New Journal of Glass and Ceramics
Vol.2 No.1(2012), Article ID:16981,10 pages DOI:10.4236/njgc.2012.21007
Effect of Ceramic Crucibles on Magneto-Optical PbO-Bi2O3-B2O3 Glasses Properties
![]()
Department of Material Science & Chemical Engineering, Politecnico di Torino, Torino, Italy.
Email: qiuling.chen@polito.it
Received October 2nd, 2011; revised November 23rd, 2011; accepted December 3rd, 2011
Keywords: Magneto Optical Glass; Ceramic Crucibles; Verdet Constant
ABSTRACT
Heavy-metal-oxide (HMO) glasses attract much interest in many applications such as Faraday rotators, current sensors, etc., in the area of magneto-optic effects due to their unique magnetic-optical property, high refractive index and other interesting properties. However, during the melt-quenching process of these glasses, the high corrosive nature of the melt to the crucibles makes the fabrication of HMO glasses complicated and the properties of the obtained glasses show strong dependence on the crucible materials. Literatures reported that the gold and platinum crucibles are not suitable due to their contamination to the melt glasses, ceramic crucible was considered suitable for the melting of HMO glasses. In this work, magnetic-optical glasses within the system of PbO-Bi2O3-B2O3 have been prepared using different kinds of ceramic crucibles for the aim of finding the most suitable crucible for melting HMO glasses. The glass properties in terms of Verdet constant, thermal stability and UV-Vis-IR transmittance in function of different crucibles were studied and reported. It was found that the same batch of glasses prepared under same conditions (melting temperature, melting time and annealing process), but in different ceramic crucibles (coded as C1, C2 and C3) showed significant difference in properties such as glass forming ability, thermal stability, optical absorption in UV-Vis-IR and Verdet constant (0.0812 - 0.1483 min/G·cm). The ceramic crucible made of 25% Al2O3 and 75% SiO2 (C2) was found to be the most suitable for PbO-Bi2O3-B2O3 glass preparation, compared with platinum, gold, C1 and C3. Glasses melted with C2 exhibit good performance in magneto and optical property, as well as good thermal stability.
1. Introduction
Recently, studies on the magnet-optical property of HMO glasses attract many interests due to their potential applications as Magneto Optical Current Transducer (MOCT) for high voltage electric current system, optical rotator and isolator with large aperture, circulators, switches, polarimeters, etc. Heavy metal oxide glasses based on PbO and Bi2O3 have attracted considerable attention due to their high Verdet constant, interesting physical and optical properties leading to different applications [1,2].
The high density, high refractive index, and low transformation temperature of these glasses make them advantageous for some applications for optical and electronic devices [3].
It is well known that the technique used for fabrication of PbO and Bi2O3 based heavy metal oxide glasses is the traditional melt-quenching, which is normally a simple and low cost technique for glass making. However, meltquenching technique shows some problem with the preparation of PbO and Bi2O3 based glasses, since these glasses in the molten state are chemically corrosive and they react with the crucible materials to dissolve some crucible elements into the glass melts, especially for the widely used gold and platinum crucibles. Due to the incorporation and subsequent aggregation of nanoparticles of metals into the glass matrix, the gold and platinum crucibles were found not suitable for preparation of PbO and Bi2O3 based magnetic-optical glasses, because the incorporation of metal nanoparticles of into the glass matrix makes the glass easy to crystallize [4] and the darkening will impact greatly the optical transmittance.
It has been noted that the Verdet constant changes with the change of glass composition and could be affected by several intrinsic and extrinsic factors [5].
When HMO glass is used as an Faraday isolator materials, not only the Verdet constant is required to be high, but also platinum incorporation coming from the crucible during melting is to be avoided: when the glass having a platinum incorporation is irradiated with laser light of high intensity, the platinum absorbs the laser light and local heat is generated, thus causing thermal stress, cracks and general damage on the glass. Due to the induced damage, the laser light is scattered, the output is greatly decreased and disorder of the laser mode is caused, which make the laser light not correctly focused [6]. In addition, for Bi2O3 based glasses, there exists a strong absorption in the range 470 - 600 nm due to platinum contamination [7].
Ceramic crucibles are therefore chosen for fabrication of HMO glasses by different researchers [8-16]. Some of them are pure Al2O3 [11,12,14,15], other are Al2O3 and SiO2 based [8,9,16], while in some cases [10,13], the chemical composition is not mentioned [17,18]. Even though different kinds of crucible materials have different effects on the glass properties, for using crucible, it is one-time for each melting which will increase the cost of research greatly, especially for the highly repeatedly study on such glass. Literature [9,16] reported the effect of ceramic crucibles on the optical absorption of heavy metal oxide glass containing bismuth oxide and the effect of the melting temperature and time on the thermal stability and optical transmittance of PbO-Bi2O3-Ga2O3 glasses. The crucible materials in [16] are made of zirconia and tin oxide, which are not commonly used due to their high cost and low stability of the obtained glasses [19].
