = 0.0112).
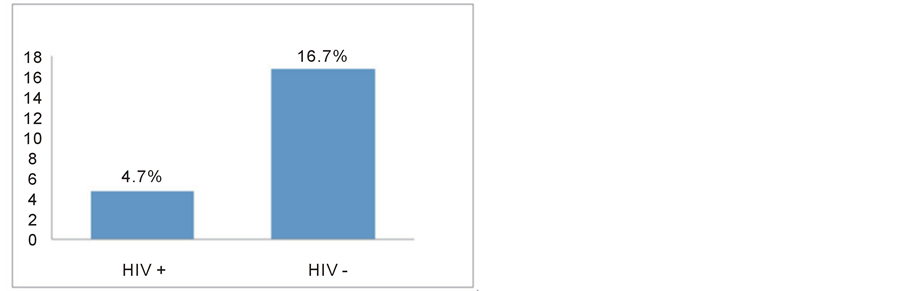
Figure 2. Rates of gestational hypertension and preeclampsia in African-American Black HIV-positive and HIV-negative women (n.s. p = 0.1187).
HIV-infected women than in controls matched for age and parity.
We prospectively analyzed women enrolled at two Italian hospital, both tertiary centers with high risk obstetrical unit, with a high prevalence of obstetrical complications.
As far as we know, this is the first report that analyzes association of HIV infection with both gestational hypertension and preeclampsia. We also excluded, among cases and controls, patients with chronic hypertension to rule out any risk factors linked to this previous medical condition.

Figure 3. Rates of gestational hypertension and preeclampsia in HIV-positive women who started HAART before or during pregnancy (n.s.).
Association of HIV infection with different rate of preeclampsia was first investigated by Wimalasundera et al. in 2002 [48] , but they found an association with lower rate of preeclampsia only in untreated HIV-positive women, with a borderline significance. They stratified by treatment their 214 HIV-positive women and they showed that within the HIV-positive cohort the rate of preeclampsia was significantly lower in women who were untreated or on mono or dual therapy than in women who took triple ART; on the contrary, compared with the HIV-negative control cohort of 214 women, there was no significant difference in the rate of preeclampsia with triple ART, or with mono or dual therapy.
A similarly low rate (0.8%, five of 634 cases) has been recorded in a demographically comparable, predominantly untreated, HIV-positive cohort in the USA [49] .
Our data disagree with Wimalasundera’s observations, although they are incomparable because all our patients were receiving HAART and were well controlled. Our results also showed that there were no difference on rates of gestational hypertension and preeclampsia in women who started treatment during pregnancy (after the first trimester) and in women already treated before pregnancy.
A lower rate of preeclampsia in HIV-infected pregnant women is also reported by few other previous studies [50] -[52] .
De Groot et al. [50] in a South African case-control study evaluated the effect of HIV-infection on the clinical course of 440 critically ill obstetrical patients and they showed that more complications occurred in the HIVnegative group: eclampsia recorded for the HIV-negative group was 17.1% and 4.7% for the HIV-positive group (p = 0.04; 95% CI: 17.1- 0.9) and lung edema was 18.2% and 6.2%, respectively (p = 0.01; 95% CI: 19.3 - 3.5); preeclampsia also occurred more often in the HIV-positive group, although it was not statistically significant.
A Brazilian study by Mattar et al. [51] compared 123 HIV-positive women receiving mono-therapy or HAART to 1708 healthy controls and a lower rate of preeclampsia in HIV group (0.8%) was detected as compared to controls (10.6%) (Fisher exact test p 1/4 0:0017).
Even thought with a different approach, Kalumba et al. in a retrospective recent study [52] found that the rate of HIV/AIDS was lower in a preeclamptic group of 492 cases than in a control group of 500 normotensive healthy pregnant women (p = 0.005, OR = 0.658).
Also Martinelli et al. [53] reported few cases of preeclampsia in their epidemiological survey on HIV infection (only two cases among 159 HIV-positive women).
On the contrary, our findings are different from those of other studies that failed to show any association between HIV infection and preeclampsia.
