Optics and Photonics Journal
Vol.3 No.1(2013), Article ID:28893,5 pages DOI:10.4236/opj.2013.31007
Fluorometric Viability Assessment of Capacitated and Acrosome-Reacted Boar Spermatozoa by Flow Cytometry
1Departamento de Ciencias de la Salud, Universidad Autónoma Metropolitana-Iztapalapa, Mexico City, Mexico
2Laboratoire d’Histologie, Embryologie et Microscopie Électronique, Université Lorraine, Nancy, France
Email: *reynacarmen2@hotmail.com
Received November 15, 2012; revised December 16, 2012; accepted December 23, 2012
Keywords: Boar Sperm; Flow Cytometry; Propidium Iodide; Rhodamine 123; Viability
ABSTRACT
Sperm capacitation involves functional changes, such as the removal or appearance of specific molecules and changes in the plasma membrane; the acrosome reaction (AR) is an exocytotic event induced by calcium influx, enabling the spermatozoa to penetrate the zona pellucida. These processes can be achieved only if the spermatozoa have good viability; indeed, determination of sperm viability is used for the assessment of semen quality. Membrane integrity and mitochondrial activity are important viability parameters of spermatozoa and fluorescent techniques based on membrane permeability to dyes have been developed to determine these parameters. The aim of this work was to determine the viability of boar sperm (fresh, one hour of capacitation induction and 20 min of AR induction) by flow cytometry using propidium iodide (PI) (1.25 μg/mL) and rhodamine 123 (R123) (0.20 μg/mL). Aliquots of 5 × 105 sperm were incubated with each fluorochrome separately and simultaneously for 10 or 20 min, respectively, at 38˚C. The proportion of labeled spermatozoa and their fluorescence intensities were measured using a flow cytometer. The fluorescence index (FI) with PI gradually increased during the incubation and we found significant differences between all the groups. With R123, the FI increased in the capacitated sperm but decreased in the acrosome-reacted sperm, with significant differences between the fresh and capacitated spermatozoa. Our results suggest that the increase in the R123 fluorescence intensity in capacitated spermatozoa is due to changes in the mitochondrial membrane activity because the spermatozoa experienced changes in membrane fluidity and flagellar activation during capacitation. The use of fluorochromes and flow cytometry is a good tool for monitoring many markers of sperm function. Although capacitation and AR processes have been well studied, there is still much information to be elucidated with regard to these complex processes.
1. Introduction
Fertilization in mammalian species is a process with sequential steps, including sperm capacitation in the female genital tract, binding of capacitated sperm to the zona pellucida (ZP), induction of the acrosome reaction (AR), penetration of the ZP and fusion of sperm with the egg vitelline membrane [1]. Sperm capacitation involves metabolic and functional changes such as the removal or appearance of specific molecules and important changes in the plasma membrane; the acrosome reaction is an exocytotic event induced by calcium influx that renders the spermatozoa capable to penetrate the ZP and fuse with the plasma membrane of the egg [2].
However, these processes can be achieved only if the spermatozoa are viable, in addition to other parameters. The determination of sperm viability is a useful technique for the assessment of semen quality; membrane integrity and mitochondrial activity are important viability parameters of spermatozoa. Different fluorescent techniques based on the permeability of the cell membrane to dyes have been developed to determine these parameters; it is possible to detect one or more different fluorochromes in a cell, enabling the simultaneous quantification and analysis of the fluorescence intensity of one or more populations [3,4]. Propidium iodide (PI) is a vital fluorescent dye that binds DNA in a non-covalent manner, indicating plasma membrane damage when cells emit red fluorescence [5]. Rhodamine 123 (R123) is a vital fluorescent dye that directly stains mitochondria, providing low-background high-resolution green fluorescence; because there are no apparent cytotoxic effects, it is used often to assess mitochondrial function [6,7]. Flow cytometry has been used to quantify the fluorescence intensity emitted by large populations of stained sperm cells in a short period of time [8-10].