In this study, glasses of the same composition within the system of PbO-Bi2O3-B2O3 have been prepared for magneto-optical applications [20] by using three low cost ceramic crucibles in order to get a suitable crucible for one-time melting for heavy metal oxide glass study. The effects on the glass properties, particularly the magneticoptical properties of the glasses were selected as factors to evaluate the crucibles.
2. Experimental
The crucible compositions employed in this study were listed in Table 1. They are pure alumina (coded as C1), silica doped with 25% Al2O3 (coded as C2), and silica doped with 15% Al2O3 and 10% Na2O and other minor components (coded as C3).
Three glasses with composition in weight percent of 37% PbO-61% Bi2O3 -2% B2O3 (coded as PBB01), 26% PbO-69% Bi2O3-5% B2O3 (PBB04), and 10% PbO-86% Bi2O3-4%B2O3 (PBB07) were prepared with the three different crucibles respectively(C1, C2 and C3 in Table 1).
Reagents were weighted using an electronic balance (Ohaus-AR2140) (accuracy 0.0001 g) and batched into the three different crucibles. The chemical purity of used regents was 99.9+% for PbO, 99.99% for Bi2O3, 99.99% for H3BO3 respectively.
The batched powders for the same glass composition (PBB01, PBB04 and PBB07) were melted C1, C2 and C3, respectively, under the same conditions (same melting Temperature for 30 minutes in electric furnace in air, Carbolite 1200), for comparison purposes. The melting temperature for different glass systems ranged from 900˚C to 1100˚C. The glass melts were stirred 5 - 10 seconds for homogenization during melting. The melted glasses were casted onto a brass mould preheated at 150˚C. The obtained glass was cut into 10 mm × 2 mm slabs after 4 hours annealing at 250˚C, and optically polished for characterization by using a Logitech PM5.
The glasses were observed by scanning electron microscopy (SEM), coupled with an energy dispersive Xray analyzer (EDX).
Differential scanning calorimetry (DSC) (Perkin-Elmer DSC-7) was used to measure the glass transformation temperature Tg, and the onset of crystallization peak, Tx. The density of samples was determined at room temperature using the Archimedes’ principle. Bulk glasses without flaws were used with distilled water as the immersion liquid.
The refractive indexes of samples were measured using a prism coupling method (Metricon Model 2010M). Spectral absorption in the ultraviolet, visible and infrared was recorded on each polished sample using a UV-Visible-IR spectrophotometer (Varian Cary 500) and FT-IR (Tensor 37). The slope line was extended to cross the horizontal axis (wavelength), the crossing-point taken as the absorption cutoff wavelength.
The crucible material incorporation into the glasses was studied by scanning electron microscopy (SEM), coupled with an energy dispersive X-ray analyzer (EDX).
The Verdet constant of the samples were tested with a custom-made single light optical bench, Figure 1 shows the schematic drawing and setup of the apparatus.
As shown in Figure 1, the static magnetic field is generated by solenoids on which a DC-potential of 12 V of voltage was applied. The samples were put into the center of the solenoids, the polarized laser light (λ = 632.8 nm, 15 mW, polarization ratio larger than 500:1) go through the sample and after the second polarizer can be detected by using the powermeter in terms of I0 and I, where I0 is the intensity of laser light propagating through the sample under the magnetic field, and I is the intensity which go through samples without magnetic field applied.
Based on Faraday Effect, the relationship between I and I0 is I = I0 cos2(α + θ), α is the angle between polarized light and analyzer axis without applied magnetic

Table 1. Compositions for melting glass crucibles.

Figure 1. Schematic diagram of optical bench set-up for Faraday rotation measurement. 1. He-Ne Laser; 2. Polarizers; 3. Bulk glass; 4. DC supplier; 5. Solenoide; 6. Power meter.
field, θ is the angle due to Faraday Effect. The difference ΔI = I – I0 can be derived as: ΔI = I0 sin2 (θ)∞I0θ2 (while α = 90°). In this way, the Faraday rotation angle (θ) of the samples under the magnetic field can be calculated by: θ2 = ΔI/I0.
Basing on the equation θ = VHL, the pure silica glass is set as the reference material and for calculating the Verdet constant of the measured sample. Pure silica will be used as the reference during the measurement and calculation according to the following formula.
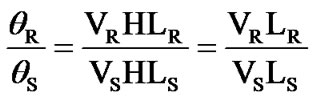
The subscript R means pure silica glass as reference, subscript S means the measured samples, and the Verdet constant of pure silica (VR) is 0.01354 min/G·cm at 633 nm [21]. As a validation test for this apparatus, PbOBi2O3-GeO2-B2O3 glasses in literature were fabricated and tested by this bench, and the Verdet constant were very close to literature report. For example, the tested Verdet constant of sample 6 PBGB glass system is 0.1248 min/G·cm, and the reported value literature [22] is 0.127 min/G·cm.
3. Results
It is found that the same batch of glasses prepared under same conditions (melting temperature, melting time and annealing process) but in three different crucibles C1, C2 and C3 showed significant difference in properties such as glass forming ability, thermal stability, optical absorption in UV-Vis-IR and Verdet constant etc. The effects of crucible materials on glasses are reported in the following paragraphs.