A large South African study by Frank et al. [54] showed that the rates of preeclampsia-eclampsia were 5.2% in HIV-negative and 5.7% in untreated HIV-positive women (p = 0.61), showing no reduction in the risk of developing preeclampsia-eclampsia amongst untreated HIV-positive women.
A similar result was reported by Bodkin et al. [55] : they compared 212 HIV positive pregnant women with 101 controls and they did not show any difference in the prevalence of eclampsia (2.83 vs. 0.99%; p = 0.44).
A Spanish group [56] even reported a significant higher risk for preeclampsia (adjusted OR, 5.6; 95% CI, 1.7 - 18.1; p = 0.004) and fetal death in HIV-positive women treated with HAART prior to pregnancy and a very low rate of preeclampsia among HIV-infected women who were not on HAART.
In 2008 Conde-Agudelo et al. wrote a systematic review and meta-analysis [57] , analyzing results from seven studies [48] [50] [51] [54] -[56] [58] that evaluated the association between HIV infection during pregnancy and the risk of preeclampsia yielding conflicting results. Overall, treated and untreated HIV infection was not associated with the risk of developing preeclampsia (pooled OR, 0.76, 95% CI, 0.46 - 1.26 and pooled OR, 0.97; 95% CI, 0.67 - 1.39), respectively.
A large nationwide cross-sectional study from the United States [58] reported no difference in hospitalization rates for preeclampsia between HIV-infected women and uninfected women.
In the AmRo study performed in the Netherlands to explore the pregnancy outcome of HIV-1 positive and negative women [59] the incidence of preeclampsia did not differ significantly (2% versus 1%, respectively); this was a case-control study where the 143 HIV-infected women received different forms of HAART and were compared to 98 controls.
Another report with a discordant result was a recent study by Boyajian [60] : 91 HIV-positive pregnant women receiving HAART and 273 HIV-negative pregnant women were compared; there was no difference in the odds of preeclampsia (3.3% vs. 5.1%; adjusted odds ratio [aOR] 0.59; 95% CI 0.11 to 3.08).
The results from various studies are conflicting, probably due to differences in study design, approach and study population, since some studies included patients with underlying chronic medical conditions; another significant difference is in population treatment, because in more recent studies, subsequent to improvements in clinical care, the proportion of infected pregnant women receiving antiretroviral treatment greatly increased.
Many of the previous cited studies are conducted in African hospitals [50] [52] [54] [55] , but there are no studies that compared rates of preeclampsia in HIV-positive women of different ethnic group. We showed a trend of a higher rate of hypertensive complications in HIV-positive African-American Black women compared to HIV-positive Caucasian white women, and a trend of a lower rate of gestational hypertension and preeclampsia in African-American Black HIV-positive women compared to HIV-negative women of the same ethnic group.
Black race is known to be a factor that may predispose to hypertensive disorders of pregnancy, although the association between Black race and preeclampsia may partially be explained by the high prevalence of obesity and chronic hypertension in this population.
African-American Black women have higher rates of preeclampsia and higher prevalence of preeclampsia risk factors (e.g., elevated BMI) than Caucasians [17] -[19] [61] .
In Central and South Africa, rates of HIV infection are very high [5] [42] -[46] . Hence, African-American Black people represent an ideal population for studies involving HIV and preeclampsia.
The pathophysiological basis of the association between HIV infection and hypertensive disorders in pregnancy remains to be clarified. Our results confirmed that HIV infected pregnant women have probably some immunological features that protect them to hypertensive complications, even in a high risk group such as African-American Black women.
The immunological hypothesis of the origin of preeclampsia was first analyzed by Need in 1975 [25] and then expanded by other authors [26] -[28] ; their hypothesis was that the induction of the immune tolerance should be brought about by contact between paternal antigens and the female genital tract through sexual intercourse and that semen should trigger an influx of antigen-presenting cells into the female genital tract, so that primipaternity and short duration of exposure to sperm antigens should be risk factors for preeclampsia [62] .
Preeclampsia is furthermore characterized by immunological abnormalities which show analogies to organ rejection after allograft transplantation and in graft-versus-host disease (GVHD) [63] .
