Advances in Biological Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17277 , 6 pages DOI:10.4236/abc.2012.21007
Evaluation of eco-friendly coagulant from Trigonella foenum-graecum seed
![]()
1Department of Biochemistry and Molecular Biology, School of Life Sciences, Pondicherry University, Puducherry, India
2Centre for Advanced Studies in Bioinformatics, School of Life Sciences, Pondicherry University, Puducherry, India
3Department of Chemistry, Periyar University, Salem, India
Email: *tchinnasamy@hotmail.com
Received 29 August 2011; revised 4 November 2011; accepted 11 January 2012
Keywords: Natural Coagulants; Water Purifier; T. foenum-graecum; Fenugreek
ABSTRACT
The ability of seed extracts of Trigonella foenum-graecum (T. foenum-graecum) and Cuminum cyminum (C. cyminum) to act as natural coagulants was tested using natural turbid water. Seed extracts were prepared using distilled water and NaCl (0.5 M and 1.0 M) solution. Only 1.0 M NaCl extract of T. foenum-graecum had coagulation capability and did not depend on pH values. Further it showed that natural coagulant obtained from T. foenum-graecum is temperature (up to 100˚C) and pH stable (pH 4.0 - 10.0). Extract of C. cyminum had very minimal (16 ± 2) coagulation property. The seed extract of T. foenum-graecum showed about 80% coagulation properties, where as the best known natural coagulants such as Strychnos potatorum and Moringa oleifera, and chemical coagulant such as Al2(SO4)3 showed around 90%, 65% and 95% respectively, which are used as standards for the present study. When compared with pond water, T. foenum-graecum extract treated water shows decrease in alkalinity, turbidity, KMnO4 demand and total coliform. This study reveals that seed extract of T. foenum-graecum can be used as natural water coagulant.
1. INTRODUCTION
Growing population, increased economic activity and industrialization have not only created an increased demand for fresh water but also resulted in severe misuse of natural resource. Water resources all over the world are threatened not only by over exploitation and poor management but also by ecological degradation. According to a survey conducted by United Nation Environment Programme (UNEP), 20% of world’s population lacks access to safe drinking water and 50% lacks access to safe sanitation. Polluted water is estimated to affect the health of about 1200 million people and contributes to the death of 15 million children (per year) under the age of five [1].
The use of plant materials as natural coagulants to clarify turbidity of water is common practice since ancient times. Seeds of Strychnos potatorum (S. potatorum) and Moringa oleifera (M. oleifera) have shown promising result as the source of natural coagulant in the clarification of turbid water [2-4]. Direct filtration with S. potatorum seeds as coagulant appeared effective in clarifying turbid water [5]. This property is attributed due to the presence of polyelectrolytes, proteins, lipids, carbohydrates and alkaloids containing the -COOH and free -OH surface groups in the seed [6]. Among all plant materials investigated, seeds of M. oleifera were found to be one of the most effective sources of primary coagulant for water treatment [3].
T. foenum-graecum (Fenugreek) belongs to the family Leguminosae that grows predominantly in Asia, Northern Africa and the Middle East. Fenugreek seed contains 23% - 26% protein, 6% - 7% fat and 58% carbohydrates of which 25% is dietary fiber, saponins [7] and rich in flavonoids. Fenugreek has been widely used as a flavoring agent and in folk medicine. Several beneficial effects, such as appetite stimulation [8], anti-inflammatory, antipyretic [9], antimicrobial [10], antioxidant, antidiabetic [11], anticancer [12] and antiatherogenic properties [13] have been reported. C. cyminum, an aromatic plant from the family Umbelliferae is used as a flavoring and seasoning agent in foods [14,15]. To our knowledge no studies have been carried out to find out, whether seeds of T. foenum-graecum and C. cyminum can serve as water coagulant. Hence, this study was ventured to investigate the applicability of natural coagulants extracted from seeds of above plants that are abundantly available in Asia.
