International Journal of Organic Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17854 , 6 pages DOI:10.4236/ijoc.2012.21007
Claisen and Intermolecular Rearrangement of Cinnamyloxynaphthalenes
Department of Applied Chemistry, Ritsumeikan University, Kusatsu-Shi, Japan
Email: *ygvictor@sk.ritsumei.ac.jp
Received November 29, 2011; revised January 6, 2012; accepted February 1, 2012
Keywords: Claisen Rearrangement; Intermolecular Rearrangement; Solvent Effect
ABSTRACT
Rearrangements of the 1- and 2-cinnamyloxynaphthalenes undergo in diethylene glycol and decalin with solvent dependence. In 2-cinnamyloxynaphthalene, the Claisen rearrange ment occurs regardless of the solvents. However, for the 1-analogue, the Claisen rear rangement occurs in decalin, while both the Claisen and intermolecular rearrangements occur at higher temperatures in diethylene glycol.
1. Introduction
The Claisen rearrangement takes place when the O-allyl ether of phenols or enols is heated, and the C-allyl compound is produced [1]. For example, allyloxybenzene (AOB) gives 2-allylphenol by such a rearrangement. When both ortho positions of the benzene ring are substituted by methyl groups, the allyl group does not migrate to the meta positions, but to the para position [2,3]. Moreover, the substrates bearing only one orthosubstituent produced the ortho rearranged product when the substituent is electron withdrawing, but the pararearranged products are produced in addition to the ortho-rearranged one when the substituent is electron releasing (Scheme 1) [4].
The Claisen rearrangement of cinnamyloxybenzene (COB), which has a phenyl group at the 3-position of the allyl group, produces the normal para-rearranged products in addition to the ortho-rearranged one in decalin (Scheme 2) [5]. It is very interesting that COB produces the para-rearranged products in spite of absence of substituent at the ortho-position. On the other hand, when COB is heated in diethylene glycol (DEG), 4-cinnamylphenol, 2-cinnamylphenol and diethylene glycol monocinnamyl ether are produced by an intermolecular process via the cinnamyl cation, and the normal Claisen rearrangement does not take place (Scheme 3) [6].
In this paper, the Claisen rearrangements of naphthalene derivatives bearing a cinnamyloxy group at position 1 or 2 are discussed. The solvents used were DEG as the polar solvent and decalin as the non-polar one. The results are compared to these known in the case of the benzene analogue, COB. need to create these components, incorporating the applicable criteria that follow.
2. Results and Discussion
2.1. 2-Cinnamyloxynaphthalene (2-CON)
When 2-cinnamyloxynaphthalene (2-CON) was heated in decalin at 120˚C or 160˚C, only 1-(1-phenylallyl)-2-naphthol (1PA2N), which is the normal ortho-Claisen rearranged product, was recognized as the product. In DEG at 120˚C or 160˚C also, 1PA2N was the sole product (Table 1). The result in decalin was similar to that of COB. However, the result in DEG was in contrast to the reaction of COB which produces just an intermolecular rearranged product [6].
The Claisen rearrangement proceeds via a cyclic transition state [7,8]. At the transition state, the reaction is accelerated with an increase in the electron density on the ipsoand ortho-carbons, i.e., the double bond character between these two carbons. Actually, the allyloxynaphthalenes are more reactive than AOB due to the higher double bond character between the 1- and 2-positions of the naphthalene [9].