The fetus in half of its antigenicity is a paternal allograft and it is not rejected by the maternal immune system, even if mechanisms are still not well understood [20] [64] -[67] .
Women who conceive through in vitro fertilization (IVF), using surgically obtained semen from initially azoospermic males (these women of course have not been previously exposed to their partners’ semen), demonstrate a 3-fold increase in preeclampsia [68] and IVF cycles involving donated gametes also demonstrate a significantly elevated rate of preeclampsia [69] -[71] .
The fetus is, of course, not only an allograft but also an autograft [64] and the immune system’s adjustment to pregnancy, therefore, does not only involve tolerance of the paternal allogenic, but also of the maternal autoimmunogenic, components of the fetus.
All these concepts supported our observation that HIV-infected pregnant women had a decreased prevalence of hypertensive disorders than controls, also because the immunologic impairment with HIV is mostly of cellular nature.
Another correlated hypothesis on the basis of the association between HIV infection and risk of preeclampsia is that normal pregnancy itself is a pro-inflammatory state and that preeclampsia might be an exaggeration of this maternal inflammatory response [20] [72] .
It has been proposed that successful pregnancy may depend, at least in part, on the bias of the maternal immune response shifting away from cell mediated inflammatory T-helper-1 type responses towards a T-helper-2 phenotype with humoral responses [67] [73] -[75] . Some data suggest that HIV infection in pregnancy accentuate this Th2 state. On the other hand, preeclampsia seems to be due to an excessive maternal inflammatory response to pregnancy and it is characterized by Th1 cytokine pattern.
Therefore, under conditions of acquired or induced immune deficiency such as the state induced by the human immunodeficiency virus, not only immune hyper-reactivity but also inflammatory responses may be inhibited and thus development of preeclampsia prevented.
We also analyzed immunological status of our study population through CD4+ cell count during pre-conceptional period, or at least at the beginning of pregnancy, but our data were uncompleted. However we founded a good immunological status (CD4+ > 500 mm3) in all our HIV-positive women who develop gestational hypertension or preeclampsia.
Few previous studies on the association between HIV and preeclampsia reported CD4+ cell count results in HIV-infected women and however data were often available for a small proportion of women [51] [52] [54] . Mattar et al. [51] reported in their patients group of 123 treated HIV seropositive women a mean value of CD4+ at the time of delivery of 510 (200 - 1378) with a median of 531; they detected a lower incidence of preeclampsia in HIV group (0.8%) as compared to controls (10.6%), but they not compared CD4+ cell count levels between groups.
Only in the study from Kalumba et al. [52] the CD4+ count levels between the preeclamptic and control groups were compared to test the hypothesis that immune-suppression could have a protective effect against preeclampsia. However, the results of the CD4+ cell count were available in only 66 cases (of a total of 492 preeclamptic HIV-positive women) and 75 controls (HIV-positive women without preeclampsia). The median CD4+ count was lower in the control group without preeclampsia (median CD4+ count = 208 cells/μl) than in the preeclamptic women (median CD4+ count = 304 cells/μl) (p = 0.008), suggesting that among HIV-infected women, the immunity was less affected in those who developed preeclampsia.
5. Conclusion
According to our data, some immunological features of even treated HIV-positive pregnant women seem to be protective to hypertensive disorders of pregnancy and this could give new insight into the pathophysiological mechanisms of preeclampsia. Pregnant women with HIV infection seem to be protected against gestational hypertension and preeclampsia and this protective effect remains also in a high risk population, such as AfricanAmerican Black ethnic group. The effect is present independently from treatment received and immunological status. The lack of immune response since the conception period should account for unopposed trophoblast invasion resulting in a better placentation.
References
- Centre for Maternal and Child Enquiries (CMACE) (2011) Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer: 2006-08. The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG, 118, 1-203.
- Chang, J., Elam-Evans, L.D., Berg, C.J., Herndon, J., Flowers, L., Seed, K.A. and Syverson, C.J. (2003) PregnancyRelated Mortality Surveillance—United States, 1991-1999. MMWR Surveillance Summaries, 52, 1-8.
