Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio n, 2011, 2, 1058-1069 doi:10.4236/msa.2011.28143 Published Online August 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) Janaina Aline Galvão Barros*, Antonio Jedson Caldeira Brant, Luiz Henrique Catalani Institute of Chemistry, University of São Paulo, São Paulo, Brazil Email: catalani@usp.br, *janabarros@usp.br Received August 6th, 2011; revised December 1st, 2010; accepted June 2nd, 2011 ABSTRACT PVP, a synthetic polymer and chitosan, a natural polymer are biocompatible and have presented a great variety of in- teresting properties for cosmetic, biomedical, pharmaceutical and biotechnological applications, Many alternatives to improve the polymer propeties has been made as bleeding polymers, for example. In this work, two different techniques of hydrogel attainment were used: one from mixtures of acid aqueous co-solutions of chitosan and PVP, whose resul- tant films and solutions were irradiated, afterwards, by ultraviolet radiation (λ = 254 nm), another one from the reac- tion of poly (N-vinyl-2-pyrrolidone-co-acrolein)-a novel copolymer synthesized in our biomaterials laboratory-and chitosan. In the first one alternatives it was possible produce hydrogels directly from mixtures of aqueous acidic co-solutions of both polymers. In the second one, the attainment of hydrogels from Schiff base has proved to be an ef- fective methodology for the production of hydrogels, showing good values of gel content and swelling. Keywords: Chitosan, Hydrogel, Copolymer, Crosslinking, Schiff Base, Poly(Vinyl-Pyrrolidone-Co-Acrolein) 1. Introduction The search for novel biomaterials, mainly polymer hy- drogels, is increasing throughout the world. In 1960, Wichterle and Lim developed the first synthetic polymer hydrogel based on 2-hydroxyethyl methacrylate (2-HEM A) as the hydrophilic monomer [1]. Throughout the last five decades numerous publications on this research topic have been released. Hydrogels play an important role in tissue engineering [2-5], dressings for burn wounds and other kinds of injuries [6,7], “intelligent” hidrogels for drug controlled release [8,9], and other biomedical ap- plications [10]. Hydrogels are three-dimensional hydrophilic polymer networks capable of swelling in water or biological fluids and retaining a great amount of fluid in the swollen state without dissolution of their structure [11,12]. They can be obtained through chemical and physical processes [13]. Dressings based on polymeric hydrogels show many advantages, such as exudate absorption, immediate pain relief, barrier to microorganisms, permeability to oxygen, adjustable transparency and mechanical proper- ties [14]. Poly(N-vinyl-2-pyrrolidone) (PVP) is a synthetic lin- ear non-toxic, biocompatible polymer, frequently used in food and cosmetics industries as well as in pharmaceuti- cal formulations [15,16]. It is very soluble in water and many organic solvents. Its use as a biomaterial in artifi- cial blood plasma was prevalent in World War II [17-19]. Nevertheless, over the last five decades, PVP has found other applications of greater scientific-biotechnological interest, such as hydrogels for drug controlled release [20,21], tissue regeneration and implants [22], wound and burn dressings [23], and others applications. Hy- drogel dressings have attracted much attention among researchers for their use in the medicinal field, chiefly in the healing of burn wounds. It must be emphasized here that there are already numerous natural and synthetic polymers being studied and/or applied as medicinal hy- drogels, and PVP is among them [24,25]. But, beyond PVP as a unique polymer, other polymer systems in- volving VP-monomers are also used in this application area: chemically modified PVP [26-28], copolymers containing units of N-vinyl-2-pyrrolidone in their chains for example, poly(methacrylamide-co-N-vinyl-2- pyrro-lidone-co-itaconic acid) [29]; poly(N-vinyl-2-pyrr- olidone- co-styrene) [30]; poly(N-vinyl-2-pyrro lidone-co- acrylic acid), [31] and blends of PVP with other bio- compatible polymers (for example, PVP-CMC [32]; PVP-PVA [33]; PVP-k-carrageenan [34]; PVP-chitosan [35].  