Indeed the known intermolecular rearrangement of COB is a reaction which proceeds via the cinnamyl cation [6], and is governed by the thermodynamic stability of the cation and phenoxy anion. In this work, if such an intermolecular reaction for 2-CON occurs, the 2- naphthoxy anion should be formed. The activation energies of the COB and 2-CON dissociations should be not significantly different because the stabilities of both the phenoxy anion and naphthoxy anion are similar. Therefore, the intermolecular rearrangement reactivities of COB
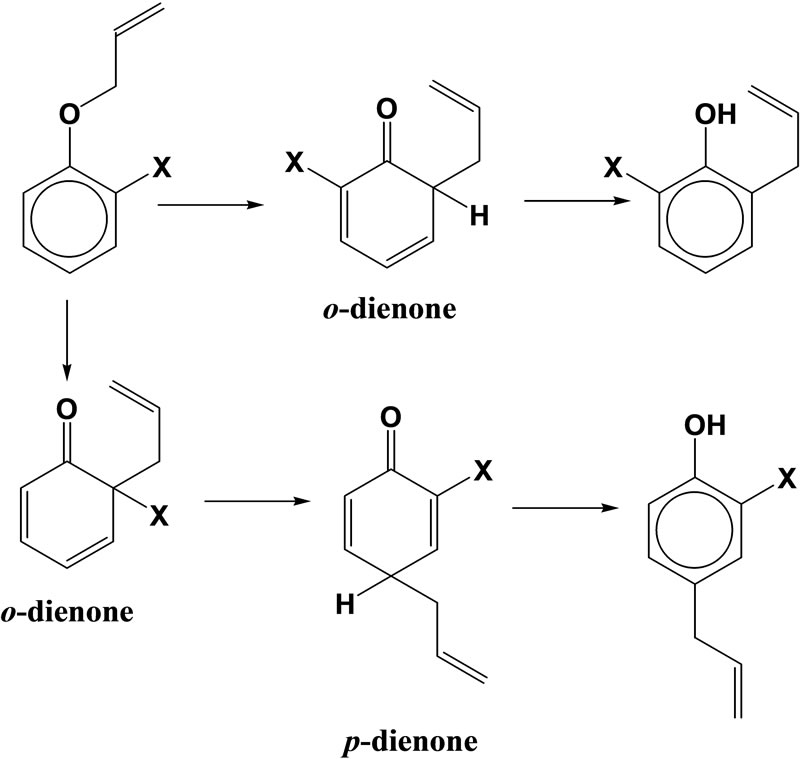
Scheme 1. Reaction mechanism of Claisen rearrangement of o-X-AOB.
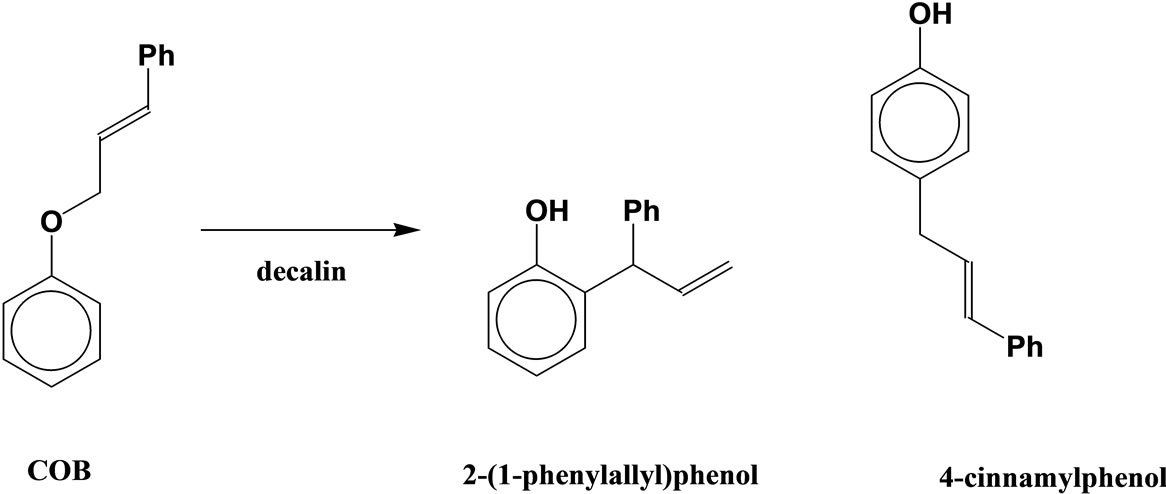
Scheme 2. Claisen rearrangement of COB in decalin.