3.1. Effects of Different Crucibles on the Glasses
The effects of different crucible materials on the glass color and glass compositions are summarized in Table 2. As it can be seen, for all the three glass samples their color changed with crucible materials from light yellow to brown. Glasses melted in C1 showed light yellow color, but they became yellow in C2 and brown in C3.
The formation of cracks after casting for the same glass composition was deeply affected by the crucible materials, as it can be seen from Table 2. The batches melted in C1 tend to crack immediately after casting, while using C2 and C3, such phenomena were avoided. The glasses melted by using C2 and C3 were less prone to crack than those melted by using C1. Figure 2 shows

Table 2. The effects of different crucible materials (C1, C2, and C3) on glasses, (*) other elements as obtained by EDS. Boron cannot be detected by this equipment.

Figure 2. XRD of the glasses PBB04 (26% PbO-69% Bi2O3-5% B2O3, wt%) when melted with different crucibles at 1000˚C: a clear change in XRD spectra can be noted (PBB01 and 07, not reported here, gave similar XRD).
the comparison of XRD spectra of glass PBB04 melted in different crucibles.
It can be seen that the batches melted with C1 showed several peaks due to crystalline Al2PbO4. When batches were melted with C2, two broad wide bands can be seen which indicated the glassy character. For C3, even through the peaks are not so many as that of C1, but there still four peaks and they are still Al2PbO4. According to the Debye-Scherrer formula Dhkl = kλ/βcosθ, the approximate size of all the crystals are calculated to be 4.6 nm.
The changes in glass compositions were analyzed by EDX and the approximate amount of crucible materials incorporated in the glass melt was calculated and listed in the Table 2. Figure 3 illustrated the EDX of the same
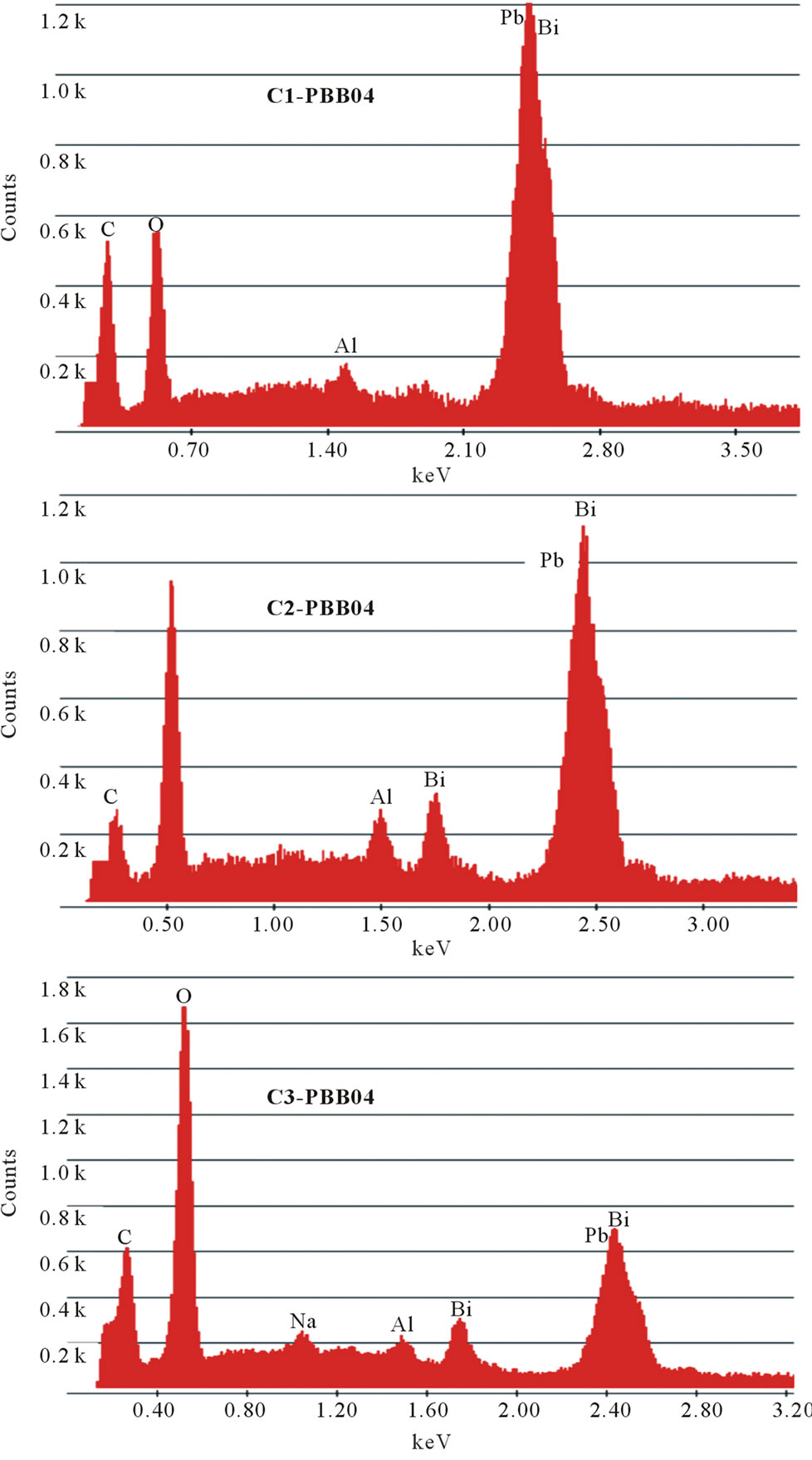
Figure 3. EDX images of compositions of glass PBB04 melted with different crucibles.
glass sample prepared by the three crucibles (C1, C2 and C3).