2. MATERIALS AND METHODS
2.1. Preparation of Seed Extracts and Collection of Turbid Water
Seeds of T. foenum-graecum, C. cyminum, M. oleifera and S. potatorum were collected locally near the city of Puducherry, India. The whole seeds of T. foenum-graecum, and C. cyminum were ground to fine powder using a laboratory mill. All ground materials were sieved through 0.4 mm membrane sieve and the fraction with particle size less than 0.4 mm was used in experiments. 10 g of prepared powder was suspended in 1 L of distilled water or NaCl (0.5 M and 1.0 M) solution and the suspension was stirred using a magnetic stirrer for 10 min to extract the coagulation active components. The seeds of M. oleifera and S. potatorum were made into fine pieces and soaked in water or NaCl for 1 hr, grinded in mortar and pestle, 1% suspension of S. potatorum and 5% suspension of M. oleifera were made with distilled water [3,4] or 1.0 M NaCl solution [2,16,17]. The suspension was then gravity filtered through a rugged filter paper. The filtered solutions, called extracts, were kept in refrigerator at 4˚C.
Water was collected from pond (stagnant rain water in red soil) near Pondicherry University, Puducherry, India, in the month of February 2010. The turbid pond water was left undisturbed for 48 hr to check for any initial spontaneous particle settlement. But the turbidity of the water did not change with increase in time.
2.2. Coagulation Test
Coagulation efficiency of each seed extract was determined by the jar test. The pond water (300 ml) was filled into the beakers (600 ml) and mixed at 200 rpm at constant room temperature (25˚C). Various doses of seed extracts were added into the beakers and mixed for 1 min. The mixing speed was then reduced to 80 rpm and kept for another 30 min. Then the suspensions were left for sedimentation. After 1 hr, 3 hr, 6 hr, 12 hr and 24 hr of sedimentation, clarified samples were collected from the top of the beakers, and turbidity was measured as TS (Turbidity of Sample). The turbidity in the control was defined as TB (Turbidity of Blank). Turbidity was measured using a turbidimeter (TURB 550 IR) and it was expressed in nephelometric turbidity units (NTU). Coagulation efficiency was calculated as:
Coagulation efficiency (%) = (TB – TS) × 100/TB
Each experiment was performed as triplicates and the same was repeated three times. The values are expressed as mean ± S.D.
2.3. Analytical Analysis
The phytochemical analysis of the seed extract of T. foenum-graecum and C. cyminum were done as described by Trease et al. [18]. Protein concentration in crude extracts was measured by Lowry et al. [19]. The quality parameters of the different water were determined using standard methods [20]. Organic matter concentration in water after coagulation was determined as KMnO4 demand by Kübel-Tiemann [21]. Each experiment was carried out in triplicates and experiment was repeated thrice.
3. RESULTS AND DISCUSSION
3.1. Characteristics of Natural Coagulant
Water extract of T. foenum-graecum and C. cyminum samples were screened for the presence of phytochemical compounds (Table 1). As expected, two plant species (used for the present study) showed the presence of various phytochemicals. Plant sterol and steroid presence was observed in T. foenum-graecum, which was absent in C. cyminum. The coagulating active agents can be extracted from seeds by water, different salt solutions, buffer solutions or organic solvents [4,22,23]. Numerous researchers reported that proteins are the active coagulating components in plant extracts [22,24]. Ndabigengesere et al. [4] reported that the coagulation active agents in the seed extract of M. oleifera are dimeric cationic proteins. However Okuda et al. [25] and Sanghi et al. [26] suggested that the coagulation active agent in the extract of M. oleifera was neither a protein, nor a polysaccharide, nor a lipid, but an organic polyelectrolyte.