The aim of this work was to determine the viability of boar sperm (fresh, one hour post-capacitation induction and acrosome reacted) by flow cytometry using PI and R123.
2. Materials and Methods
All chemicals were purchased from Sigma Chemical Company (St. Louis, MO), unless otherwise indicated.
Semen samples were obtained from the sperm-enriched fraction of the ejaculates from 3 healthy Landrace fertile boars using the gloved-hand method, followed by the removal of the gelatinous fraction.
Semen analysis was performed under a light microscope. All the samples were classified as normozoospermic according to established criteria [11]. The semen was diluted in Beltsville liquid extender (BL-1) to improve the viability of the sperm during the 12 h required for transportation at 16˚C [12,13].
2.1. Fresh Sperm
To remove the semen plasma, the semen was washed twice by adding 1 mL of phosphate-buffered saline (PBS) to an equal volume of semen, followed by centrifugation at 600× g for 5 min. The pellet was resuspended in 1 mL of PBS.
2.2. Capacitation Induction
Following the two washes described above, the pellet was resuspended in 1 mL of PBS. Aliquots of 8 × 106 cells were seeded in a Nunc 4-well multidish (Nunc, Denmark) with 1 mL of capacitation medium (TALPHEPES) supplemented with 6 mg/mL of bovine serum albumin fraction V (BSA) and 7 mM sodium pyruvate, pH 7.4 [14,15].
The cells were incubated for 1 h at 39˚C in a humid atmosphere with 5% CO2 [16].
2.3. Acrosome Reaction (AR) Induction
Progesterone was added to the samples at a final concentration of 10 µg/mL to induce the AR and incubated for 20 min under the same conditions as above [17,18].
2.4. Flow Cytometry
Aliquots of 5 × 105 sperm in 200 µL were incubated under three conditions:
1) 25 µL of IP (1.25 μg/mL) for 10 min at 38˚C. The samples were then washed twice in 1 mL of PBS and centrifuged for 5 min at 600× g.
2) 5 µL of R123 (0.20 μg/mL) for 10 min at 38˚C. The samples were then washed twice in 1 mL of PBS and centrifuged for 5 min at 600× g.
3) 25 µL of IP (1.25 μg/mL) and 5 µL of R123 (0.20 μg/mL) for 20 min at 38˚C. The samples were then washed twice in 1 mL of PBS and centrifuged for 5 min at 600× g.
The labeled pellets were resuspended and fixed in 1% paraformaldehyde in PBS. The proportion of labeled spermatozoa and their fluorescence intensities were measured using a FACScan flow cytometer (Becton Dickinson, Immunocytometry System, CA, USA). Five thousand cells per sample were analyzed. The data were analyzed using a paired Student’s T test; probability values P < 0.05 were considered significant [4,16].
2.5. Light Microscopy
Sperm viability was evaluated by eosin-nigrosin staining (1% eosin and 5% nigrosin), mixing three parts of semen and one part of stain. Two hundred cells of each dried preparation were analyzed using a light microscopy (magnification × 400) [19,20].
3. Results and Discussion
There are many interrelated physiological aspects of spermatozoa, including viability, motility and mitochondrial function and such characteristics can be assessed by flow cytometry. Using live and dead cells, the optimal final stain concentrations specific for the sperm concentrations and culture conditions used in this study were determined in preliminary assays (data not shown).
We analyzed the sperm viability of samples transported in BL-1 under three conditions: fresh (without incubation), capacitated (one hour of incubation in capacitation medium) and acrosome reacted (acrosome reaction induced with progesterone).
We expressed the results of flow cytometry as the Fluorescence index (FI) by multiplying the percentage and fluorescence intensity of the labeled sperm [10]. When stained only with PI, we observed that the FI of the reacted sperm was higher than in the capacitated sperm and still higher than in the fresh sperm, with statistically significant differences. There was no difference between the fresh and capacitated sperm (Table 1).
