Journal of Applied Mathematics and Physics
Vol.04 No.08(2016), Article ID:69728,7 pages
10.4236/jamp.2016.48153
Gas-Phase Conversion of the U(VI), Sr, Mo, and Zr Oxides in Nitrating Atmosphere
Sergey A. Kulyukhin1, Yurii M. Nevoin1, Natalya A. Konovalova1, Andrey V. Gordeev1, Alexey A. Bessonov1, Margarita P. Gorbacheva1, Andrey Yu. Shadrin2, Konstantin N. Dvoeglazov2
1Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Moscow, Russia
2Open Joint-Stock Company VNIINM, Moscow, Russia



Received 9 February 2016; accepted 8 August 2016; published 15 August 2016

ABSTRACT
The gas-phase conversion of U3O8, MoO3, SrO, and their mechanical mixtures, and also of ZrO2 into water-soluble compounds in the atmosphere of (NOx + vapor H2O) or HNO3 (vapor) was studied. In the course of gas-phase conversion, U3O8 and SrO transform into water-soluble compounds (nitrates, hydroxonitrates), whereas MoO3 and ZrO2 undergo no changes. The principal possibility of separating U from Mo and Zr by gas-phase conversion of the oxides in the atmosphere of (NOx + vapor H2O) or HNO3 (vapor) was demonstrated.
Keywords:
Metal Oxides, Nitrates, Gas-Phase Conversion, Nitrogen Oxides

1. Introduction
The current plans calling for the transition to fast-neutron reactors, as well as to reactors with a high fuel burn- up have stimulated an active search of spent nuclear fuel (SNF) reprocessing techniques that would be alternative to the classical Purex process. One of the promising technologies of short-cooled SNF reprocessing is voloxidation (volume oxidation) of both SNF and zircalloy fuel cladding, followed by treatment of voloxidation products in the atmosphere of NOx gases. This technology is being developed in Russia and other countries. Voloxidation allows volatile components (3H, 14C, 129I, radioactive noble gases) to be removed before starting the radiochemical reprocessing of the fuel; in addition, strong zirconium fuel claddings transform into ZrO2 [1], and UO2 transforms into U3O8. However, this treatment does not eliminate regular problems with colloid formation in the step of SNF dissolution in HNO3. It seems more promising that the oxidative recrystallization be followed not by the dissolution of SNF voloxidation products and fuel rods in nitric acid, but by their treatment with nitrogen oxides to obtain weakly hydrated water-soluble uranium compounds.
The main relationships of the reaction of uranium oxides with liquid N2O4, gaseous nitrogen oxides, and solutions of N2O4 in organic solvents were studied in [2]-[6]. The formation of NO[UO2(NO3)3] in the reaction of uranium oxides with liquid N2O4 was proved. It was found that the reactions with UO2 and U3O8 occur considerably more slowly than with UO3. However, the reaction rates considerably increase with hydration of the oxides. Revenko et al. [7] studied the conversion of uranium oxides into nitrates with an N2O4-H2O mixture in an autoclave at a pressure of 0.5 - 1 MPa and a temperature of 100˚C - 140˚C and with an N2O4-H2O mixture using supercritical extraction with CO2 in an autoclave at a pressure of 7 - 15 MPa at a temperature of 10˚C - 75˚C. As a result, uranyl nitrate solutions with a U concentration of 1000 - 1100 g/L and HNO3 content of 1.5 - 7 M were obtained. Liyang et al. [8] demonstrated the possibility of direct conversion of ceramic UO2 fuel into nitrates in the N2O4-H2O system. Voloxidation performed in an O2 atmosphere prior to starting the conversion considerably simplifies the further process. A two-step scheme of reprocessing oxide SNF from light water reactors was presented in a US patent [9]. The first step involves the oxidation of UO2 to U3O8 using NO2, and the second step, the treatment of U3O8 with NO2 vapor. It is stated in [9] that U and Pu in the course of the gas- phase conversion will transform into water-soluble compounds, whereas the fission products, in particular, Tc, will remain in the oxide form. Bondin et al. [10] studied the conversion of the real irradiated fuel from a WWER-1000 reactor and of its simulants in the N2O4-H2O system. The possibility of the conversion of real SNF to obtain nitric acid solutions with high uranium concentration was demonstrated.