- The Lancet Maternal Survival Steering Group (2006) Strategies for Reducing Maternal Mortality: Getting on with What Works. Lancet, 368, 1284-1299. http://dx.doi.org/10.1016/S0140-6736(06)69381-1
- The Lancet Maternal Survival Steering Group (2006) Maternal Mortality: Who, When, Where and Why. Lancet, 368, 1189-1200. http://dx.doi.org/10.1016/S0140-6736(06)69380-X
- (2002) Saving Mothers. Second Report on Confidential Enquiries into Maternal Deaths in South Africa, 1999-2001. Department of Health, Pretoria.
- Ujah, I.A., Aisien, O.A., Mutihir, J.T., Vanderjagt, D.J., Glew, R.H. and Uguru, V.E. (2005) Factors Contributing to Maternal Mortality in North-Central Nigeria: A Seventeen-Year Review. African Journal of Reproductive Health, 9, 27-40. http://dx.doi.org/10.2307/3583409
- ACOG Practice Bulletin No. 33 (2002) American College of Obstetricians and Gynecologists. Diagnosis and Management of Preeclampsia and Eclampsia. Obstetrics & Gynecology, 99, 159-167.
- (2000) Report of the NHBPEP Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics & Gynecology, 183, S1-S22. http://dx.doi.org/10.1067/mob.2000.107928
- National Collaborating Centre for Women’s and Children’s Health Commissioned by the National Institute for Health and Clinical Excellence (NICE) (2010) Hypertension in Pregnancy, the Management of Hypertensive Disorders during Pregnancy.
- (2001) The International Society for the Study of Hypertension in Pregnancy: The Classification and Diagnosis of the Hypertensive Disorders of Pregnancy.
- Cunningham, F.G., Gant, N.F., Leveno, K.J., Gilstrap III, L.C., Hauth, J.C. and Wenstrom, K.D. (2001) Hypertensive Disorders in Pregnancy. In: Williams Obstetrics, 21st Edition, McGraw-Hill, New York, 567-618.
- Hauth, J.C., Ewell, M.G., Levine, R.J., Esterlitz, J.R., Sibai, B., Curet, L.B., Catalano, P.M. and Morris, C.D. (2000) Pregnancy Outcomes in Healthy Nulliparas Who Developed Hypertension. Calcium for Preeclampsia Prevention Study Group. Obstetrics & Gynecology, 95, 24-28. http://dx.doi.org/10.1016/S0029-7844(99)00462-7
- Ward, K. (2008) Searching for Genetic Factors Underlying Pre-Eclampsia: Recent Progress and Persistent Challenges. Minerva Ginecologica, 60, 399-419.
- Lachmeijer, A.M., Dekker, G.A., Pals, G., Aarnoudse, J.G., ten Kate, L.P. and Arngrímsson, R. (2002) Searching for Preeclampsia Genes: The Current Position. European Journal of Obstetrics & Gynecology and Reproductive Biology, 105, 94-113. http://dx.doi.org/10.1016/S0301-2115(02)00208-7
- Smith, R.A. and Baker, P.N. (2005) Risk Factors, Prevention and Treatment of Hypertension in Pregnancy. Minerva Ginecologica, 57, 379-388.