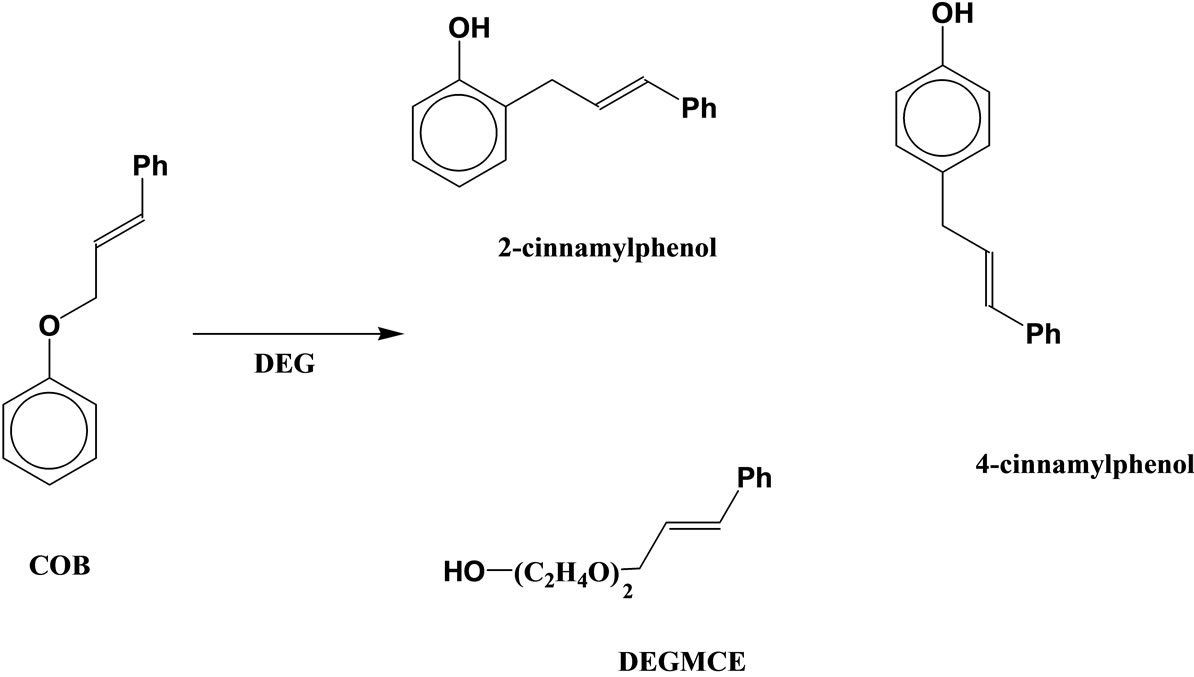
Scheme 3. Intermolecular rearrangement of COB in DEG.
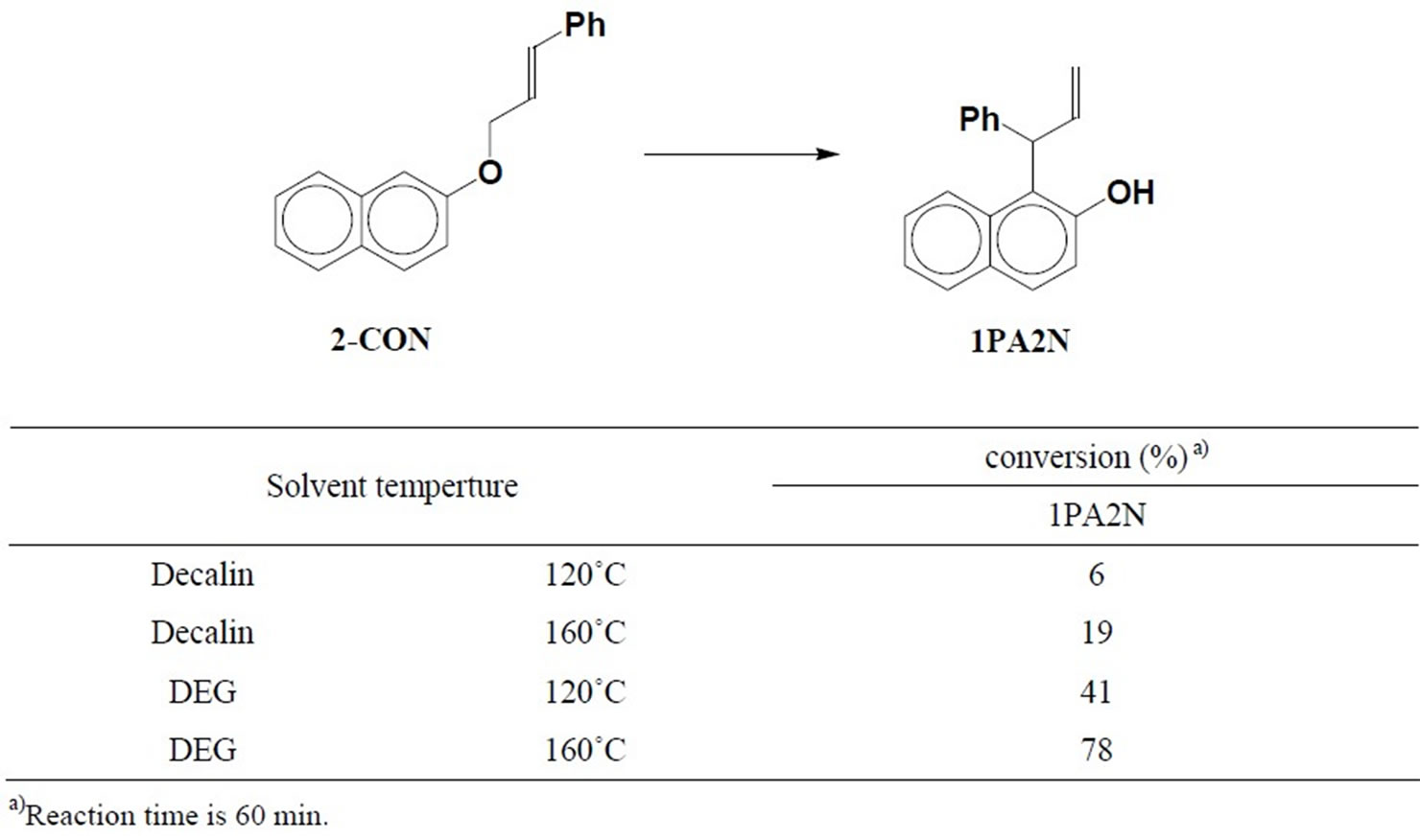
Table 1. Conversion from 2-CON to 1PA2N.
and 2-CON should be similar. However, on the Claisen rearrangement, 2-CON is more reactive than COB as mentioned above. Therefore, the Claisen rearrangement would be preferred in 2-CON, whilst the intermolecular rearrangement proceeds in COB.
2.2. 1-Cinnamyloxynaphthalene (1-CON)
As reported by Tsai et al. [10], 1-CON affords both 2- (1-phenylallyl)-1-naphthol (2PA1N) and 4-cinnamyl-1- naphthol (4C1N) as products in spite of no orthosubstituent. This result is in accord with our previous result concerning COB [5,6]. In this work, the reaction of 1-CON was carried out in the same conditions with those of COB [6]. As a result, 2PA1N was yielded as major product, and 4C1N was by-product in decalin at 110˚C or 160˚C (Table 2). That is, the normal Claisen rearranged products were formed by this reaction as in the case of COB. As shown in a previous paper [5], the ratio of the orthoand para-rearranged products of COB was 2:8. The present result for 1-CON was 2:1; the para-rearrangment of 1-CON was rather difficult to proceed. It is presumed that the Cope rearrangement from the orthodienone to the para-dienone does not easily occur due to the low double bond character between the 2- and 3- positions of the naphthalene.
When 1-CON was reacted in DEG at 110˚C, 2PA1N and 4C1N was the main product, and 2C1N, intermolecular rearranged product, was only slightly obtained (Table 2). That is, at 110˚C, the Claisen rearrangement was preferred over the intermolecular rearrangement in the same way as 2-CON.
The reaction of 1-CON was carried out in DEG at 160˚C. The main product was 4C1N, and the by-products were 2PA1N, 2C1N and DEGMCE, a condensation product with the solvent (Table 2). Thus, at higher temperatures, both the Claisen and intermolecular rearranged products were obtained.