We observed significant differences between the groups of fresh and capacitated sperm, between the fresh and reacted sperm and between the capacitated and reacted sperm when using R123 (Table 2).
Regarding the combined staining with PI and R123, it was observed that the FI emitted by PI gradually increased and we found significant differences between the fresh and reacted and between the capacitated and reacted sperm. Using R123, the FI increased in the capacitated but decreased in the acrosome-reacted sperm, with
Table 1. Sperm viability evaluated with propidium iodide and flow cytometry (fluorescence index).

a, b: P < 0.005.
Table 2. Sperm viability evaluated with Rhodamine 123 and flow cytometry (fluorescence index).
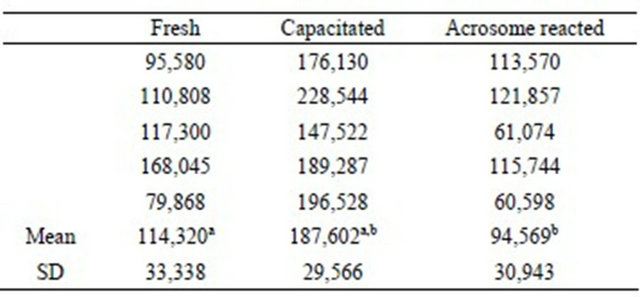
a: P < 0.05; b: P < 0.005.
significant differences between the fresh and capacitated sperm (Table 3); although there was no significant difference between the capacitated and acrosome-reacted groups, there was a tendency toward a decrease of fluorescence in acrosome-reacted spermatozoa. These data are consistent with those found when using PI and R123 separately. It is important to note that by increasing the temperature to 25˚C, Medrano et al. detected changes in the permeability of the spermatozoa plasma membrane, and these authors reported many fluorescent cells using PI [21]. We think that PI is not the best vital stain for capacitated and acrosome-reacted spermatozoa studies because the plasma and acrosome membranes become fluid and permeable during these processes, resulting in inaccurate data.
The staining intensity of R123 is concentration dependent under equilibrium conditions over a range of 0.2 - l50 µg/mL. Some authors report the use of 10 µg/mL [7,22], however we used a lower concentration and found a good fluorescent signal. The use of R123 is advantageous because it measures the mitochondrial activity of spermatozoa that are still alive, even though the plasma membrane is permeable because of the acrosome reaction and these spermatozoa could be counted as dead cells by PI staining [23].
Our results suggest that the increase in R123 fluorescence intensity in capacitated sperm is due to changes in the mitochondrial activity rather than the mitochondrial number because the spermatozoa underwent flagellar
Table 3. Sperm viability evaluated simultaneously using PI and R123 and flow cytometry (fluorescence index).

a,b: P < 0.05; c: P < 0.001.
activation during the capacitation process [24]. The capacitation status has been observed through calciummediated changes using chlortetracycline or by changes in membrane fluidity monitored by the binding of the fluorescent amphiphilic probes Merocyanine 540, annexin-V, C6NBD and Ro-09-0198 [25]. To our knowledge, there are no reports of the status of mitochondrial activity of boar sperm measured with R123 after one hour of capacitation.
To verify and to compare our flow cytometry data, we also utilized light microscopy with eosin-nigrosin staining to evaluate the sperm samples. Using this technique, the dead sperm stained red or pink because the integrity of their plasma membranes had been compromised, causing an increase in membrane permeability that led to the uptake of the dye; in contrast, the live sperm remained white. We found a gradual decrease in live sperm, i.e., the fresh sperm group had a greater percentage of living cells (80 ± 12), which decreased after one hour of capacitation induction (62 ± 9) and decreased further in the acrosome-reacted group (54 ± 12); the differences between the fresh and the other two groups were significant (P < 0.05, n = 5). These data are in agreement with those obtained with the PI-flow cytometry method.
The use of fluorochromes and flow cytometry is a good tool for monitoring many markers of sperm function. Although the capacitation and acrosome reaction processes have been studied for many years, being that these processes are so complex, there is still much information to be elucidated.