Despite the advantages of the technology of gas-phase SNF conversion in an atmosphere of nitrogen oxides, the behavior of fission elements in the course of conversion is still poorly understood and requires additional study. Therefore, this study was aimed at checking the possibility of gas-phase conversion of U3O8 (simulating the voloxidation product of oxide SNF), MoO3, SrO (both simulating fission element oxides), ZrO2 (simulating the product of oxidative recrystallization of fuel rod claddings), and their mechanical mixtures into water-so- luble compounds in the atmosphere of (NOx + vapor H2O) or HNO3 (vapor) atmosphere (later-nitrating atmosphere).
2. Experimental
Experiments were performed with SrO, MoO3, and monoclinic ZrO2 (all chemically pure grade). U3O8 was prepared by the decomposition of UO2(NO3)2・6H2O in air at 900˚C for 4-6 h. Mechanical mixtures U3O8-MoO3 (10 wt%) and U3O8-MoO3 (5 wt%)-SrO (5 wt%) were prepared by mixing weighed portions of U3O8, MoO3, and SrO.
Gas-phase conversion experiments were performed in nitrating atmosphere. Weighed portions of U3O8, MoO3, SrO, and their mechanical mixtures, and also of ZrO2 were placed in glass cups, which, in turn, were arranged in the system. The system was either left in a fume hood at room temperature or placed into a furnace with forced evacuation of the gas-phase. The desiccators were left closed for 1 to 12 d at room temperature (20˚C - 30˚C) or for 1 - 10 h at a temperature of 70˚C to 150˚C in nitrating atmosphere. After a definite time, the desiccators were cooled, opened, and ventilated, and the samples were taken off. The final products were weighed, and samples for X-ray diffraction analysis were taken. The remaining part of the final product was treated with distilled water. At incomplete conversion, a water-insoluble precipitate remained in the system. It was separated from the mother liquor by centrifugation. The precipitate was dried to the airdry state and weighed. The content of metals and 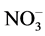 in the mother liquor was determined. The U(VI) and
in the mother liquor was determined. The U(VI) and 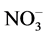 content was determined by spectrophotometry. The absorption spectrum was taken on a Specord М40 spectrophotometer in quartz cells with the working space thickness of 0.1 - 5 cm. The
content was determined by spectrophotometry. The absorption spectrum was taken on a Specord М40 spectrophotometer in quartz cells with the working space thickness of 0.1 - 5 cm. The 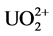 concentration was calculated from the absorption intensity at λ = 413 nm (ε = 7.8 L/mol∙cm), and the
concentration was calculated from the absorption intensity at λ = 413 nm (ε = 7.8 L/mol∙cm), and the 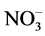 concentration, from that at λ = 301 - 302 nm (ε = 7.0 L/mol∙cm). The content of Zr, Mo, and Sr in the mother liquors was determined by ICP-MS.
concentration, from that at λ = 301 - 302 nm (ε = 7.0 L/mol∙cm). The content of Zr, Mo, and Sr in the mother liquors was determined by ICP-MS.
The powder X-ray diffraction patterns of the initial oxides and their nitration products were obtained with an ADP-10 diffractometer (Philips) using CuKα radiation.
Thermal gravimetric analysis of the products of gas-phase conversion of SrO, MoO3, and U3O8, and also of UO2(NO3)2∙6H2O was performed with a Q-1500D derivatograph (MOM, Hungary) in platinum crucibles in air. The heating rate was 10 deg/min.
The IR absorption spectrum of the gas-phase was recorded with a Specord M80 spectrometer in the 4000 - 400 cm− 1 range in a 125 cm3 cell with KBr windows and working space length of 100 mm.
3. Results and Discussion
3.1. U3O8 Conversion
The gas-phase conversion of U3O8 with the formation of water-soluble compounds in nitrating atmosphere can be described by the following reaction equations:
U3O8 + 6NO2 + 2O2 + 3nH2O = 3UO2(NO3)2・nH2O (n = 0, 1, 3 or 6) (1)
U3O8 + 3H2O + 3NO2 + 1/2O2 = 3UO2(OH)(NO3) (2)
U3O8 + 8HNO3 = 3UO2(NO3)2×nH2O + 2NO2 + 4H2O (n = 0, 1, 3 or 6) (3)
U3O8 + 5HNO3 = 3UO2(OH)(NO3) + 2NO2 + H2O. (4)
The formation of a mixture of uranyl nitrates and hydroxonitrates cannot be ruled out.