The other two glass systems (PBB01 and PBB07) melted with different crucibles gave similar results and are not reported here. It can be seen from the Figure 3, during the melting process, some components from crucibles entered into the glass and changed the glass composition; in particular, they decreased the percentage of heavy metal oxides (PbO and Bi2O3) responsible of a high Verdet costant. The samples melted with C3 Figure 3, had large amount of light component (13.59% in weight) coming from the crucible. On the contrary, few light components (1.28%) such as alumina were introduced into the samples melted in C1; a few light components such as alumina and silica were introduced in the samples melted in C2.
3.2. Effects of Different Crucibles on the Glass Thermal Stability
The glass stability against devitrification can be in a first approximation expressed by ∆T = Tx – Tg. Figure 4 shows the plot of ∆T vs the difference of crucible for the three glasses.
From the Figure 4 and Table 3, the ∆T factor of three glass systems showed the same trend: as expected, the glass samples prepared by crucible C2 and C3 showed better thermal stability than that of C1 which showed the lowest thermal stability.
3.3. Effects of Different Crucibles on the Magneto-Optical Property
The Verdet constant measurement was carried out for each glass system. Table 4 reports the Verdet constant (V) and refractive index (n) at 633 nm vs different crucibles employed.
From the measurement results, the glass PBB01 with the compositions of 37% PbO-61% Bi2O3-2% B2O3 (wt%) exhibited the best performance of Verdet constant, due to its high amount of lead and bismuth oxides. However, a significant influence of crucible materials on the Verdet constant for the same glass sample was observed in Table 4: the Verdet constant and the refractive index n as well for all the three glass samples showed the largest value when prepared by the crucible C1 and smallest one when prepared by crucible C3, while the value for C1 and C2 are quite close.
3.4. Effects of Different Crucibles on the Glass Optical Absorption and FT-IR Transmittance
The absorption spectra between 200 nm to 800 nm for all the three glass samples prepared by crucible C1, C2 and C3 respectively were measured. The absorption coefficient (a) and cutoff value was calculated and shown in Table 4. Figure 5 shows the absorption of the glasses melted with C1, C2 and C3.
As in Table 5, samples melted with C2 have smaller absorption coefficient compared to C1 and C3. The samples melted in C1 had very high absorption coefficient.
As in Figure 5, the crucible used did not change the shape of the cutoff (all of them has a steep cutoff), but the cutoff for C2 and C3 is shifted to shorter wavelength compared to that of C1.
A typical FT-IR spectrum of PBB01, PBB04 and PBB07 glasses melted with different crucibles are shown in Figure 6. A wide band near 3000 cm–1 was observed and is due to the fundamental vibration of OHabsorption band [23]. The peak near 2350 cm–1 is probably due to the heavy metal oxide element vibration in the glass [24].

Table 3. Density and DSC measurement results on the glasses melted in different crucibles.
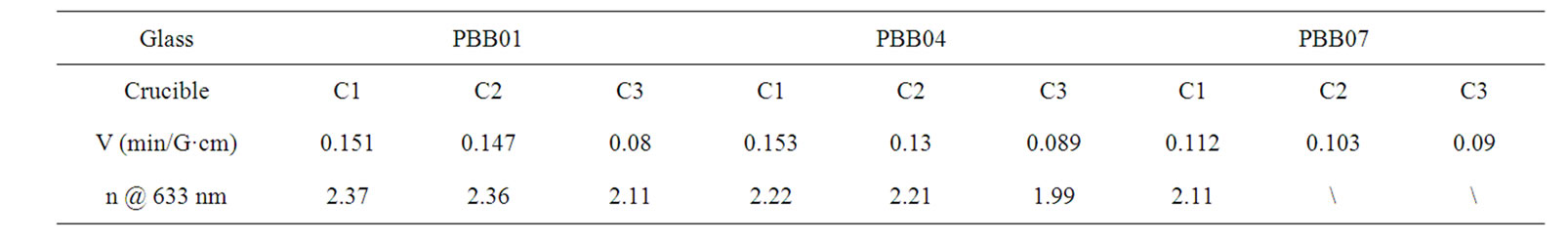
Table 4. Magneto-optical behavior of glass melted in different crucibles.

Table 5. Optical absorption coefficient and cutoff of the glasses melted in different crucibles.

Figure 4. Glass stability factor ∆T vs melting crucible.
In summary, the transmittance of glasses melted in crucible C2 exhibited the best performance.