- Moodley, J. (2011) Maternal Deaths Associated with Hypertension in South Africa: Lessons to Learn from the Saving Mothers Report, 2005-2007. Cardiovascular Journal of Africa, 22, 31-35. http://dx.doi.org/10.5830/CVJA-2010-042
- Knuist, M., Bonsel, G.J., Zondervan, H.A. and Treffers, P.E. (1998) Risk Factors for Preeclampsia in Nulliparous Women in Distinct Ethnic Groups: A Prospective Cohort Study. Obstetrics & Gynecology, 92, 174-178. http://dx.doi.org/10.1016/S0029-7844(98)00143-4
- Tanaka, M., Jaamaa, G., Kaiser, M., Hills, E., Soim, A., Zhu, M., Shcherbatykh, I.Y., Samelson, R., Bell, E., Zdeb, M. and McNutt, L.A. (2007) Racial Disparity in Hypertensive Disorders of Pregnancy in New York State: A 10-Year Longitudinal Population-Based Study. American Journal of Public Health, 97, 163-170. http://dx.doi.org/10.2105/AJPH.2005.068577
- Tucker, M.J., Berg, C.J., Callaghan, W.M. and Hsia, J. (2007) The Black-White Disparity in Pregnancy-Related Mortality from 5 Conditions: Differences in Prevalence and Case-Fatality Rates. American Journal of Public Health, 97, 247-251. http://dx.doi.org/10.2105/AJPH.2005.072975
- Redman, C.W.G., Sacks, G.P. and Sargent, I.L. (1999) Preeclampsia: An Excessive Maternal Inflammatory Response to Pregnancy. American Journal of Obstetrics & Gynecology, 180, 499-506. http://dx.doi.org/10.1016/S0002-9378(99)70239-5
- Roberts, J.M. and Lain, K.Y. (2002) Recent Insights into the Pathogenesis of Pre-Eclampsia. Placenta, 23, 359-372. http://dx.doi.org/10.1053/plac.2002.0819
- Walker, J.J. (2000) Pre-Eclampsia. Lancet, 356, 1260-1265. http://dx.doi.org/10.1016/S0140-6736(00)02800-2
- Redman, C.W. and Sargent, I.L. (2000) Placental Debris, Oxidative Stress and Pre-Eclampsia. Placenta, 21, 597-602. http://dx.doi.org/10.1053/plac.2000.0560
- Brosens, I.A., Robertson, W.B. and Dixon, H.G. (1972) The Role of the Spiral Arteries in the Pathogenesis of Preeclampsia. Obstetrics and Gynecology Annual, 1, 177-191.
- Need, J.A. (1975) Pre-Eclampsia in Pregnancies by Different Fathers. British Medical Journal, 1, 548-549. http://dx.doi.org/10.1136/bmj.1.5957.548
- Robillard, P.Y., Hulsey, T.C., Perianan, J., Janky, E., Miri, E.H. and Papiernik, E. (1994) Association of PregnancyInduced Hypertension with Duration of Sexual Cohabitation before Conception. Lancet, 344, 973-975. http://dx.doi.org/10.1016/S0140-6736(94)91638-1
- Dekker, G.A., Robillard, P.Y. and Hulsey, T.C. (1998) Immune Maladaption in the Etiology of Preeclampsia: A Review of Corroborative Epidemiologic Studies. Obstetrical & Gynecological Survey, 53, 377-382. http://dx.doi.org/10.1097/00006254-199806000-00023
- Robertson, S.A., Bromfield, J.J. and Tremellem, K.P. (2003) Seminal “Priming” for Protection from Pre-Eclampsia— A Unifying Hypothesis. Journal of Reproductive Immunology, 59, 253-265. http://dx.doi.org/10.1016/S0165-0378(03)00052-4
- Centers for Disease Control and Prevention (1982) Unexplained Immunodeficiency and Opportunistic Infections in Infants: New York, New Jersey, California. MMWR Morbidity and Mortality Weekly Report, 31, 665-667.
- Cowan, M.J., Hellmann, D., Chudwin, D., Wara, D.W., Chang, R.S. and Ammann, A.J. (1984) Maternal Transmission of Acquired Immune Deficiency Syndrome. Pediatrics, 73, 382-386.
- Centers for Disease Control and Prevention (2006) Achievements in Public Health: Reduction in Perinatal Transmission of HIV Infection: United States, 1985-2005. MMWR Morbidity and Mortality Weekly Report, 55, 592-597.
- Centers for Disease Control and Prevention (2005) U.S. Public Health Service Task Force’s Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States, November 17. Centers for Disease Control and Prevention, Atlanta.
- Public Health Service Task Force (2005) Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1 Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. U.S. Department of Health and Human Services, National Institutes of Health, Health Resources and Services Administration, Rockville. http://www.aidsinfo.nih.gov
- Abrams, E.J. (2004) Prevention of Mother-to-Child Transmission of HIV—Successes, Controversies and Critical Questions. AIDS Reviews, 6, 131-143.