In the present study protein concentrations in extracts
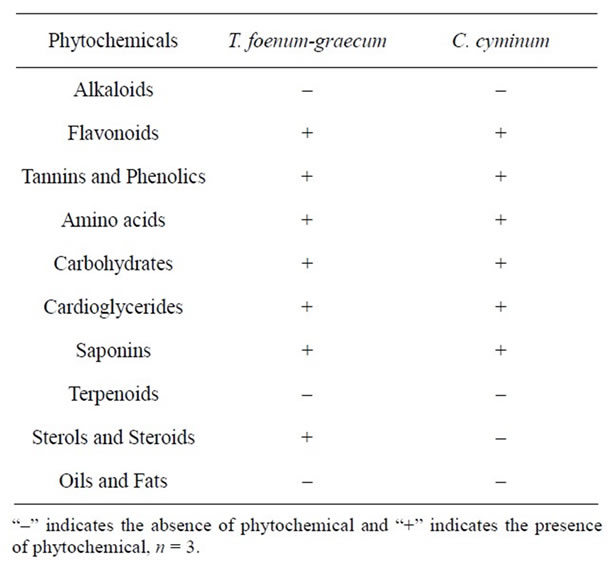
Table 1. Phytochemical screening of seed extracts of T. foenum-graecum and C. cyminum.
of the different samples were determined. The efficiency of protein extraction from seeds of T. foenum-graecum and C. cyminum by distilled water, 0.5 M or 1 M NaCl solutions was done (Figure 1). Results showed the water extracts obtained from different samples has the highest values of protein concentration compared to 0.5 M and 1.0 M NaCl extracts. Water extract of T. foenum-graecum showed the highest (2.14 mg/ml) concentration of protein. The coagulating active agents were extracted by distilled water and two different salt concentrations (0.5 M and 1.0 M NaCl). 1.0 M NaCl extract of T. foenum-graecum showed highest coagulation efficiency (~81%) compared to 0.5 M NaCl (~25%) and water (~8%) extracts on pond water (Figure 2). C. cyminum also showed highest coagulation efficiency in 1.0 M NaCl extract (~16%). This coagulation efficiency of C. cyminum was negligible compared to that of T. foenum-graecum and hence the latter was used for further studies.
Figure 3 shows the coagulation efficiency of T. foenum-graecum along with 3 standard natural coagulants and 1 synthetic coagulant used in the study. The coagulation efficiency was maximum for synthetic coagulant Al2 (SO4)3 followed by S. potatorum, T. foenum-graecum and M. oleifera seed extracts. To verify that coagulation active components contributed to the coagulation efficiency, the authors performed coagulation test with NaCl alone (no added extracts). No significant changes were observed in the turbidity levels of control pond water and NaCl alone treated pond water (data not shown) even after 48 hr. This observation suggests the presence of coagulation active compounds in the seed extract of T. foenum-graecum, which is responsible for the coagulation of turbid pond water and not by other mechanisms [25].
3.2. Properties of Treated and Untreated Water
Table 2 shows a few essential properties of the tap water, untreated and treated pond water with different seed extracts. Comparison between tap and pond water shows higher values of alkalinity, turbidity, permanganate demand (presence of organic matter) and total coliform. These marked differences suggest the increased impurity and turbidity of pond water. When comparing the above properties between pond and treated water, there is a significant decrease in T. foenum-graecum and other seed extracts treated water (Table 2). To our surprise the total coliform is higher in T. foenum-graecum treated sample when compared to tap water, however it is low when compared with pond water (untreated); this may be due to the presence of organic and inorganic matter that promotes the microbial growth. The organic matter in water might cause color, odor, taste as well as microbial changes during storage of treated water. It might consume additional chlorine in the water treatment plant and can act as a
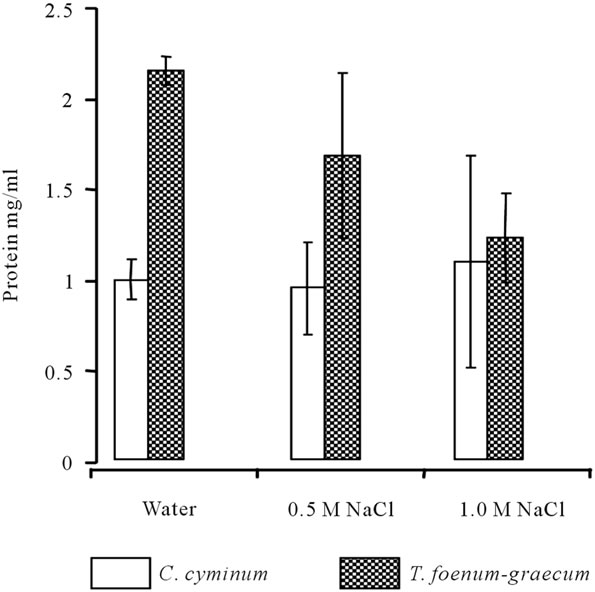
Figure 1. Protein concentration in extracts obtained from seeds of T. foenum-graecum and C. cyminum with distilled water or NaCl solution (sample: 1 g/100 ml; n = 3).