4. Acknowledgements
This study was partially supported by the CONACYT (México) grant 0105961/10110/194/09. The authors thank Jacqueline Chanel for technical assistance.
REFERENCES
- R. Yanagimachi, “Mechanisms of Fertilization in Mammals,” In: L. Mastroianni and J. D. Biggers, Eds., Fertilization and Embryonic Development in Vitro, Plenum Press, New York, 1981, pp. 81-182.
- R. Yanagimachi, “Mammalian Fertilization,” In: E. Knobil and J. D. Neill, Eds., The Physiology of Reproduction, Vol. 1, Raven Press, New York, 1994, pp. 189-317.
- P. Talbot and R. Chacon, “A Triple Stain Technique for Evaluating Normal Acrosome Reactions of Human Sperm,” Journal of Experimental Zoology, Vol. 215, No. 2, 1981, pp. 201-211. doi:10.1002/jez.1402150210
- R. Fierro, M. C. Bene, B. Foliguet, G. C. Faure and G. Grignon, “Evaluation of Human Sperm Acrosome Reaction and Viability by Flow Cytometry,” Italian Journal of Anatomy and Embryology, Vol. 103, No. 41998, pp. 75- 84.
- D. L. Garner, D. Pinkel, L. A. Johnson and M. M. Pace, “Assessment of Spermatozoal Function Using Dual Fluorescent Staining and Flow Cytometric Analyses,” Biology of Reproduction, Vol. 34, No. 1, 1986, pp. 127-138. doi:10.1095/biolreprod34.1.127
- L. V. Johnson, M. L. Walsh and L. B. Chen, “Localization of Mitochondria in Living Cells with Rhodamine 123,” Proceedings of the National Academy of Sciences USA, Vol. 77, No. 2, 1980, pp. 990-994. doi:10.1073/pnas.77.2.990
- D. P. Evenson, Z. Darzynkiewicz and M. R. Melamed, “Simultaneous Measurement by Flow Cytometry of Sperm Cell Viability and Mitochondrial Membrane Potential Related to Cell Motility,” The Journal of Histochemistry & Cytochemistry, Vol. 30, No. 3, 1982, pp. 279-280. doi:10.1177/30.3.6174566
- M. A. Van Dilla, B. L. Gledhill, S. Lake, P. N. Dean, J. W. Gray, V. Kachel, et al., “Measurement of Mammalian Sperm Deoxyribonucleic Acid by Flow Cytometry. Problems and Approaches,” The Journal of Histochemistry & Cytochemistry, Vol. 25, No. 7, 1977, pp. 763-773. doi:10.1177/25.7.70455
- M. Kerker, D. S. Wang and H. W. Chew, “An Optical Model for Fluorescence of Mammalian Sperm in Flow Cytometry,” Cytometry, Vol. 1, No. 2, 1980, pp. 161-167. doi:10.1002/cyto.990010212
- R. Fierro, B. Foliguet, G. Grignon, M. Daniel, M. C. Bene, G. C. Faure, et al., “Lectin-Binding Sites on Human Sperm During Acrosome Reaction: Modifications Judged by Electron Microscopy/Flow Cytometry,” Archives of Andrology, Vol. 136, No. 3, 1996, pp. 187-196. doi:10.3109/01485019608987095
- D. L. Garner and E. S. E. Hafez, “Spermatozoa and Seminal Plasma,” In: E. S. E. Hafez, Ed., Reproduction in Farm Animals, Lea & Febiger, Philadelphia, 1993, pp. 165-187.
- L. A. Johnson, J. G. Aalbers, C. M. T. Willems, J. H. M. Rademaker and C. E. Rexroad Jr., “Use of Boar Spermatozoa for Artificial Insemination. III. Fecundity of Boar Spermatozoa Stored in Beltsville Liquid and Kiev Extenders for Three Days at 18˚C,” Journal of Animal Science, Vol. 54, No. 1, 1982, pp. 132-136.