In accordance with Equations (1)-(4), the conversion should lead both to an increase in the sample weight and to a change in its color. Indeed, depending on the experiment conditions, samples of U3O8 conversion products had either black or yellow color. The change in the sample color from black to yellow was accompanied by a noticeable increase in the sample weight.
The results of an experimental study of the gas-phase conversion of U3O8 in nitrating atmosphere show that in virtually all the cases the sample weight increases, suggesting the occurrence of the gas-phase conversion with the formation of either uranyl nitrates or uranyl hydroxonitrates.
The analysis of the angles 2θ for the strongest lines of the X-ray diffraction pattern of products of gas-phase conversion, obtained at the maximal degree of U3O8 conversion in nitrating atmosphere, show that the strongest reflections (Imax = 100) characteristic of UO2(NO3)2∙6H2O in the range 2θ = 13.408˚-5.032˚ [11] are also present in the X-ray diffraction patterns of the U3O8 conversion products (13.465˚, I = 90; 3.195˚, I = 74). In addition, a number of diffraction lines of the conversion products are close in positions to the lines given in the literature for uranyl hydroxonitrate [12] [13]. Analysis of the black samples revealed reflections corresponding to the initial U3O8 [14].
It is necessary noted that TG curves for uranyl nitrate and products of U3O8 in nitrating atmosphere have a similar course. In addition, similar endothermic effects associated with the elimination of water molecules are observed in the DTA curves at 50˚C - 60˚C and 240˚C - 260˚C. The data obtained suggest that the major product of the U3O8 conversion is hydrated uranyl nitrate.
On the other hand, it is known from the published data [2] that one of the products formed in the reaction of uranium oxides with anhydrous N2O4 is nitrosonium trinitratouranylate (NTN) NO[UO2(NO3)3]. It was interesting to examine the possibility of the NTN formation in our reaction system. The NTN formation is possible in the following reaction:
U3O8 + 20NO2 = 3NO[UO2(NO3)3] + 4N2O + 4O2 (5)
accompanied by the formation of N2O in the gas-phase. To identify N2O, we recorded the IR spectrum of the gas-phase formed in the course of the U3O8 conversion in the NOx?H2O (vapor)?air atmosphere (Figure 1). The spectrum obtained contains a weak absorption peak at ν = 2236 cm−1, corresponding to published data for N2O [15]. The presence of N2O in the gas-phase suggests that one of possible intermediates in U3O8 conversion in the NOx?H2O (vapor)?air atmosphere is NTN, which subsequently undergoes hydrolysis to form hydrated uranyl nitrate or hydroxonitrate.
After the contact with water, the yellow conversion product rapidly dissolves to form a yellow solution. The UO22+ absorption bands are clearly seen in the absorption spectrum of the aqueous solution obtained by dissolving the U3O8 conversion products in nitrating atmosphere. It can be concluded from the absorption spectra that the gas-phase conversion results in the formation of water-soluble uranyl compounds.
For the water-soluble conversion products, we determined the [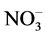 ]: [U(VI)] ratio by spectrophotometry. In the case of the U3O8 conversion in, the [
]: [U(VI)] ratio by spectrophotometry. In the case of the U3O8 conversion in, the [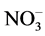 ]: [U(VI)] ratio varies from 1 to 2 in the experiments performed both at room temperature and on heating. There is no correlation between the [
]: [U(VI)] ratio varies from 1 to 2 in the experiments performed both at room temperature and on heating. There is no correlation between the [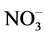 ]: [U(VI)] ratio and reaction time in these experiments. The observed [
]: [U(VI)] ratio and reaction time in these experiments. The observed [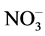 ]: [U(VI)] ratio suggests the formation of a mixture of uranyl nitrate and uranyl hydroxonitrate, which is relatively readily soluble in water [14]. The degree of the U3O8 conversion increased both with the reaction time and with the temperature of the medium. At room temperature, the conversion increased from 87.2% to 100% as the reaction time was increased from 1 to 6 d. At 70˚C, the conversion was higher than 50% at all the reaction times, and at 110˚C - 150˚C it was close to 100% irrespective of the time of keeping U3O8 in nitrating atmosphere.