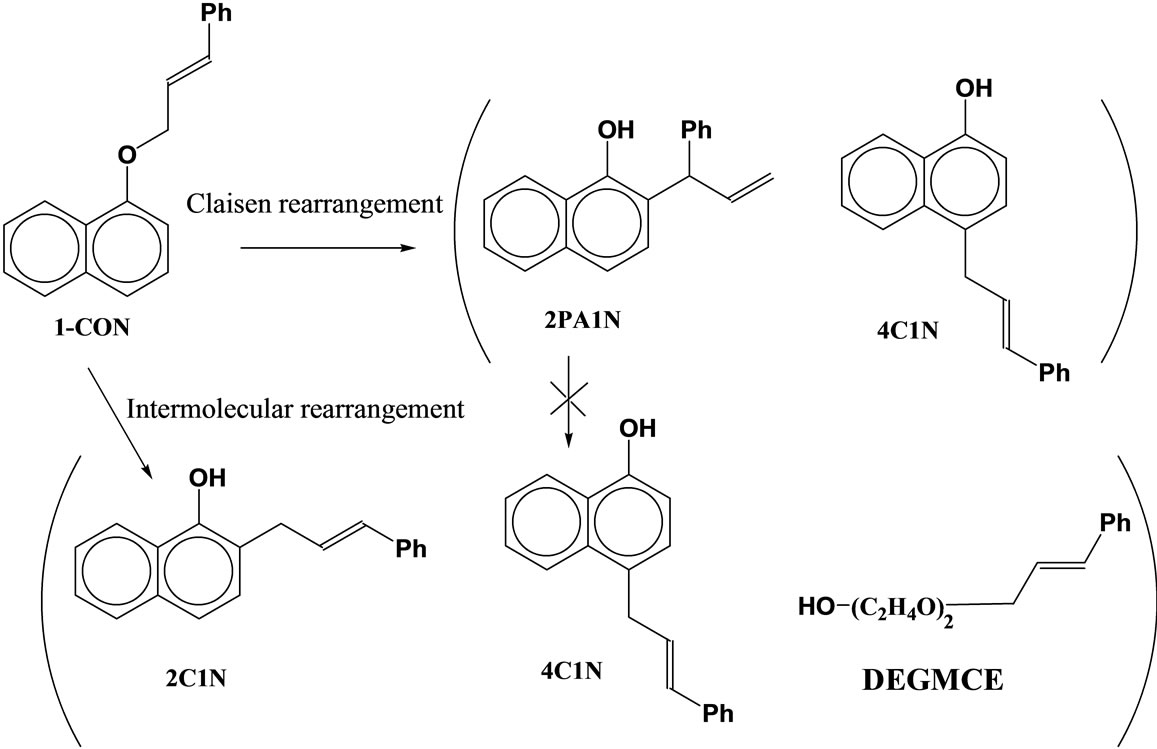
No reaction occurred when the formed 2PA1N was heated to 160˚C. Namely, the thermodynamically stable 4C1N is not produced via 2PA1N, but directly from the substrate. The intermolecular rearrangement is initiated by the formation of DEGMCE which forms by attachment of DEG as a nucleophile. Therefore, this intermolecular rearrangement would be preferred at the higher temperatures (Scheme 4).
Comparison of 1- and 2-CON’s reactivity was done. The reactivity of 1-CON was higher than that of 2-CON based on the decreasing amount of the substrate. In general, the reactivity of 1-naphthyl derivative is higher than that of 2-naphthyl derivative. 1-Naphthyl derivative is destabilized by an intramolecular mutual repulsion between allyloxy group and peri-proton, and, therefore, the activation energy is reduced. Therefore 1-CON would be more reactive than 2-CON.
3. Experimental
All reagents were of commercial quality and were used as received. Solvents were dried and purified using standard techniques.
3.1. Measurement Apparatus
The 1Hand 13C-NMR spectra were recorded on a JEOL A-400 spectrometer (400 MHz for 1H, 100 MHz for 13C) using TMS as internal standard and CDCl3 as solvent. The mass spectra were obtained using a Shimadzu LCMSQP8000. The direct ionization mode was that of the atmospheric pressure chemical ionization (APCI) method, and the positive mode was used.