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1059 Chitosan is another polymer that has found use in biomedical applications over the last three decades [36]. Chemically, it is constituted of units of 2-amine-2-deoxy- D-glycopyranose and 2-acetamide-2-deoxy-D-glycopy- ranose linked by β- (1→ 4) glycoside linkages [37] along a linear chain. Commercially, it is obtained from alkaline deacetylation of chitin through a hydrolysis reaction us- ing an aqueous solution with ca. 50% KOH at tempera- tures near to 100˚C. Chitin is a linear polysaccharide present mainly in exoskeletons of crustaceans (shrimps, crabs, lobsters, etc.) [38]. After cellulose, it is the most abundant natural biopolymer on the earth [39]. Thus, chitosan is a renewable and sustainable material. Also, due to its biocompatibility, non-toxic properties and it having inhibitory effects on the growth of fungi and bac- teria, there is a growing interest in its potential applica- tion in the biomaterials field. Chitosan is practically insoluble in water and organic solvents. Although it is a hydrophilic polymer, its disso- lution in water only takes place in dilute acidic solutions, in which organic acids (for example, formic acid, acetic acid) or mineral acids (for example, HCl, HNO3) are utilized. These protonate its available amine groups, leading to the formation of a hydrosoluble polycation at pHs < 6. Solubilization of chitosan constitutes, therefore, an important step for its use in diverse types of biomate- rials. Chitin derivatives containing more than 50% of free amine in their structure are usually denominated chitosan [40]. The majority of commercial chitosans have degrees of acetylation between 15% and 30%. The degree of acetylation (DA) and the molecular weight of chitosan define practically all its physicochemical properties and determine its use for applications in medicine, biotech- nology and other fields involving biomaterials [41]. Average degree of acetylation (DA ) is defined as the average percentage of remaining acetyl groups along the chain of chitin after its deacetylation. There are several methods to determine it, from simple to very sophisti- cated ones: elemental analysis (EA), infrared spectrome- try (IR), hydrogen nuclear magnetic resonance (1H-NM- R), UV- spectrophotometry, linear potenciometric titra- tion, ninhydrin test, circular dichroism measurements, others [42-43]. Among these, IR is widely used because it is relatively simple, non-destructive, cheap and gener- ally gives reliable results. It mainly makes use of base- line (a) expressed by: 1665 3450 (%) 100 1.33 A A DA where DA is the degree of acetylation, i.e. the percent- age of amide groups acetylated; 1655 A and 3450 A are the absorbance at 1655 cm–1 of the amide-I band as a measure of the N-acetyl group content and the hydroxyl absorbance band at 3450 cm–1 as an internal standard to correct film thickness or differences in chitosan concen- tration in powder form. This ratio of 1655 A / 3450 A is used as an internal standard peak; the factor “1,33” is the value of the ratio of 1655 A / 3450 A for fully N-acetylated chitosan [43,44] The degree of deacetylation, DD , is then obtained by equation: (%) 100DD DA Evidently, a higher DD of chitosan indicates more free amine groups in its structure. It may contribute not only to a lowering of chitosan crystallinity up to a certain extent - crystallinity is maximum for both chitin (i.e. 0% deacetylated) and fully deacetylated chitosan (i.e. 100%) [45], but also to an improvement of its solubility in water, what is achieved by the formation of salts soluble in wa- ter after the protonation of amine