]: [U(VI)] ratio suggests the formation of a mixture of uranyl nitrate and uranyl hydroxonitrate, which is relatively readily soluble in water [14]. The degree of the U3O8 conversion increased both with the reaction time and with the temperature of the medium. At room temperature, the conversion increased from 87.2% to 100% as the reaction time was increased from 1 to 6 d. At 70˚C, the conversion was higher than 50% at all the reaction times, and at 110˚C - 150˚C it was close to 100% irrespective of the time of keeping U3O8 in nitrating atmosphere.
Thus, our experiments show that in the course of the gas-phase conversion in nitrating atmosphere U3O8 transforms into water-soluble nitrate compounds (uranyl nitrate and/or hydroxonitrate).
Figure 1. IR spectrum of the gas-phase formed in the course of the U3O8 conversion in the NOx-H2O (vapor)-air atmosphere.
3.2. SrO Conversion
The gas-phase conversion of SrO with the formation of water-soluble compounds in nitrating atmosphere can be described by the following reaction equations:
SrO + 2NO2 + 1/2O2 = Sr(NO3)2, (6)
2SrO + 2NO2 + H2O + 1/2O2 = 2Sr(NO3)OH (7)
SrO + 2HNO3 = Sr(NO3)2 + H2O (8)
SrO + HNO3 = Sr(OH)NO3 (9)
The formation of strontium nitrate and hydroxonitrate is also possible through the reaction of SrO with water vapor:
SrO + H2O = Sr(OH)2 (10)
Sr(OH)2 + HNO3 = Sr(OH)NO3 + H2O (11)
Sr(OH)2 + 2HNO3 = Sr(NO3)2 + 2H2O (12)
In accordance with Equations (6)-(12), the SrO conversion in nitrating atmosphere should lead to an increase in the sample weight.
Preliminary experiments showed that keeping SrO in water vapor at a high temperature does not lead to the sample weight gain. This fact suggests that the major conversion product is strontium nitrate. Also, the formation of strontium hydroxonitrate cannot be ruled out.
The experimental results obtained in the course of studying the gas-phase conversion of SrO in nitrating atmosphere show that in virtually all the cases the sample weight increases, suggesting the occurrence of the gas-phase conversion with the formation of strontium nitrate and hydroxonitrate.
The phase composition of the conversion products was studied by powder X-ray diffraction. The analysis of diffraction data show that the 2θ angles for the strongest lines of the X-ray diffraction pattern shown that the reflections in the 2θ ranges 19.642˚ - 22.718˚ and 38.127˚ - 39.893˚, characteristic of Sr(NO3)2 [16], are also present in the X-ray diffraction patterns of the SrO conversion products (19.7648˚, I = 84; 19.7198˚, I = 93; 38.3489˚, I = 100; 38.3048˚, I = 100; 40.1048˚, I = 81; 40.0598˚, I = 76). In addition, it should be noted that in some experiments we detected the diffraction lines characteristic of Sr(OH)2 [17] and SrO [18].
In all the experiments, the SrO samples did not change their color, remaining white, and a colorless solution was formed upon their dissolution in water. In some experiments, the dissolution was incomplete, and a white insoluble precipitate remained in the system. At room temperature, the degree of the SrO conversion in nitrating atmosphere increased from 67.7% to 82.6% with increasing time of keeping SrO in the nitrating atmosphere. An increase in the temperature of the system led to a noticeable increase in the rate of the SrO conversion. The degree of the SrO conversion on keeping for 1 - 10 h at 70˚C - 150˚C was in the range from 26.8% to 75.4%. However, we cannot speak of any rigorous correlation between the experimental conditions (time, temperature) and degree of conversion.
Thus, the gas-phase conversion of SrO in nitrating atmosphere yields water-soluble products: Sr(NO3)2 (major product), Sr(OH)NO3, and Sr(OH)2.
3.3. MoO3 Conversion
The gas-phase conversion of MoO3 in nitrating atmosphere with the formation of water-soluble compounds can be described by the following hypothetical reaction equations:
MoO3 + 2NO2 + 1/2O2 = MoO2(NO3)2 (13)
MoO3 + 4NO2 + O2 = MoO(NO3)4 (14)
MoO3 + 2HNO3 = MoO2(NO3)2 + H2O (15)
MoO3 + 4HNO3 = MoO(NO3)4 + 2H2O (16)
In accordance with Equations (13)-(16), the conversion of MoO3 in nitrating atmosphere should lead to an increase in the sample weight due to the formation of molybdenum oxonitrates. However, no weight gain was observed in the course of the experiment.