Figure 2. Coagulation efficiency of extracts obtained from seeds of T. foenum-graecum and C. cyminum. (coagulant dose 10 ml/L of pond water; pH 7.0; at room temperature; at 6 hr; n = 3).
precursor of byproducts during the disinfection process. It can be noticed from Table 2 that extract treatment decreased organic matter concentration (4 ± 0.8 mg KMnO4/L) when compared to pond water (7 ± 1 mg KMnO4/L). The decrease in organic matter of treated water is due to the formation of flocks that settle during the coagulation process [25,27]. However, the authors are unable to explain, almost equal amount of organic matter content (it is anticipated more) in pond water and M. oleifera treated samples. Electrical conductivity observed in water is the result of ions of mineral salts and carbon dioxide dissolved in it. Pond water has low electrical
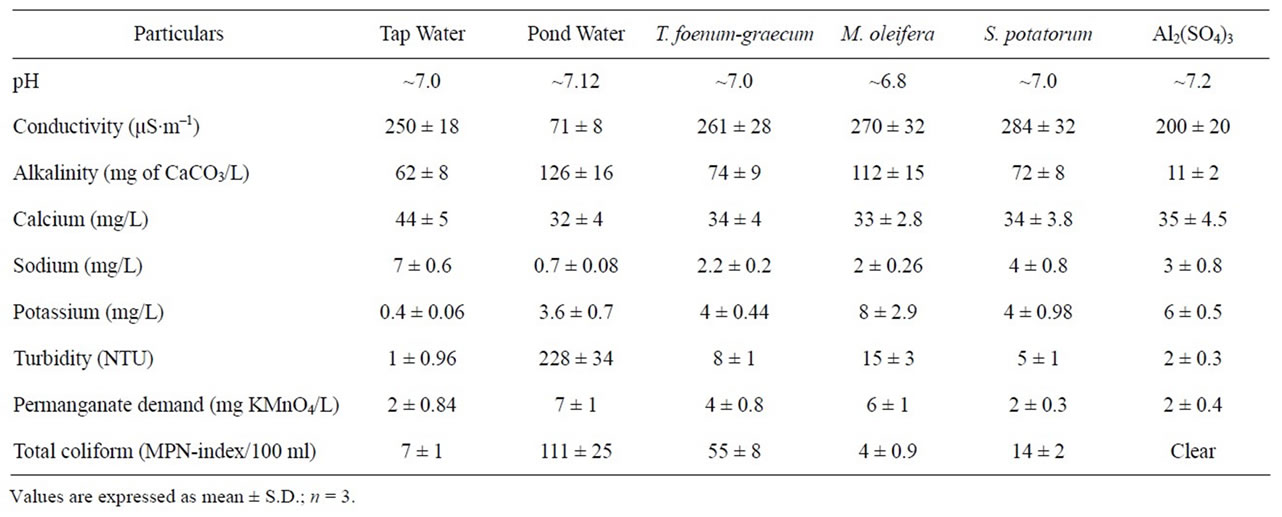
Table 2. Properties of untreated and treated water with different seed extracts.