groups. Average molecular weight of chitosan is another pa- rameter that plays an important role in determining ap- plications for this polymer. Chen and Hwa [46] have demonstrated the influence of molecular weight of di- verse chitosan samples with the same DA on its ther- mal, mechanical and permeability properties. Other properties which depend upon the chitosan molecular weight are antioxidant activity [47], antimicrobial activ- ity [48], film water absorption [49], etc. Determination of chitosan molecular weight can be performed by several methods [50] usually employed in polymer chemistry: capillary viscometry [51,52], membrane osmometry [53], laser light scattering [54] exclusion size chromatography (SEC) [55] others. Additionally, chitosan is a non-toxic, biocompatible and biodegradable linear polymer [56]. It has presented a great variety of interesting properties for cosmetic, bio- medical, pharmaceutical and biotechnological applica- tions [57]. Chemical modification of chitosan mainly aims at an improvement of its solubility in water or in other types of solvent, however it can also aim at the incorporation of special physicochemical properties to the polymer for determined applications [58]. The most common reactions of chitosan chemical modification encompass N-deacetylation, N-acetylation and O-acetyla- tion [37], N-acylation and O-acylation [59-60], and N-phtalation [61]. Chitosan, chemically modified chito- san, and chitosan in blends with hydrophilic polymers such as poly(vinyl alcohol), poly(N-vinyl-2-pyrrolidone), poly( ethylene oxide), starch, cellulose [62] have raised interest in the research and development of biomate- rials with promising results. However, in the last four — baseline (a), Copyright © 2011 SciRes. MSA  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1060 decades, much attention has been given to alternative methods in blending polymers to create new materials with improved physicochemical and mechanical proper- ties for determined applications. Such methods generally are simpler and cheaper than the synthesis of new poly- mers. In this case, miscibility of blends is crucial in their stability and performance. Miscibility depends on poly- mer-polymer interactions at molecular level, mainly through H-bonds [63-64], and can be evaluated by sev- eral methods: infrared spectroscopy, differential scanning calorimetry (DSC), dynamical mechanical analysis (DMA), scanning electron microscopy (SEM), etc. [65-67,32]. Blends of chitosan and PVP can become good alterna- tives for producing new biomaterials, once the inherent properties of both polymers herein discussed as well as their high miscibility show very favorable performance properties for this application area. In this work, two different techniques of hydrogel at- tainment were used: one from mixtures of acid aqueous co-solutions of chitosan and PVP, of which the resultant films and solutions were afterwards irradiated by ultra- violet radiation (λ = 254 nm), that is capable of inducing cross-linking of PVP by recombination of free macro- radicals and subsequent hydrogel formation [25,17]; an- other from the reaction of poly (N-vinyl-2-pyrrolidone- co-acrolein) a novel copolymer synthesized in our bio- materials laboratory and chitosan. Cross-linking, in this case, is supposed to occur through Schiff base formation by the aldehyde group of the acrolein units of the co- polymer and available amine groups of chitosan [68]. In both techniques, stable hydrogels were produced at low polymer concentrations (2% m/v, polymer/water, respec- tively). Some physical properties of films resulting from chitosan and PVP blends and the hydrogels from the co-solutions of both polymers as well as the hydrogels from the reaction of chitosan with the novel copolymer were characterized in order to previously evaluate their potential use as biomaterials in the near future. 