The powder X-ray diffraction pattern of the products of the MoO3 conversion in nitrating atmosphere is well consistent with the patterns calculated theoretically from the crystallographic data available in JCPDS-ICDD for MoO3 [19].
Analysis of data on the Mo content in the aqueous phase after the contact of the products of the MoO3 conversion in nitrating atmosphere with water shows that the Mo concentration in the aqueous solution obtained is on the level of the MoO3 solubility in water, equal to 7.4 × 10−3 M at 18˚C [20].
The results obtained show that the gas-phase conversion of MoO3 into water-soluble compounds in nitrating atmosphere does not occur to noticeable extent.
3.4. ZrO2 Conversion
The gas-phase conversion of ZrO2 in nitrating atmosphere with the formation of water-soluble compounds can be described by the following hypothetical reactions:
ZrO2 + 2NO2 + 1/2O2 = ZrO(NO3)2, (17)
ZrO2 + 4NO2 + O2 = Zr(NO3)4, (18)
ZrO2 + 2HNO3 = ZrO(NO3)2 + H2O, (19)
ZrO2 + 4HNO3 = Zr(NO3)4 + 2H2O. (20)
In accordance with Equations (17)-(20), the conversion of ZrO2 in nitrating atmosphere should lead to an increase in the sample weight due to the formation of oxonitrates and nitrates of zirconium. However, no weight gain was observed in the course of the experiment.
The powder X-ray diffraction pattern of the products of the ZrO2 conversion in nitrating atmosphere is in good agreement with the X-ray diffraction pattern presented in the JCPDS-ICDD database for ZrO2 [21].
The solubility of the products of the ZrO2 conversion in nitrating atmosphere was evaluated by ICP-MS. No Zr was detected in the aqueous phase (its content was below the detection limit of the ICP mass spectrometer used). Thus, in contact of the products of the ZrO2 conversion in nitrating atmosphere with water, Zr does not noticeably pass into the aqueous phase.
The results obtained show that the gas-phase conversion of ZrO2 into water-soluble compounds in nitrating atmosphere does not occur to noticeable extent. The behaviour of ZrO2 is similar to behaviour of MoO3.
3.5. Conversion of the U3O8-MoO3 Mixture
Despite the fact that real SNF is not a mechanical mixture of oxides but is a solid solution of Mo, Sr, and Zr in uranium dioxide, in the course of voloxidation this solid solution can partially transform into a mixture of oxides. Therefore, we studied the behavior of the U3O8?MoO3 (10 wt%) mechanical mixture in nitrating atmosphere.
The results of experiments on the gas-phase conversion of the U3O8-MoO3 (10 wt%) mixture in nitrating atmosphere show that in virtually all the cases the mixture undergoes weight gain. In most cases, the color of the mixture changed from black to yellow. When the products of the conversion of the U3O8-MoO3 (10 wt%) mixture in nitrating atmosphere were treated with water, a yellow solution formed, and a black or white precipitate remained. The black precipitate remained in the case of incomplete conversion of U3O8. In the case of complete conversion of U3O8, the precipitate had white color characteristic of MoO3.
The yellow solution formed upon interaction of the conversion products with water had the absorption spectrum identical to that obtained upon the gas-phase conversion of U3O8. The optical absorption spectrum of the solution corresponded in the shape to the absorption spectrum of UO22+.
The powder X-ray diffraction pattern of the products of conversion of the U3O8-MoO3 (10 wt%) mixture in nitrating atmosphere contains lines observed previously in the X-ray diffraction patterns of the products of the gas-phase conversion of U3O8 and MoO3, taken separately, in nitrating atmosphere, i.e., the gas-phase conversion of the mechanical mixture of the oxides occurs by the same mechanisms as the conversion of the individual oxides.
Analysis of data on the content of U and Mo in the aqueous phase shows that the aqueous solutions contain virtually no Mo. The U amount in the aqueous phase increases as the temperature of the gas-phase in the course of the experiment and the time of keeping the mixture in nitrating atmosphere are increased. In the experiments performed at room temperature for a time from 1 to 12 d, the observed values of the U3O8 conversion varied from 50% to 88%, with the MoO3 conversion being as low as 1%-2%. In the experiments performed at 70˚C - 150˚C, the U3O8 conversion was in the range from 70% to 88% at all the keeping times, and the MoO3 conversion was also within 2%. No significant correlation can be revealed between the U3O8 conversion and experimental conditions. The Mo concentration in the solution in all the experiments was on the level of the MoO3 solubility at room temperature.