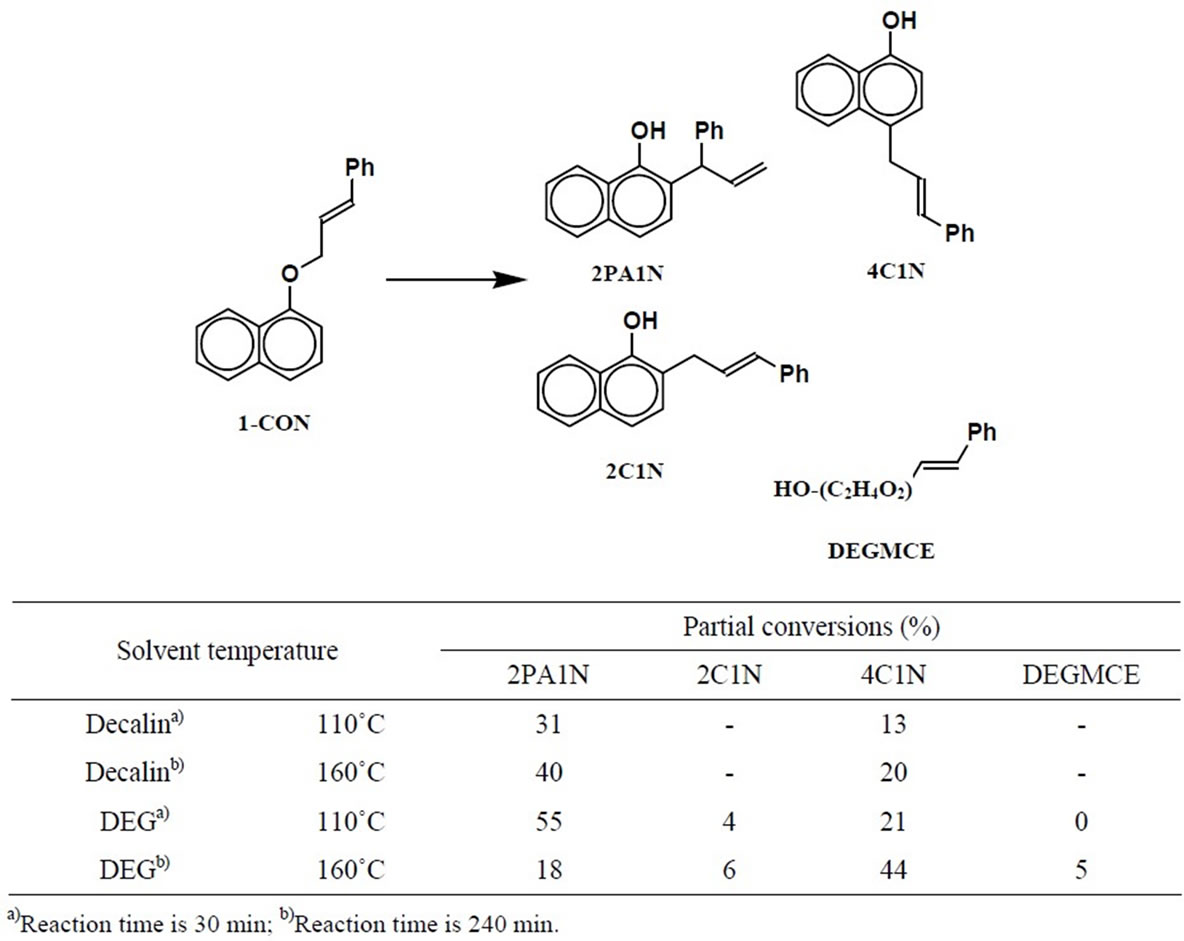
Table 2. Partial conversions from 1-CON to products.
Scheme 4. Claisen and intermolecular rearrangement of 1-CON.
3.2. Synthesis
3.2.1. Synthesis of 1- and 2-Cinnamyloxynaphthalenes (1- and 2-CON)
The CONs were synthesized in the same way with COB [6]. 1- or 2-Naphthol (0.5 mol), cinnamylbromide 0.5 mol), potassium iodide (0.5 mol), and potassium carbonate (0.75 mol) were put in a 500 mL round-bottom flask with 300 mL of acetone. The reaction mixture was refluxed for 6 hours with stirring by a magnetic stirrer. After the completion of the reaction, the reaction mixture was cooled to room temperature, and the solvent was evaporated. The residue was dissolved in toluene, and washed with 10% NaOH and water. The purification was done by aluminum column chromatography (50 g of 50 mesh alumina) using toluene-hexane (1:1) as eluent.