2. Experimental 2.1. Materials For the experiments, the following materials were used: two commercial chitosan samples referred to as high- molecular weight and medium-molecular weight from Aldrich (Brookfield viscosity 800.000 cP and 200.000 cP, respectively, data from the supplier), poly(N-vinyl- 2-pyrrolidone) known as Luviskol® K-90 from BASF (n M = 360,000; w M = 1.200,000), acetic acid and so- dium hydroxide (Vetec, São Paulo), anhydrous sodium acetate (Sigma), sodium chloride (Synth), monomers of N-vinyl-2-pyrrolidone and acrolein (Aldrich). Except for the polymers and monomers here cited, all reagents used in the experiments were of analytical grade. 2.2. Methods 2.2.1. Chitosan Characterization 1) Chitosan purification Chitosan samples were dissolved in 2% aqueous acetic acid solution (v/v) at polymer concentration of 2% (w/v) under constant stirring overnight at room temperature. The obtained solutions were filtered through cellulose acetate MILLIPORE® membrane of 0.45 m pore size and neutralized with 10% aqueous NaOH solution (w/v) for up to three cycles. The precipitated polymer was then washed extensively with distilled water, brought to pH about 7.0 and lyophilized up to constant mass. 2) Chitosan average degree of dacetylation (DD ) 3) Elemental analysis Around 20 mg of each purified chitosan in duplicate were taken for percentage determination of C, H and N in elemental analysis test carried out using a Perkin Elmer CHN analyzer. Samples were taken in duplicate. a) Infrared spectrometry (FT-IR) Samples of chitosan were dispersed in KBr (ca. 2% polymer, w/w) and pressed into transparent discs for analysis by FT-IR spectroscopy. Transmittance spectra were obtained through a FT-IR Bomem MB 100 spec- trometer operating in a range of 350 - 4000 cm–1, 2 cm–1 resolution, 32 runs. For DA/DD calculation, the two standard absorption bands [69] were taken into account: amide- I (1655 cm–1) as a measure of the N-acetyl group content and hydroxyl (3450 cm–1 as an internal standard to correct film thickness or differences in chitosan con- centration. The factor ‘1.33’ denoted the value of the ratio of A1655/A3450 for fully N-acetylated chitosan. b) 1H nuclear magnetic resonance (1H-NMR) 1H-NMR spectra were obtained on a Bruker DRX 500 spectrometer at 70˚C from chitosan solutions prepared with 10 mg of chitosan suspended in a solution com- posed of 1.96 mL of D2O and 0.04 mL of DCl under constant stirring at room temperature, having 3-(trimeth- ylsilyl)-1-propanesulfonic-d4 acid (TSPA, Aldrich) as an external reference. The calculations of DA were per- formed from the ratios of areas of peaks in accordance with equation 3 2 (%) 100, 3 CH H A DA A where: 3 CH A = area of the peak at 2 ppm, attributed to the nuclei of the hydrogens of methyl group; AH2= area of the peak at 3.2 ppm, attributed to the nucleus of hy- drogen at position 2 of the glycosamine ring. By deduct- ing from 100 the value of DA, one obtains the value of DD, that is, DD (%) = 100 – DA (%). Copyright © 2011 SciRes. MSA  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1061 2.2.2. Hydrogel Attainment from Chitosan and PVPAC 1) Preparation and characterization of poly(vinylpy- rrolidone-co-acrolein) (PVPAC) Mixtures containing 90% of N-vinyl-2-pyrrolidone and 10% of acrolein (w/w) were prepared. The copolymeri- zation was carried out in 10 mL test tubes flushed with nitrogen and duly sealed, using as an initiator –0.05% of azoisobutyronitrile (AIBN) by weight, based on the mo- lar ratio of N-vinyl pyrrolidone/acrolein = 9. The mix- tures were kept for one day at 65˚C. The obtained co- polymer was purified by dissolving it in absolute ethanol and, next, precipitating it in ethyl ether. Its separation was performed by filtration. This procedure was per- formed up to three cycles. Afterwards, the copolymer was dried under vacuum at 60˚C to constant weight and washed with ethyl ether to remove unreacted monomer. Then, it was lyophilized for withdrawal of all remaining solvent. The percentage of the incorporated co-monomer was gotten by elemental analysis of C, H, and N on a Perkin Elmer CHN analyzer. 