Thus, in the course of conversion and subsequent dissolution, U3O8 partially transformed into water-soluble nitrates, whereas MoO3 virtually fully remained in the precipitate phase. The data obtained suggest the principal possibility of separating U and Mo in the course of conversion of the U3O8-MoO3 mechanical mixture in nitrating atmosphere.
3.6. Conversion of the U3O8-MoO3-SrO Mixture
The results of experiments on the gas-phase conversion of the U3O8-MoO3 (5 wt%)-SrO (5 wt%) mixture in nitrating atmosphere show that in all the cases the sample weight increases. After the experiment completion, the products of the conversion of the U3O8-MoO3 (5 wt%)-SrO (5 wt%) mechanical mixture had yellow or black color.
The observed weight gain suggests the occurrence of the gas-phase conversion of U3O8 and SrO with the formation of uranyl nitrates and hydroxonitrates and of strontium nitrate, hydroxonitrate, and hydroxide.
The powder X-ray diffraction pattern of the products of conversion of the U3O8-MoO3 (5 wt%)-SrO (5 wt%) mixture in nitrating atmosphere show that the positions and intensities of the main reflections contains lines observed previously in the X-ray diffraction patterns of the products of gas-phase conversion of U3O8, MoO3, and SrO.
Treatment of the product mixture with water resulted in the formation of a yellow solution, with a white or black precipitate remaining. The black precipitate remained in the case of incomplete conversion of U3O8. In the case of complete conversion of U3O8, the precipitate had white color characteristic of MoO3 and SrO.
The absorption spectrum of aqueous solutions formed when the products of conversion of the U3O8?MoO3 (5 wt%)?SrO (5 wt%) mixture in nitrating atmosphere were treated with water was identical to the spectra obtained upon gas-phase conversion of U3O8 and U3O8?MoO3 (10 wt%) mixture in nitrating atmosphere.
Analysis of data on the content of U, Mo, and Sr in the aqueous phase shows that, when the experiments were performed at room temperature for 1 - 12 d, the U3O8 conversion varied from 74% to 89%, the SrO conversion, from 37% to 75%, and the MoO3 conversion did not exceed 2%. In the experiments performed at 70˚C-150˚C, the U3O8 conversion was in the range from 75% to 100%, the SrO conversion, in the range from 67% to 90% at all the treatment times, and the MoO3 conversion was low. No significant correlation was revealed between the degree of conversion of U3O8 and SrO and the experimental conditions. The Mo content in the solution was on the level of the MoO3 solubility at room temperature in all the experiments.
Thus, in the course of conversion and subsequent dissolution, U3O8 and SrO partially transformed into water-soluble nitrates, whereas MoO3 virtually fully remained in the precipitate phase. The data obtained suggest the principal possibility of the separation of U from Mo in the U3O8-MoO3-SrO mechanical mixture, but the separation of U from Sr under the same conditions of conversion in nitrating atmosphere is impossible. Thus, the gas-phase treatment of the U3O8-MoO3 (5 wt%)-SrO (5 wt%) mechanical mixture in nitrating atmosphere does not allow quantitative separation of U and Sr. However, the gas-phase conversion of the oxides allows virtually complete separation of Mo, because the major fraction of Mo remains in the water-insoluble precipitate.
To conclude, the gas-phase conversion of U and Sr oxides into water-soluble compounds allows not only separation of U from Mo and Zr, but also solution of a number of problems associated with the behavior of Mo and Zr in hydrometallurgical reprocessing of SNF.
Cite this paper
Sergey A. Kulyukhin,Yurii M. Nevoin,Natalya A. Konovalova,Andrey V. Gordeev,Alexey A. Bessonov,Margarita P. Gorbacheva,Andrey Yu. Shadrin,Konstantin N. Dvoeglazov, (2016) Gas-Phase Conversion of the U(VI), Sr, Mo, and Zr Oxides in Nitrating Atmosphere. Journal of Applied Mathematics and Physics,04,1475-1481. doi: 10.4236/jamp.2016.48153
References
- 1. Nikulin, S.L., Lukonin, G.A., Panov, G.A. and Zelenkin, M.A. (2013) Innovative Developments in Main Spent Fuel Reprocessing Operations. Nuclear and Environment Safety, 2, 40-42.