4. Discussion
4.1. Effects of Crucible Materials on the Glass Colour, Compositions and Density
The change of glass properties and colour by using different crucible materials are mainly due to the change of glass compositions through the reaction between the crucible materials and glass melts and the incorporation of crucible materials into the glass melts, as shown in the Table 2.
The change of glass colour due to the change of crucible materials is also reported in [9,16]. In this study, glass prepared by C3 showed the brown colour while the samples prepared by C1 shown light yellow colour.
With the increase of incorporation of light oxides from the crucible, the change of glass nominal composition in terms of PbO and Bi2O3 increased. The change of compositions is in good agreement to the change of density. The presence of heavy metal oxide in glasses contributes to glass density value. Since the atomic weight of bismuth and lead is higher than the atomic weight of Al and Si, equimolar replacement of PbO and Bi2O3 by Al2O3, Na2O and SiO2 leads to the increasing of volume of mass glass and the decreasing of density.
4.2. Effects of Crucible Materials on the Glass Nature and Thermal Stability
It is well known that the introduction of the alumina, Na2O and silica improves the glass-forming ability. This is because their partial dissolution in the melt modifies the original compositions of the samples and inhibits their crystallization [9]. This can be observed from Figure 5. The crystallization temperature increases when
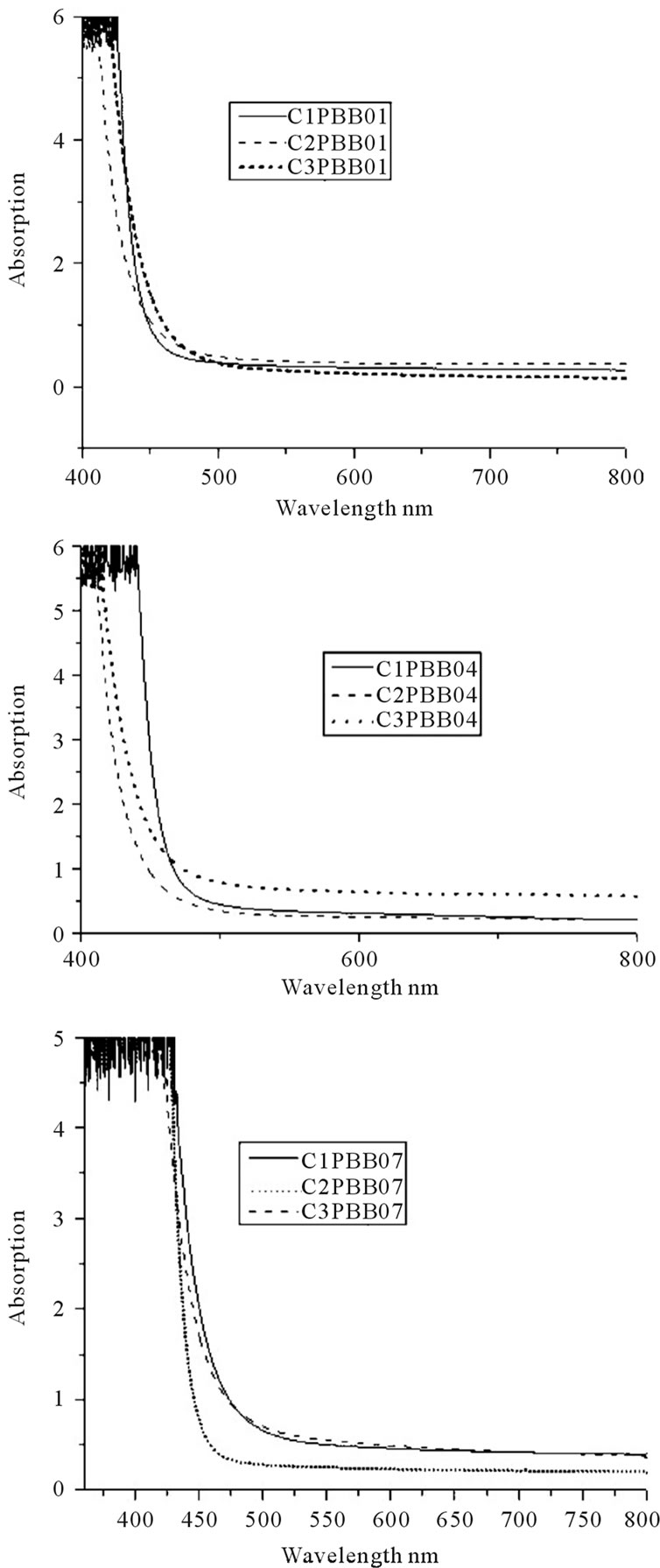
Figure 5. UV Absorption spectra vs crucibles for the three glasses.
samples melted with C2 and C3. The role of Al2O3 in the glass matrix effectively lowers the liquidus temperature of the system and improved the thermal stability to hinder the devitrification [25]. In other words, Al or Na ions break the Pb-O or Bi-O bonds and reduce the chance of crystallization [26].