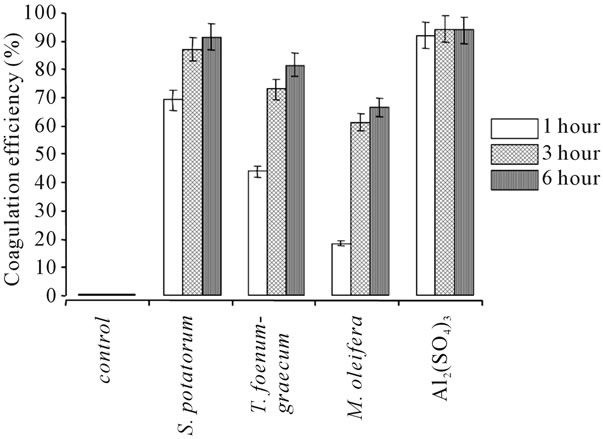
Figure 3. Turbidity removal of extracts obtained from seeds of S. potatorum, T. foenum-graecum (1 g/100 ml of water or NaCl solution; coagulant dose 10 ml/L of pond water; pH 7.0) and M. oleifera (5 g/100 ml of water or NaCl; coagulant dose 50 ml/L of pond water; pH 7.0) and Al2(SO4)3 (coagulant dose 5 g/L of pond water; pH 7.0) at room temperature, n = 3.
conductivity when compared to tap water, where as different extract treated pond water has more or less similar electrical conductivity to that of the tap water.
3.3. Effect of pH, Temperature and Coagulant Dose on Coagulation
Many parameters may have an influence on coagulation process, which includes nature and composition of coagulant, coagulant dose, composition of water, initial water turbidity, pH, temperature, jar test conditions etc. Since pH is a very important parameter with respect to the process of coagulation and protein charge, the influence of pH on coagulant efficiency was investigated. Figure 4 shows the effect of different pH on the coagulation efficiency of T. foenum-graecum. The pond water (~200

Figure 4. Coagulation efficiency of extracts obtained from seeds of T. foenum-graecum as a function of pH (coagulant dose 10 ml/L of pond water; at room temperature; at 6 hr; n = 3).
NTU initial turbidity) was adjusted to the desired pH range (4.0 - 12.0) and seed extract of coagulant dose 10.0 ml/L of pond water was added and the coagulation efficiency was calculated. Results showed that the most appropriate pH to perform coagulation for T. foenumgraecum was 7.0. Also the extract showed good coagulation efficiency in the pH range of 6.0 to 10.0. Further increase (above pH 10.0) or decrease (below pH 6.0) in pH of pond water made it coagulated without an added natural coagulant. These results are not in accordance with reports, that higher pH values are optimal for other natural coagulants derived from M. oleifera [25], P. juliflora and C. latifaria [28], C. angustifolia [26], and common bean [29]. However, T. foenum-graecum extract can be used as natural coagulant within the recommended pH range for tap water, which is from 6.5 to 8.5 [30]. It was found that the pH of clarified water in our experiment was around 7.0.
The seed extract of T. foenum-graecum being an excellent natural coagulant, the authors tried to establish the stability of this coagulation active component for temperature and pH change. Table 3 shows the stability of coagulation active components in the extract. Temperature stability was assessed by heating the extract at 50˚C and 100˚C for 10 min and then cooled to room temperature. pH stability was also assessed by changing the pH of extract from 4.0 to 10.0 using 1 N HCl or 1 N NaOH, incubated for 10 min and then neutralized (pH 7.0). It is evident from the results (Table 3) that change in pH or increase in temperature of the extract does not alter the stability of the coagulation active components in the extract and thereby the coagulation efficiency.
The other interesting factor that affects the coagulation efficiency is the dose of the natural coagulant. The extract with initial turbidity of around 200 NTU, pH 7.0 was investigated with range (coagulant dose) from 0.5 ml to 12.0 ml /L of pond water and the results are presented in Figure 5. Increase in coagulant dose (0.5, 1.0, 2.0, 4.0, 8.0 and 12.0 ml/L of pond water) and incubation time (1, 3, 6, 12 and 24 hr) resulted in increase of coagulation efficiency. The extract at a dose of 12.0 ml/L of pond water produced clear water at 6 hr. When compared to other natural coagulants [6,25-27] T. foenum-graecum extract showed coagulation property at very low concentration. Generally, it can be concluded that lower doses of investigated natural coagulants are better than higher doses. Lower doses are not only quite economic, but also low organic matter load in the processed water results in reduced microbial growth.