1-Cinnamyloxynaphthalene (1-CON): yield: 13%; mp 88.0˚C - 90.3˚C; 1H-NMR: 4.87 (2H,d, 6.0 Hz), 6.56 (1H, m), 6.86 (1H, d, 16.0 Hz), 7.15 - 7.33 (5H, m), 7.25 - 7.81 (7H, m); 13C-NMR: 68.8, 105.1 - 136.5, 137.8, 154.3; APCI-MS(+): 261([M+1]+).
2-Cinnamyloxynaphthalene (2-CON): yield: 13%; mp 122.7˚C - 124.3˚C; 1H-NMR: 4.77 (2H, d, 6.0 Hz), 6.48 (1H, m), 6.75 (1H, d, 16.0 Hz), 7.13 - 7.30 (5H, m), 7.22-7.77(7H, m); 13C-NMR: 68.6, 107.0 - 134.5, 136.4, 156.5; APCI-MS(+): 261 ([M+1]+).
3.2.2. Synthesis of 1-(1-Phenylallyl)-2-naphthol (1PA2N)
2-Cinnamyloxynaphthalene (0.012 mol) was dissolved in N,N-dimethylaniline (14 g). The solution was heated at 120˚C for 5 hours. After the cooling, the reaction mixture was dissolved in chloroform, and washed by 3N HCl to remove N,N-dimethylaniline. The product was extracted with 5% NaOH from the chloroform solution.
1-(1-Phenylallyl)-2-naphthol (1PA2N): yield: 32%; 1HNMR: 5.60 (1H,d, 6.6 Hz), 6.52 (1H, m), 5.09 (1H, d, 17.0 Hz), 5.31 (1H, d, 10.6 Hz), 5.65 (1H, s), 7.00 - 7.30 (5H, m), 7.25 - 7.84 (6H, m); 13C-NMR: 46.1, 118.8 - 137.8, 118.6, 138.9, 140.8, 152.6; APCI-MS(+): 261 ([M+1]+).
3.2.3. Synthesis of 2-(1-Phenylallyl)-1-naphthol (2PA1N)
1-Cinnamyloxynaphthalene (0.012 mol) was dissolved in decalin (20 g). The solution was heated at 110˚C for 5 hours. After the cooling, chloroform was added in the reaction mixture. The product was extracted with 5% NaOH from the chloroform solution.
1-(1-Phenylallyl)-2-naphthol (2PA1N): yield: 25%; 1HNMR: 5.38 (1H, d, 6.5 Hz), 6.38 (1H, m), 5.01 (1H, d, 17.0 Hz), 5.29 (1H, d, 10.6 Hz), 5.40 (1H, s), 7.11 - 7.38 (5H, m), 7.31 - 8.06 (6H, m); 13C-NMR: 50.2, 117.7, 120.4 - 139.1, 139.6, 141.5, 153.4; APCI-MS(+): 261 ([M+1]+).
3.2.4. Synthesis of 2-Cinnamyl-1-naphthol (2C1N) and 4-Cinnamyl-1-naphthol (4C1N)
1-Cinnamyloxynaphthalene (0.012 mol) was dissolved in diethylene glycol (20 g). The solution was heated at 160˚C for 5 hours. After the cooling, chloroform was added in the reaction mixture. The products were extracted with 5% NaOH from the chloroform solution as mixture of the two isomers. The separation of the isomers was done by preparative HPLC (20φ × 250 mm ODS column).
2-Cinnamyl-1-naphthol (2C1N): yield: 5%; 1H-NMR: 3.03 (2H, d, 5.8 Hz), 4.91 (1H, s), 5.63 (1H, m), 6.15 (1H, d, 16.0 Hz), 7.05 - 7.21 (5H, m), 7.17 - 7.44 (6H, m); 13C-NMR: 45.3, 124.2 - 137.2, 137.8, 143.6, 145.5; APCIMS(+): 261 ([M+1]+).