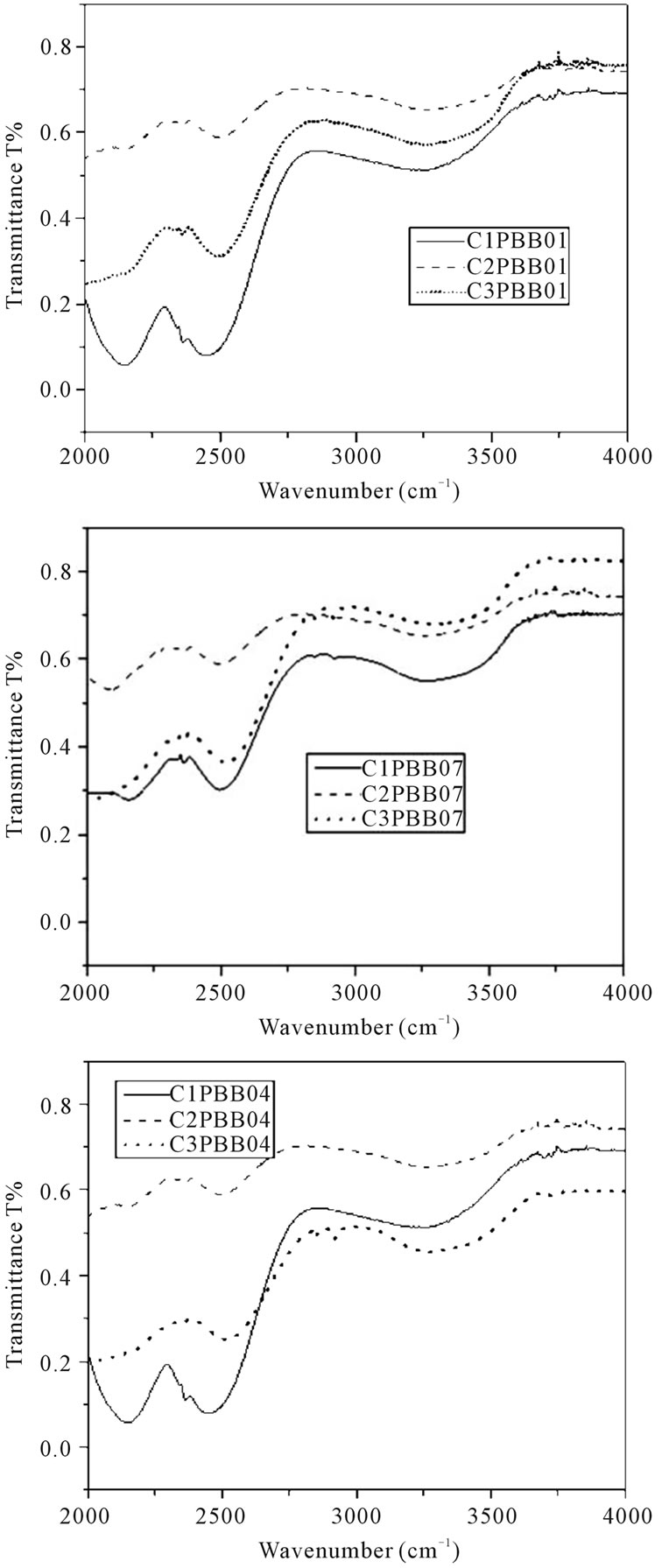
Figure 6. FT-IR spectrum of glasses (2 mm thickness) melted in three crucibles.
This can be confirmed by the XRD test of the samples using different crucible: the samples melted with C2 has good glass-forming ability, this is proved not only during their casting process as in Table 1, but also from the XRD spectra in Figure 2.
According to literature [27], the glass-forming ability appears to be in a first approximation determined by the difference between crystallization and glass transition temperatures (∆T = Tx – Tg). According to this, the glass forming ability has close relationship with the thermal stability of the glass. From Figure 4 and Table 4, the ∆T factor of three glass systems showed, as expected, the same clear trend: the glass samples prepared by crucible C2 and C3 showed better thermal stability than that of C1 which showed the lowest thermal stability.
4.3. Effects of Crucible Materials on the Magneto-Optical Property
According to literature [20] and [28], the Verdet constant of glasses linearly increased with the sum of HMO concentration due to the high optical dispersion and strong polarization of heavy metal ions. In this case, the Verdet constant has close relation to the amount percent of HMO in glass matrix and the polarization density of the glass. This can be explained by the decrease of polarization density induced by the incorporation into the glass of crucible components. The high concentration of HMO component glass has a big polarizability which will cause a higher Verdet constant and refractive index. In this study, when the PbO and Bi2O3 were substituted with other component such as Al and Si and Na oxides from the crucible materials, the sum of polarizability of the constituent ions and the molecular volumes will be decreased [26], because the larger the radius of the cation (Pb2+ and Bi3+), the stronger the polarizability of the cation will be (Pb2+, Bi3+ > Al3+, Si4+). It was found in this study that glasses prepared by crucible C3, showed the lowest value of Verdet constant since the incorporation of Al, Si and other oxide impurities from C3 into the glass matrix enlarges the volume and decrease the weight percentage of HMO ions in the glass. Such change of structure and composition reduces V and n values.