4. CONCLUSION
Seeds of T. foenum-graecum contain materials that can act as effective natural coagulant. The natural coagulant
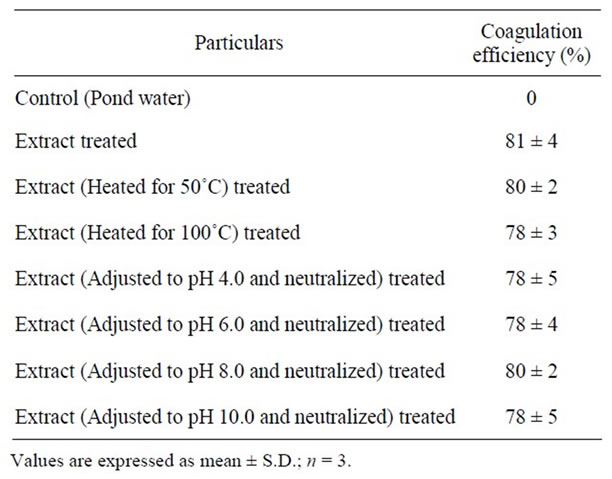
Table 3. Effect of temperature and pH on coagulant of T. foenum-graecum seed extract. Extract was kept at 50˚C and 100˚C (water bath) for 10 min and then cooled. The extract was adjusted to pH 4.0, 6.0, 8.0 & 10.0 by using 1.0 N HCl or 1.0 N NaOH, waited for 10 min then neutralized (coagulant dose 10 ml/L of pond water; at room temperature; at 6 hr).

Figure 5. Effect of coagulant dose on coagulation efficiency of extracts obtained from seeds of T. foenum-graecum (coagulant dose 0.5 ml to 12 ml/L of pond water; pH 7.0; at room temperature; n = 3).
present in T. foenum-graecum shows its coagulation efficiency at neutral pH. Further it was found that this natural coagulant is temperature and pH stable. It may not produce total coliform-free water. It is to be noted that even with well-designed and maintained systems it is unable to produce zero total coliform without chlorination. The present study shows obvious presence of natural water coagulant in seed of T. foenum-graecum. However, further studies pertaining to the precise mechanism of action of water coagulant is warranted.
5. ACKNOWLEDGEMENTS
The authors acknowledge the Department of Science and Technology, Government of India, India for the financial support in the form of DST-FIST. The authors also thank the water testing laboratory, public health division, P. W. D. Pondicherry-12, India for providing various facilities.
REFERENCES
- WHO (World Health Organization) (1997) Surveillance and control of community supplies. In: Guidelines for Drinking-Water Quality, World Health Organization, Geneva.
- Beltran-Heredia, J. and Sanchez-Martin, J. (2008) Azo dye removal by Moringa oleifera seed extract coagulation. Coloration Technology, 124, 310-317. doi:10.1111/j.1478-4408.2008.00158.x
- Folkard, G. and Sutherland, J. (2002) Development of a naturally derived coagulant for water and wastewater treatment. Water Science and Technology: Water Supply, 2, 89- 94.
- Ndabigengesere, A. and Narasiah, K.S. (1998) Quality of water treated by coagulation using Moringa oleifera seeds. Water Research, 32, 781-791. doi:10.1016/S0043-1354(97)00295-9
- Babu, R. and Chaudhuri, M. (2005) Home water treatment by direct filtration with natural coagulant. Journal of Water and Health, 3, 27-30.
- Tripathi, P.N., Chaudhury, M. and Bokil, S.D. (1976) Nirmali seed—A naturally occurring coagulant. Indian Journal of Environmental Health, 18, 72-81.
- Lu, F.R., Shen, L., Qin, Y., et al. (2008) Clinical observation on Trigonella foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chinese Journal of Integrative Medicine, 14, 56-60. doi:10.1007/s11655-007-9005-3
- Petit, P., Sauvaire, Y., Ponsin, G., et al. (1993) Effects of a fenugreek seed extract on feeding behaviour in the rat: Metabolic-endocrine correlates. Pharmacology Biochemistry and Behavior, 45, 369-374. doi:10.1016/0091-3057(93)90253-P
- Ahmadiani, A., Jayan, M., Semnanjan, S., et al. (2001) Anti-inflammatory and antipyritic effects of Trigonella foenum-graecum leaves extract in the rat. Journal of Ethnopharmarcology, 75, 283-286. doi:10.1016/S0378-8741(01)00187-8
- Randhir, R., Lin, Y.T. and Shetty, K. (2004) Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pacific Journal of Clinical Nutrition, 13, 295-307.