- British HIV Association (2012) Management of HIV Infection in Pregnant Women. British HIV Association, London.
- Thompson, M.A., Aberg, J.A., Hoy, J.F., Telenti, A., Benson, C., Cahn, P., Eron, J.J., Gunthard, H.F., Hammer, S.H., Reiss, P., Richman, D.D., Rizzardini, G., Thomas, D.L., Jacobsen, D.M. and Volberding, P.A. (2012) Antiretroviral Treatment of Adult HIV Infection: 2012 Recommendations of the International Antiviral Society-USA Panel. JAMA, 308, 387-402. http://dx.doi.org/10.1001/jama.2012.7961
- WHO/UNAIDS Working Group on Global HIV/AIDS and STI Surveillance (2013) Guidelines for Assessing the Utility of Data from Prevention of Mother-to-Child Transmission.
- Bulterys, M., Weidle, P.J., Abrams, E.J. and Fowler, M.G. (2005) Combination Antiretroviral Therapy in African Nursing Mothers and Drug Exposure in Their Infants: New Pharmacokinetic and Virologic Findings. Journal of Infectious Diseases, 192, 709-712. http://dx.doi.org/10.1086/432490
- Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents (2006) Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. http://AIDSinfo.nih.gov
- Public Health Service Task Force (2006) Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1 Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. http://AIDSinfo.nih.gov
- Ellis, J., Williams, H., Graves, W. and Lindsay, M.K. (2002) Human Immunodeficiency Virus Infection Is a Risk Factor for Adverse Perinatal Outcome. American Journal of Obstetrics & Gynecology, 186, 903-906. http://dx.doi.org/10.1067/mob.2002.123407
- Centers for Disease Control and Prevention (2011) HIV Surveillance—United States, 1981-2008. MMWR Morbidity and Mortality Weekly Report, 60, 689-693.
- Humes, K., Jones, N.A. and Ramirez, R.R. (2011) Overview of Race and Hispanic Origin: 2010. U.S. Census Bureau. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf
- Centers for Disease Control and Prevention (2008) HIV Prevalence Estimates—United States, 2006. MMWR Morbidity and Mortality Weekly Report, 57, 1073-1076.
- Centers for Disease Control and Prevention (2008) Persons Tested for HIV—United States, 2006. MMWR Morbidity and Mortality Weekly Report, 57, 845-849.
- Black AIDS Institute (2012) Passing the Test: The Challenges and Opportunities of HIV Testing in Black America. http://www.blackaids.org/docs/6_09_passing_test.pdf
- Centers for Disease Control and Prevention (1992) 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS among Adolescent and Adults. MMWR Morbidity and Mortality Weekly Report, 41.
- Wimalasundera, R.C., Larbalestier, N., Smith, J.H., de Ruiter, A., McG Thom, S.A., Hughes, A.D., Poulter, N., Regan, L. and Taylor, G.P. (2002) Pre-Eclampsia, Antiretroviral Therapy, and Immune Reconstitution. Lancet, 360, 1152- 1154. http://dx.doi.org/10.1016/S0140-6736(02)11195-0
- Stratton, P., Tuomala, R.E., Abboud, R., Rodriguez, E., Rich, K., Pitt, J., Diaz, C., Hammill, H. and Minkoff, H. (1999) Obstetric and Newborn Outcomes in a Cohort of HIV-Infected Pregnant Women: A Report of the Women and Infants Transmission Study. Journal of Acquired Immune Deficiency Syndromes & Human Retrovirology, 20, 179-186. http://dx.doi.org/10.1097/00042560-199902010-00011
- de Groot, M.R., Corporaal, L.J., Cronje, H.S. and Joubert, G. (2003) HIV Infection in Critically Ill Obstetrical Patients. International Journal of Gynecology & Obstetrics, 81, 9-16. http://dx.doi.org/10.1016/S0020-7292(02)00399-5