- 2. Kobets, L.V., Klavsut, G.N. and Umreiko, D.S. (1981) Physico-Chemical Investigation Waterless Uranyl Nitrate and Nitrosonium Trinitratouranylate. Zh. Neorganicheskoi Khimii (Russia), 26, 173-178.
- 3. Kobets, L.V., Klavsut, G.N., Dolgov, V.M. and Umreiko, D.S. (1983) The Study of the Interaction of Uranium Oxides with Liquid Nitrogen Tetraoxide. Radiokhimiya (Russia), 25, 48-51.
- 4. Kobets, L.V., Klavsut, G.N. and Dolgov, V.M. (1983) The Nehaviour of Uranium Fioxide in Liquid Nitrogen Tetra- oxide. Radiokhimiya (Russia), 25, 661-665.
- 5. Kobets, L.V. and Klavsut, G.N. (1990) The Interaction of Uranium Oxides with Liquid Nitrogen Tetraoxide. Uspekhi Khimii (Russia), 59, 1251-1264.
- 6. Ryabkova, N.V., Lumpov, A.A., Mikhalev, V.A., Murzin, A.A. and Shadrin, A.Yu. (2010) Dissolution of UO2 in the N2O4-H2O System. Radiochemistry, 52, 399-402. http://dx.doi.org/10.1134/S1066362210040132
- 7. Revenko, Yu.A., Romanovskii, V.N., Kudryavtsev, E.G., Shadrin, A.Yu., Bondin, V.V., Bychkov, S.I., Efremov, I.G., Murzin, A.A. and Babain, V.A. (2005) Method of Actinide Nitrate Synthesis (Variants). RF Patent 2295789.
- 8. Zhu, L.Y., Duan, W.H., Xu, J.M. and Zhu, Y.J. (2010) Conversion of Ceramic UO2 Pellets for HTR-10 Fuel Elements into Nitrate with N2O4. Ind. Eng. Chem. Res., 49, 11195-11199. http://dx.doi.org/10.1021/ie101325n
- 9. Collins, E.D., Delcul, G.D., Hunt, R.D., Johnson, J.A. and Spencer, B.B. (2013) Advanced Dry Head-End Reprocessing of Light Water Reactor Spent Nuclear Fuel. US Patent 8574523.
- 10. Bondin, V.V., Bychkov, S.I., Revenko, Yu.A., Efremov, I.G., Murzin, A.A., Shadrin, A.Yu., Babain, V.A., Roma- novskii, V.N. and Kudryavtsev, E.G. (2008) Conversion of Uranium Dioxide and Real Spent Nuclear Fuel into Nitrates. Radiochemistry, 50, 253-255. http://dx.doi.org/10.1134/S1066362208030065
- 11. JCPDS—Int. Centre for Diffraction Data, PDF 27-0936, UO2(NO3)2·6H2O.
- 12. Kulyukhin, S.A., Kamenskaya, A.N. and Rumer, I.A. (2009) Mechanism of UO2(NO3)2·6H2O Decomposition under the Action of Microwave Radiation: Part 2. Radiochemistry, 51, 469-478. http://dx.doi.org/10.1134/S1066362209050063
- 13. JCPDS—Int. Centre for Diffraction Data, PDF. 34-0630, UO2(OH)(NO3)·2H2O.
- 14. JCPDS—Int. Centre for Diffraction Data, PDF 76-1850, U3O8.
- 15. (2015) IR-Spektrensammlung der ANSYCO GmbH. http://www.ansyco.de
- 16. JCPDS—Int. Centre for Diffraction Data, PDF 01-087-0557, Sr(NO3)2.
- 17. JCPDS—Int. Centre for Diffraction Data, PDF 01-074-0407, Sr(OH)2.
- 18. JCPDS—Int. Centre for Diffraction Data, PDF 01-075-0263, SrO.
- 19. JCPDS—Int. Centre for Diffraction Data, PDF 82-2405, MoO3.
- 20. Lidin, R.A., Molochko, V.A. and Andreeva, L.L. (2006) Chemical Properties of Inorganic Substances. KolosS, Moscow.
- 21. JCPDS—Int. Centre for Diffraction Data, PDF 03-065-2357, ZrO2.