- Broca, C., Manteghetti, M., Gross, R., et al. (2000) 4- Hydroxyisoleucine: Effects of synthetic and natural analogues on insulin secretion. European Journal of Pharmacology, 39, 339-345. doi:10.1016/S0014-2999(00)00030-3
- Raju, J., Patlolla, M.R., Swamy, M.V., et al. (2004) Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiology Biomarkers and Prevention, 13, 1392-1398.
- Sowmya, P. and Rajyalakshmi, P. (1999) Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Foods for Human Nutrition, 53, 359-365. doi:10.1023/A:1008021618733
- Srinivasan, K. (2005) Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. International Journal of Food Sciences and Nutrition, 56, 399-414. doi:10.1080/09637480500512872
- Pradeep, K.U. and Geervani, P. (1994) Influence of spices on protein utilisation of winged bean (Psophocarpus tetragonolobus) and horsegram (Dolichos biflorus). Plant Foods for Human Nutrition, 46, 187-193. doi:10.1007/BF01088989
- Beltran-Heredia, J. and Sanchez-Martin, J. (2009) Removal of sodium lauryl sulphate by coagulation/flocculation with Moringa oleifera seed extract. Journal of Hazardous Materials, 164, 713-719. doi:10.1016/j.jhazmat.2008.08.053
- Beltran-Heredia, J. and Sanchez-Martin, J. (2009) Improvement of water treatment pilot plant with Moringa oleifera extract as flocculant agent. Environmental Technology, 30, 525-534. doi:10.1080/09593330902831176
- Trease, G.E. and Evans, W.C. (1978) Pharmacology. Bailliere Tindall Ltd., London, 60-75.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., et al. (1951) Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265-275.
- Clesceri, L.S., Greenberg, A.E. and Eaton, A.D. (1998) Standard methods for the examination of water and wastewater. In: APHA, AWWA and WEF, Washington DC.
- Selbstverlag der MEBAK and Freising-Weihenstephan. (1997) Analytical methods in brewing industry-MEBAK, Germany.
- Ghebremichael, K.A., Gunaratna, K.R. and Dalhammar, G. (2006) Single-step ion exchange purification of the coagulant protein from Moringa oleifera seed. Applied Microbiology and Biotechnology, 70, 526-532. doi:10.1007/s00253-005-0130-7
- Ndabigengesere, A., Narasiah, K.S. and Talbot, B.G. (1995) Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Research, 29, 703-710. doi:10.1016/0043-1354(94)00161-Y
- Ghebremichael, K.A., Gunaratna, K.R., Henriksson, H., et al. (2005) A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Research, 39, 2338-2344. doi:10.1016/j.watres.2005.04.012
- Okuda, T., Baes, A.U., Nishijima, W., et al. (2001) Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Research, 35, 830-834. doi:10.1016/S0043-1354(00)00296-7
- Sanghi, R., Bhattacharya, B. and Singh, V. (2002) Cassia angustifolia seed gum as an effective natural coagulant for decolourisation of dye solutions. Green Chemistry, 4, 252- 254. doi:10.1039/b200067a
- Okuda, T., Baes, A.U., Nishijima, W., et al. (2001) Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Research, 35, 405-410. doi:10.1016/S0043-1354(00)00290-6
- Diaz, A., Rincon, N., Escorihuela, A., et al. (1999) A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela. Process Biochemistry, 35, 391-395. doi:10.1016/S0032-9592(99)00085-0
- Šc´iban, M., Klasnja, M.T. and Stojimirovic, J.L. (2005) Investigation of coagulation activity of natural coagulants from seeds of different leguminous species. Acta Periodica Technologica, 36, 81-87.
- EPA (2003) National secondary drinking water regulations, EPA/816/F-03-016. Washington DC.
NOTES
*Corresponding author.