2) Characterization of PVPAC a) Size exclusion chromatography (SEC) The molar mass of PVPAC was evaluated in a liquid chromatograph, SHIMADZU LC-10AD equipped with refractive index and UV (254 nm) detectors (Class- LC10), utilizing Ultrahydrogel® columns (120, 250, 500 and 2000) (300 × 7,8 mm) from Waters, mobile phase of distilled water and acetonitrile (0.03 M) at a 1.0 mL/min flow. The calibration curves have been achieved with standards of poly (ethylene oxide) (PEO) with molar masses of 860,000; 348,000; 88,200; 24,800 and 1200 g·mol–1. b) Membrane osmometry The number average molecular weight of PVPAC was obtained from 4 dilute aqueous solutions at different polymer concentrations in water. Osmotic pressures of diluted solutions were measured at 30˚C in a OSMO- MAT osmometer, model 090, from GONOTEC (Ger- many), equipped with cellulose acetate membranes (cut-off 5 kDa e 10 kDa). Number average molecular weight, n M , was calculated according to equation 2 0 1 nC RTA c cM where Ris the universal gas constant, T temperature (K), A2 is the second virial coefficient, 0c c is the reduced osmotic pressure extrapolated to concentration c = 0 by linear regression. 3) Hydrogels from PVPAC and chitosan a) Attainment of chitosan-PVPAC hydrogels The hydrogels were obtained from solutions of 80 and 120 g·L–1 of PVPAC copolymer and solution of 20 g·L–1 of chitosan dissolved in dilute aqueous acetic acid (2%, v/v). Polymer solutions were then mixed at different chi- tosan: PVPAC weight ratios, at pH ca. 2.5 under mag- netic stirring until the attainment of a gel. 4) Characterization of PVPAC-chitosan hydrogels a) Cross-linking kinetics and mechanical properties of Schiff base hydrogel Cross-linking kinetics of imine hydrogels, prepared on the rheometer, between the parallel geometry plates, was performed on a cone-plate rheometer—Physica MCR 300—Paar Physica. All rheological measurements were carried out at 25˚C. The samples used in these experi- ments were 0.5 mL (80 and 120 g·L–1) of PVPAC solu- tion and 0.5 mL (20 g·L–1) of chitosan solution. b) Gel fraction and equilibrium swelling ratio The hydrogel samples obtained from chitosan and PVPAC was weighed and immersed in deionized water for 48 h at room temperature. Afterwards, they were re- moved from the solvent, dried quickly in filter paper for withdrawal of excess water, weighed and let to dry into a ventilated oven at 70˚C during 48 h to a constant weight (wd). The gel fraction (GF) was determined by equation: (%) 100 d i m GF m where % GF is the percentage of gel fraction, md , the mass of dry hydrogel after extraction, mi, initial polymer mass before the extraction in deionized water. The equilibrium swelling ratio was calculated from equation. id i d mm Sw m where Swi is the equilibrium swelling ratio, mi , the mass of the swollen gel, md, the mass of the dry gel. For these experiments, the samples were taken in triplicate. 3. Results and Discussion 3.1. Average Degree of Deacetylation of Chitosan The average degrees of deacetylation of both original and purified chitosans are listed in Table 1. They were ob- tained from the three different methods described herein. The three techniques utilized in determination of DD of the chitosans in our experiments generally showed results with good correlations for the original and purified sam- ples, except for elemental analysis of the original CM, whose results were rather discrepant. The high discrepan- cies probably are due to presence of excess of water in its samples evaluated by this method. Chitosan is a high hy- groscopic polymer in nature, and it is practically impossi- ble to totally eliminate water contained in it by conven- tional drying techniques or