- Mattar, R., Amed, A.M., Lindsey, P.C., Sass, N. and Daher, S. (2004) Preeclampsia and HIV Infection. European Journal of Obstetrics & Gynecology and Reproductive Biology, 117, 240-241. http://dx.doi.org/10.1016/j.ejogrb.2004.04.014
- Kalumba, V.M.S., Moodley, J. and Naidoo, T.D. (2013) Is the Prevalence of Pre-Eclampsia Affected by HIV/AIDS? A Retrospective Case-Control Study. Cardiovascular Journal of Africa, 24, 24-27. http://dx.doi.org/10.5830/CVJA-2012-078
- Martinelli, P., Agangi, A., Sansone, M., Maruotti, G.M., Buffolano, W., Paladini, D., Pizzuti, R. and Floridia, M. (2008) Epidemiological and Clinical Features of Pregnant women with HIV: A 21-Year Perspective from a Highly Specialized Regional Center in Southern Italy. HIV Clinical Trials, 9, 36-42. http://dx.doi.org/10.1310/hct0901-36
- Frank, K.A., Buchmann, E.J. and Schackis, R.C. (2004) Does Human Immunodeficiency Virus Infection Protect against Preeclampsia-Eclampsia? Obstetrics & Gynecology, 104, 238-242. http://dx.doi.org/10.1097/01.AOG.0000130066.75671.b2
- Bodkin, C., Klopper, H. and Langley, G. (2006) A Comparison of HIV Positive and Negative Pregnant Women at a Public Sector Hospital in South Africa. Journal of Clinical Nursing, 15, 735-741. http://dx.doi.org/10.1111/j.1365-2702.2006.01438.x
- Suy, A., Martínez, E., Coll, O., Lonca, M., Palacio, M., de Lazzari, E., Larrousse, M., Milinkovic, A., Hernández, S., Blanco, J.L., Mallolas, J., León, A., Vanrell, J.A. and Gatell, J.M. (2006) Increased Risk of Pre-Eclampsia and Fetal Death in HIV-Infected Pregnant Women Receiving Highly Active Antiretroviral Therapy. AIDS, 20, 59-66. http://dx.doi.org/10.1097/01.aids.0000198090.70325.bd
- Conde-Agudelo, A., Villar, J. and Lindheimer, M. (2008) Maternal Infection and Risk of Preeclampsia: Systematic Review and Metaanalysis. American Journal of Obstetrics & Gynecology, 198, 7-22. http://dx.doi.org/10.1016/j.ajog.2007.07.040
- Kourtis, A.P., Bansil, P., McPheeters, M., Meikle, S.F., Posner, S.F. and Jamieson, D.J. (2006) Hospitalizations of Pregnant HIV-Infected Women in the USA Prior to and during the Era of HAART, 1994-2003. AIDS, 20, 1823-1831. http://dx.doi.org/10.1097/01.aids.0000244201.11006.1c
- Boer, K., Nellen, J.F., Patel, D., Timmermans, S., Tempelman, C., Wibaut, M., Sluman, M.A., van der Ende, M.E. and Godfried, M.H. (2007) The AmRo Study: Pregnancy Outcome in HIV-1-Infected Women under Effective Highly Active Antiretroviral Therapy and a Policy of Vaginal Delivery. BJOG: An International Journal of Obstetrics & Gynaecology, 114, 148-155. http://dx.doi.org/10.1111/j.1471-0528.2006.01183.x
- Boyajian, T., Shah, P.S. and Murphy, K.E. (2012) Risk of Preeclampsia in HIV-Positive Pregnant Women Receiving HAART: A Matched Cohort Study. Journal of Obstetrics and Gynaecology Canada, 34, 136-141.
- Flegal, K.M., Carroll, M.D., Ogden, C.L. and Curtin, L.R. (2010) Prevalence and Trends in Obesity among US Adults, 1999-2008. JAMA, 303, 235-241. http://dx.doi.org/10.1001/jama.2009.2014
- Verwoed, G.R., Hall, D.R., Grové, D., Maritz, J.S. and Odendaal, H.J. (2002) Primipaternity and Duration of Exposure to Sperm Antigens as Risk Factors for Preeclampsia. International Journal of Gynecology & Obstetrics, 78, 121-126. http://dx.doi.org/10.1016/S0020-7292(02)00130-3
- Gleicher, N. (2007) Why Much of the Pathophysiology of Preeclampsia-Eclampsia Must Be of an Autoimmune Nature. American Journal of Obstetrics & Gynecology, 196, 5.e1-7.e1.