4-Cinnamyl-1-naphthol (4C1N): yield: 8%; 1H-NMR: 3.82 (2H, d, 5.8 Hz), 5.00 (1H, s), 6.38 (1H, m), 6.69 (1H, d, 16.0 Hz), 7.10 - 7.37 (5H, m), 7.28 - 7.43 (6H, m); 13CNMR: 36.0, 108.1 - 130.9, 133.7, 137.5, 139.1; APCIMS(+): 261 ([M+1]+).
3.2.5. Synthesis of Diethylene Glycol Monocinnamyl Ether (DEGMCE)
DEGMCE was synthesized as described in a previous paper [6].
3.3. Monitoring of Claisen Rearrangement [5,6]
The Claisen rearrangement was carried out in a small ampoule, which contained the substrate (0.5 mmol) and the solvent (5 mmol). The ampoule was heated at a constant temperature in an oil bath. After a specific time, 0.5 mL of methanol was added in the ampoule. The methanol layer was separated followed by evaporation of the methanol. The residue was then analyzed. The sum of the orthoand para-rearranged products was measured by reverse-phase HPLC (4.6φ × 250 mm ODS column).
REFERENCES
- L. Claisen, “Rearrangement of Phenyl Allyl Ethers in C-Allylphenols,” Berichte der Deutschen Chemischen Gesellschaft, Vol. 45, 1912, pp. 3157-3166. doi:10.1002/cber.19120450348
- C. D. Hurd and M. A. Pollack, “Mechanisms for the Rearrangements of Ethers: γ-Ethylallyl Phenyl Ether and γ-Ethylallyl Vinyl Ether,” Journal of Organic Chemistry, Vol. 3, No. 6, 1939, pp. 550-569. doi:10.1021/jo01223a004
- E. N. Marvell, B. J. Burreson and T. Crandall, “Influence of Alkyl Groups on the Rate of the Para Claisen Rearrangement,” Journal of Organic Chemistry, Vol. 30, No. 4, 1965, pp. 1030-1032. doi:10.1021/jo01015a018
- D. Y. Curtin and H. W. Johnson Jr., “Mechanism of the para Claisen Rearrangement. Evidence for a Dienone- Phenyl Ether Rearrangement,” Journal of the American Chemical Society, Vol. 78, No. 11, 1956, pp. 2611-2615. doi:10.1021/ja01592a078
- Y. Okada, M. Adachi and T. Hayashi, “Substituent Effect on Claisen Rearrangement of 1-Substituted 2-Cinnamyloxybenzenes,” Journal of Oleo Science, Vol. 51, No. 5, 2002, pp. 359-364. doi:10.5650/jos.51.359
- T. Hayashi, Y. Okada, K. Arita and H. Kuromizu, “Solvent Effect on the Thermal Rearrangement of Cinnamyloxybenzene,” Nippon Kagaku Kaishi, No. 4, 1997, pp. 255-259. doi:10.1246/nikkashi.1997.255
- H. Schmid and K. Schmid, “The Claisen Rearrangement. I. Experiments with Carbon 14. 2,” Helvetica Chimica Acta, Vol. 35, 1952, pp. 1879-1890. doi:10.1002/hlca.19520350612
- H. Schmid and K. Schmid, “The Claisen Rearrangement. II. Experiments with Carbon 14. 3,” Helvetica Chimica Acta, Vol. 36, 1952, pp. 489-500. doi:10.1002/hlca.19530360218
- T. Hayashi, Y. Okada and T. Inaba, “ Catalytic Effect of Metal Salts on the Claisen Rearrangement of 1- and 2-(Allyloxy)naphthalene and 8-(Allyloxy)quinoline,” Chemistry Express, Vol. 51, 1997, pp. 255-259.
- J. Tsai, S. Li Chen, L. Chen, P. Chen, S. Hsu, C. Lin and E. Wang, “Synthesis of Substituted 2,5-Dihydro-1-naphthoxepines from 1-Naphthol via Ring-Closing Metathesis,” Arkivoc, 2008, pp. 205-217.
NOTES
*Corresponding author.