- J. Conejo-Nava, R. Fierro, C. G. Gutierrez and M. Betancourt, “Membrane Status and in Vitro Capacitation of Porcine Sperm Preserved in Long-Term Extender at 16˚C,” Archives of Andrology, Vol. 49, No. 4, 2003, pp. 287-295.
- E. Bonilla, A. Amador and M. Betancourt, “In Vitro Capacitation of Boar Sperm in a Protein-Free Medium Supplemented with Histidine and Cysteine,” Medical Science Research, Vol. 22, 1994, pp. 725-726.
- M. Betancourt, R. Fierro and D. Ambriz, “In Vitro Fertilization of Pig Oocytes Matured in Vitro,” Theriogenology, Vol. 40, 1993, pp. 1155-1160. doi:10.1016/0093-691X(93)90286-E
- I. Jiménez, H. Gonzalez-Marquez, R. Ortiz, M. Betancourt, J. Herrera and R. Fierro, “Expression of Lectin Receptors on the Membrane Surface of Sperm of Fertile and Subfertile Boars by Flow Cytometry,” Archives of Andrology, Vol. 48, No. 2, 2002, pp. 159-166. doi:10.1080/014850102317267481
- T. Berger, “Pisum Sativun Agglutinen Used as an Acrosomal Stain of Parene and Craprine Sperm,” Theriogenology, Vol. 33, 1990, pp. 689-695. doi:10.1016/0093-691X(90)90546-6
- J. A. Herrera, R. Fierro, H. Zayas, A. García, J. Conejo, I. Jiménez, et al., “Acrosome Reaction in Fertile and Subfertile Boar Sperm,” Archives of Andrology, Vol. 48, No. 2, 2002, pp. 161-167. doi:10.1080/014850102317267445
- L. B. Emilson, K. A. Dougherty, A. T. Cockett and R. L. Urry, “Simultaneous Determination of Human Sperm Morphology and Viability: Simple Office Technique,” Urology, Vol. 11, No. 5, 1978, pp. 488-491. doi:10.1016/0090-4295(78)90165-6
- M. R. Bakst, “Fertilizing Capacity and Morphology of Fowl and Turkey Spermatozoa in Hypotonic Extender,” Journal of Reproduction and Fertility, Vol. 60, No. 1, 1980, pp. 121-127. doi:10.1530/jrf.0.0600121
- A. Medrano and W. V. Holt, “Protective Effects of Glycerol during Cold Shock in Boar Spermatozoa. A Cryomicroscope Study Using Propidium Iodide and SYBR-14,” Reproduction in Domestic Animals, Vol. 31, No. 1, 1995, pp. 281-282. doi:10.1111/j.1439-0531.1995.tb00052.x
- J. K. Graham, E. Kunze and R H. Hammerstedt, “Analysis of Sperm Cell Viability, Acrosomal Integrity, and Mitochondrial Function Using Flow Cytometry,” Biology of Reproduction, Vol. 43, No. 1, 1990, pp. 55-64. doi:10.1095/biolreprod43.1.55
- D. J. McLean, N. Korn, B. S. Perez and R. J. Thurston, “Isolation and Characterization of Mitochondria from Turkey Spermatozoa,” Journal of Andrology, Vol. 14, No. 6, 1993, pp. 433-438.
- B. Marquez and S. S. Suarez, “Different Signaling Pathways in Bovine Sperm Regulate Capacitation and Hyperactivation,” Biology of Reproduction, Vol. 70, No. 6, 2004, pp. 1626-1633. doi:10.1095/biolreprod.103.026476
- L. Gillan, G. Evans and W. M. C. Maxwell, “Flow Cytometric Evaluation of Sperm Parameters in Relation to Fertility Potential,” Theriogenology, Vol. 63, No. 2, 2005, pp. 445-457. doi:10.1016/j.theriogenology.2004.09.024.
NOTES
*Corresponding author.