- Gleicher, N., Pratt, D. and Dudkiewicz, A. (1993) What Do We Really Know about Autoantibody Abnormalities and Reproductive Failure: A Critical Review. Autoimmunity, 16, 115-140. http://dx.doi.org/10.3109/08916939308993318
- Gleicher, N. (2002) Some Thoughts on the Reproductive Autoimmune Failure Syndrome (RAFS) and the Th-1 versus Th-2 Immune Responses. American Journal of Reproductive Immunology, 48, 252-254. http://dx.doi.org/10.1034/j.1600-0897.2002.01111.x
- Clark, D.A., Blois, S., Kandil, J., Handjiski, B., Manuel, J. and Arck, P.C. (2005) Reduced Uterine Indoleamine 2,3- Dioxygenase versus Increased Th1/Th2 Cytokine Ratios as a Basis for Occult and Clinical Pregnancy Failure in Mice and Humans. American Journal of Reproductive Immunology, 54, 203-216. http://dx.doi.org/10.1111/j.1600-0897.2005.00299.x
- Szekeres-Bartho, J. (2002) Immunological Relationship between the Mother and the Fetus. International Reviews of Immunology, 21, 471-495. http://dx.doi.org/10.1080/08830180215017
- Wang, J.X., Knottnerus, A.M., Schuit, G., Norman, R.J., Chan, A. and Dekker, G.A. (2002) Surgically Obtained Sperm, and Risk of Gestational Hypertension and Pre-Eclampsia. Lancet, 359, 673-674. http://dx.doi.org/10.1016/S0140-6736(02)07804-2
- Wiggins, D. and Main, E. (2005) Outcomes of Pregnancies Achieved by Donor Egg in Vitro Fertilization: A Comparison with Standard in Vitro Fertilization Pregnancies. American Journal of Obstetrics & Gynecology, 192, 2002-2006. http://dx.doi.org/10.1016/j.ajog.2005.02.059
- Salha, O., Sharma, V., Dada, T., Nugent, D., Rutherford, A.J., Tomlinson, A.J., Philips, S., Allgar, V. and Walker, J.J. (1999) The Influence of Donated Gametes on the Incidence of Hypertensive Disorders of Pregnancy. Human Reproduction, 14, 2268-2273. http://dx.doi.org/10.1093/humrep/14.9.2268
- Tranquilli, A.L., Biondini, V., Talebi Chahvar, S., Corradetti, A., Tranquilli, D. and Giannubilo, S. (2013) Perinatal Outcomes in Oocyte Donor Pregnancies. Journal of Maternal-Fetal and Neonatal Medicine, 26, 1263-1267. http://dx.doi.org/10.3109/14767058.2013.777422
- Dietl, J. (2000) The Pathogenesis of Pre-Eclampsia: New Aspects. Journal of Perinatal Medicine, 28, 464-471. http://dx.doi.org/10.1515/JPM.2000.063
- Wegmann, T.G., Lin, H., Guilbert, L. and Mosmann, T.R. (1993) Bidirectional Cytokine Interactions in the MaternalFetal Relationship: Is Successful Pregnancy a TH2 Phenomenon? Immunology Today, 14, 353-357. http://dx.doi.org/10.1016/0167-5699(93)90235-D
- Hunt, J.S. (2006) Stranger in a Strange Land. Immunological Reviews, 213, 36-47. http://dx.doi.org/10.1111/j.1600-065X.2006.00436.x
- Sargent, I.L., Borzychowski, A.M. and Redman, C.W. (2006) NK Cells and Human Pregnancy—An Inflammatory View. Trends in Immunology, 27, 399-404. http://dx.doi.org/10.1016/j.it.2006.06.009