The reason why the V and n did not change so much for glasses prepared with C2 and C1, is that the nominal compositions of the glasses prepared by C1 and C2 did not change so much as shown in Figure 2, which means the polarization density is not reduced that much. Another reason is probably that in C2, there was no other incorporation except the Al and Si oxides. According to the literature [28], the Si4+ will show diamagnetism property in the presence of magnetic field which will contribute to the increase of V and n value. No literatures reported that the Al3+ exhibit such property.
4.4. Effects on the Glass Optical Absorption and FT-IR Transmittance
According to literature [9,16,29], with the increase of the amount of heavy metal oxide in glass, the cutoff in UV region will shift to longer wavelength. Conversely in this study, the introduction of Si4+ and more Al3+ in C2 and C3 decreased the Pb2+ and Bi3+ percentage in the glass system, and the cutoff shifted to shorter wavelength. The cutoff of glass melted in C2 showed a shift to shorter wavelength compared to C1. The reason why the cutoff of glass melts in C3 was a little bigger than that of C2, probably due to the diffusion of alkali (i.e. Na+, K+ ions) can move the cutoff to longer wavelength according to literatures [30].
In addition, the samples melted in C2 exhibited good optical absorption performance and FT-IR transmittance. On the contrary, the glass melted in C1 had high absorption, this is mainly due to the crystals in these glasses which increase the light scattering [9] (see Figure 2), and as for glass melted in C3, the impurities incorporation into the glass matrix also can increase the absorption [31], the brown colour of the samples melted in C3 contribute to the absorption as well.
5. Conclusion
A study on the effects of crucible materials on the properties of magneto-optical PbO-Bi2O3-B2O3 glasses was carried out in this paper. It is found that, three kinds of crucible materials (C1, C2 and C3) had significant effects on the glass properties in terms of the glass-forming ability, thermal stability, and density, Verdet constant and optical absorption. The changes of glass properties are mainly due to the changes of glass compositions and structures caused by the incorporation of elements from the crucibles into glass matrices. The crucible C2 with the composition of 25% Al2O3 and 75% SiO2 was found to be most suitable for PBB magnetic-optical glasses preparation because it combines a high value of Verdet constant with a clear improvement to the glass-forming ability, thermal stability and optical absorption.
REFERENCES
- Q. Chen, M. Ferraris. D. Milanese, et al., “Novel Erbium Doped PbO and B2O3 Based Glasses: Investigation of Quantum Efficiency and Non-Radiative Transition Probability for 1.5 µm Broadband Emission Fluorescence,” Journal of Non-Crystalline Solids, Vol. 324, No. 1-2, 2003, pp. 12-20. doi:10.1016/S0022-3093(03)00222-9
- Q. Chen , M. Ferraris, et al., “Novel Erbium Doped PbO and B2O3 Based Glasses with Broad 1.5 µm Absorption Line Width and Low Refractive Index,” Journal of NonCrystalline Solids, Vol. 324, No. 1-2, 2003, pp. 1-11.
- Z. D. Pan and S. H. Morgan, “Raman Spectra and Thermal Analysis of a New Lead-Tellurium-Germanate Glass System,” Journal of Non-Crystalline Solids, Vol. 210, No. 2-3, 1997, pp.130-135. doi:10.1016/S0022-3093(96)00603-5
- A. Bishay and C. Maghraby, “Properties of Bismuth Glasses in Relation to Structure,” Physics and Chemistry of Glasses, Vol. 10, 1969, pp.1-11.
- Z. Yang, S. Q. Xu, S. X. Dai, et al., “Research Development of Magneto-Optical Glass Applied to Optical Fiber Sensor,” Journal of Functional Materials And Devices, Vol. 9, No. 2, 2003, pp. 227-232.
- W. Li, K. Zou, M. Lu, B. Peng and W. Zhao, “Faraday Glasses with a Large Size and High Performance,” International Journal of Applied Ceramic Technology, Vol. 7, No. 3, 2010, pp. 369-374. doi:10.1111/j.1744-7402.2008.02344.x
- W. H. Dumbaugh, “Heavy Metal Oxide Glasses Containing Bi2O3,” Physics and Chemistry of Glasses, Vol. 27, 1986, pp. 119-123.
- S. Hazra, S. Mandal and A. Ghosh, “Properties of Unconventional Lithium Bismuthate Glasses,” Physical Review B, Vol. 56, No. 13, 1997, pp. 8021-8025. doi:10.1103/PhysRevB.56.8021
- O. Sanz, E. Haro-Poniatowski, J. Gonzalo and J. M. Fernandez Navarro, “Rare-Earth Photoluminescence in SolGel Derived Confined Glass Structures,” Journal of NonCrystalline Solids, Vol. 352, No. 6-7, 2006, pp. 761-768.
- P. S. Gahlot, A. Agarwal, V. P. Seth and S. Sanghi, “Study of EPR, Optical Properties and Electrical Conductivity of Vanadyl Doped Bi2O3·PbO·B2O3 Glasses,” Spectrochimica Acta Part A, Vol. 61, No. 6, 2005, pp. 1189-1194. doi:10.1016/j.saa.2004.06.040
- A. Pan and A. Ghosh, “A New Family of Lead-Bismuthate Glass with a Large Transmitting Window,” Journal of Non-Crystalline Solids, Vol. 271, No. 1-2, 2000, pp. 157-161.
- I. Ardelean, S. Simon and M. Peteanu, “Magnetic Susceptibility Studies on Bi2O3-PbO-As2O3-MnO2 Glasses,” Materials Letters, Vol. 39, No. 1, 1999, pp. 42-45. doi:10.1016/S0167-577X(98)00214-6
- V. C. Veeranna Gowda, C. Narayana Reddy and K. C. Radha, “Structural Investigations of Sodium Diborate Glasses Containing PbO, Bi2O3 and TeO2,” Journal of Non-Crystalline Solids, Vol. 353, No. 11-12, 2007, pp. 1150-1163.
- C. B. Pedroso, E. Munin and A. B. Villaverde, “Linear Displacements Sensor Based on the Magneto-Optical Faraday Effect,” Sensors and Actuators A: Physical, Vol. 70, No. 3, 1998, pp. 134-142.
- Y. Cheng, H. Xiao and W. Guo, “Thermal Behavior of GeO2 Doped PbO-B2O3-ZnO-Bi2O3 Glasses,” Materials Science and Engineering: A, Vol. 423, No. 1-2, 2006, pp. 184-188.
- I, M. Santos and R. C. M. Moreira, “Ceramic Crucibles: A New Alternative for Melting of PbO-BiO1.5-GaO1.5 Glasses,” Journal of Non-Crystalline Solids, Vol. 319, No. 3, 2003, pp. 304-310.
- H. Sun, L. Wen, S. Xu, et al., “Novel Lithium-BariumLead-Bismuth Glasses,” Journal of Materials Letters, Vol. 59, No. 8-9, 2005, pp. 959-962.
- N. Singh and K. J. Singh, “Comparative Study of Lead Borate and Bismuth Lead Borate Glass,” Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, Vol. 225, No. 3, 2004, pp. 305-309.
- C. M. S. Rodrigues, J. A. Labrincha and F. M. B. Marques, “Study of YSZ-Glass Composites by Impedance Spectroscopy,” Journal of The Electrochemical Society, Vol. 144, No. 12, 1997, pp. 4303-4309. doi:10.1149/1.1838182
- J. E. Shelby and J. T. Kolhi, “Rare-Earth Alumina Silicate Glasses,” Journal of the American Ceramic Society, Vol. 73, No. 1, 1990, pp. 39-42. doi:10.1111/j.1151-2916.1990.tb05087.x
- Y. Ruan, R. A. Jarvis, A. V. Rode, et al., “Wavelength Dispersion of Verdet Constants in Chalcogenide Glasses,” Optics Communications, Vol. 252, No. 1-3, 2005, pp. 39- 45.
- C. B. Pedroso, E. Munin, et al., “Magneto-Optical Rotation of Heavy-Metal Oxide Glasses,” Journal of Non-Crystalline Solids, Vol. 231, No. 1-2, 1998, pp. 134-142. doi:10.1016/S0022-3093(98)00408-6
- K. Kobayashi, “Development of Infrared Transmitting Glasses,” Journal of Non-Crystalline Solids, Vol. 316, No. 2-3, 2003, pp. 403-406.
- E. P. Golis and M. Reben, “Investigations of Tellurite Glasses for Optoelectronics Devices,” Optica Applicata, Vol. 38, No. 1, 2008, pp. 163-169.
- M. Yamane, “Glasses for Photonics,” 1st Edition, Cambridge University Press, Cambridge, 2005.
- S. Hazra, S. Mandal and A. Ghosh, “Properties of Unconventional Lithium Bismuthate Glasses,” Physical Review B, Vol. 56, No. 13, 1997, pp. 8021-8025. doi:10.1103/PhysRevB.56.8021
- X. Zhao and S. Sakka, “Glass Formation and Crystallization in Alkali-Containing Fluoride,” Journal of Non-Crystalline Solids, Vol. 95-96, No. 1, 1987, pp. 487-494.
- Y. Cheng, H. Xiao, et al., “Structure and Crystallization Kinetics of PbO-B2O3 Glasses,” Ceramics International, Vol. 33, No. 7, 2007, pp. 1341-1347. doi:10.1016/j.ceramint.2006.04.025
- N. Singh, K. J. Singh, K. Singh and H. Singh, “GammaRay Attenuation Studies of PbO-BaO-B2O3 Glass System,” Radiation Measurements, Vol. 41, No. 1, 2006, pp. 84-88.
- R. Sheibani and C. A. Hogarth. “Optical Properties of Some Borate Glasses,” Journal of Materials Science, Vol. 26, No. 2, 1991, pp. 429-433.
- J. Ballato and E. Snitzer, “Fabrication of Fibers with High Rare-Earth Concentrations for Faraday Isolator Applications,” Journal of Applied Optics, Vol. 34, No. 30, 1995, pp. 6848-6854.