even utilizing lyophilization. In- frared spectrometry showed to be a very efficient technique Copyright © 2011 SciRes. MSA 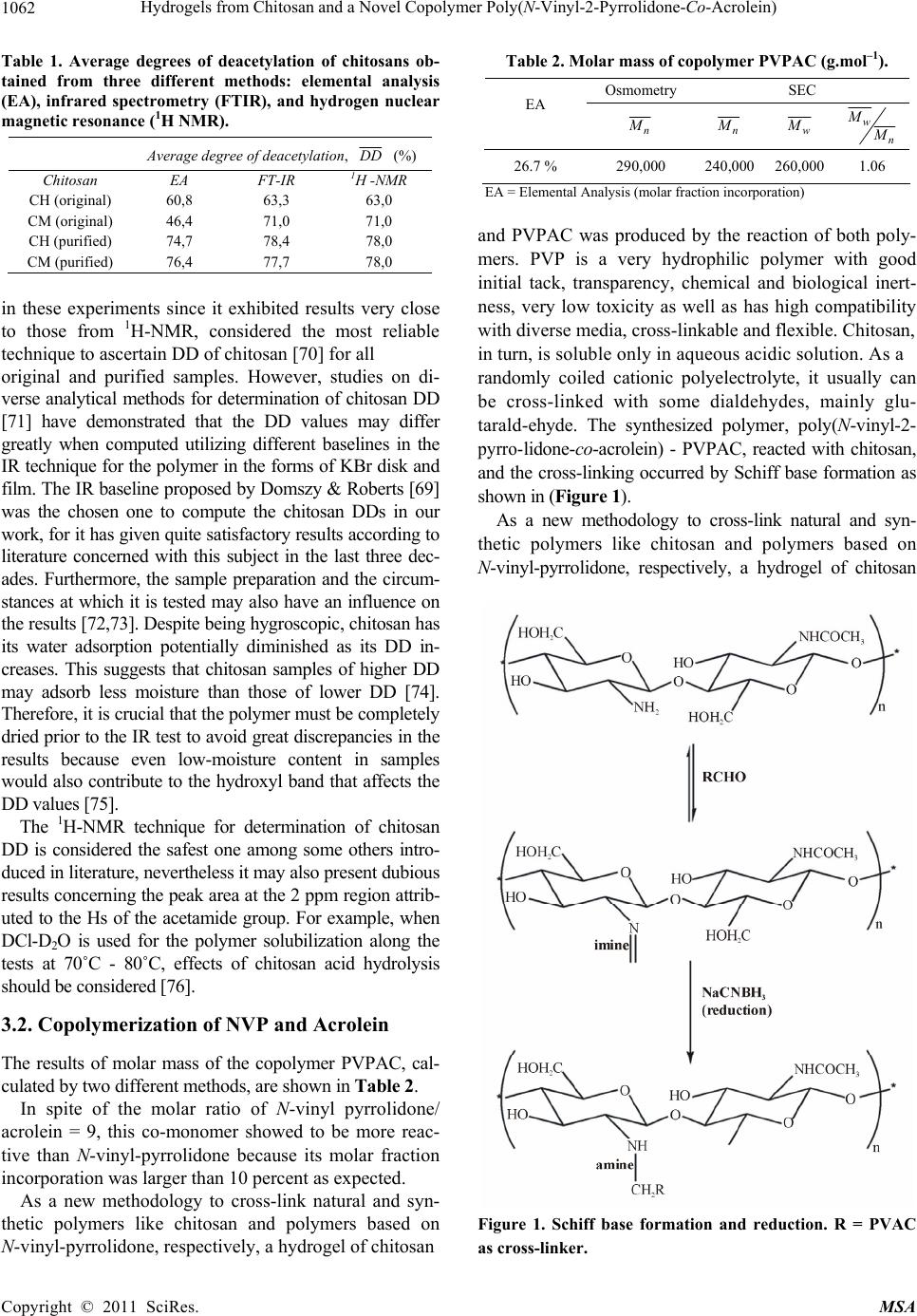 Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1062 Table 1. Average degrees of deacetylation of chitosans ob- tained from three different methods: elemental analysis (EA), infrared spectrometry (FTIR), and hydrogen nuclear magnetic resonance (1H NMR). Average degree of deacetylation, D D (%) Chitosan EA FT-IR 1H -NMR CH (original) 60,8 63,3 63,0 CM (original) 46,4 71,0 71,0 CH (purified) 74,7 78,4 78,0 CM (purified) 76,4 77,7 78,0 in these experiments since it exhibited results very close to those from 1H-NMR, considered the most reliable technique to ascertain DD of chitosan [70] for all original and purified samples. However, studies on di- verse analytical methods for determination of chitosan DD [71] have demonstrated that the DD values may differ greatly when computed utilizing different baselines in the IR technique for the polymer in the forms of KBr disk and film. The IR baseline proposed by Domszy & Roberts [69] was the chosen one to compute the chitosan DDs in our work, for it has given quite satisfactory results according to literature concerned with this subject in the last three dec- ades. Furthermore, the sample preparation and the circum- stances at which it is tested may also have an influence on the results [72,73]. Despite being hygroscopic, chitosan has its water adsorption potentially diminished as its DD in- creases. This suggests that chitosan samples of higher DD may adsorb less moisture than those of lower DD [74]. Therefore, it is crucial that the polymer must be completely dried prior to the IR test to avoid great discrepancies in the results because even low-moisture content in samples would also contribute to the hydroxyl band that affects the DD values [75]. The 1H-NMR technique for determination of chitosan DD is considered the safest one among some others intro- duced in literature, nevertheless it may also present dubious results concerning the peak area at the 2 ppm region attrib- uted to the Hs of the acetamide group. For example, when DCl-D2O is used for the polymer solubilization along the tests at 70˚C - 80˚C, effects of chitosan acid hydrolysis should be considered [76]. 3.2. Copolymerization of NVP and Acrolein The results of molar mass of the copolymer PVPAC, cal- culated by two different methods, are shown in Table 2. In spite of the molar ratio of N-vinyl pyrrolidone/ acrolein = 9, this co-monomer showed to be more reac- tive than N-vinyl-pyrrolidone because its molar fraction incorporation was larger than 10 percent as expected. As a new methodology to cross-link natural and syn- thetic polymers like chitosan and polymers based on N-vinyl-pyrrolidone, respectively, a hydrogel of chitosan Table 2. Molar mass of copolymer PVPAC (g.mol–1). Osmometry SEC EA n M n M w M w n M M 26.7 % 290,000 240,000 260,000 1.06 EA = Elemental Analysis (molar fraction incorporation) and PVPAC was produced by the reaction of both poly- mers. PVP is a very hydrophilic polymer with good initial tack, transparency, chemical and biological inert- ness, very low toxicity as well as has high compatibility with diverse media, cross-linkable and flexible. Chitosan, in turn, is soluble only in aqueous acidic solution. As a randomly coiled cationic polyelectrolyte, it usually can be cross-linked with some dialdehydes, mainly glu- tarald-ehyde. The synthesized polymer, poly(N-vinyl-2- pyrro- lidone-co-acrolein) - PVPAC, reacted with chitosan, and the cross-linking occurred by Schiff base formation as shown in (Figure 1). As a new methodology to cross-link natural and syn- thetic polymers like chitosan and polymers based on N-vinyl-pyrrolidone, respectively, a hydrogel of chitosan Figure 1. Schiff base formation and reduction. R = PVAC as cross-linker. Copyright © 2011 SciRes. MSA 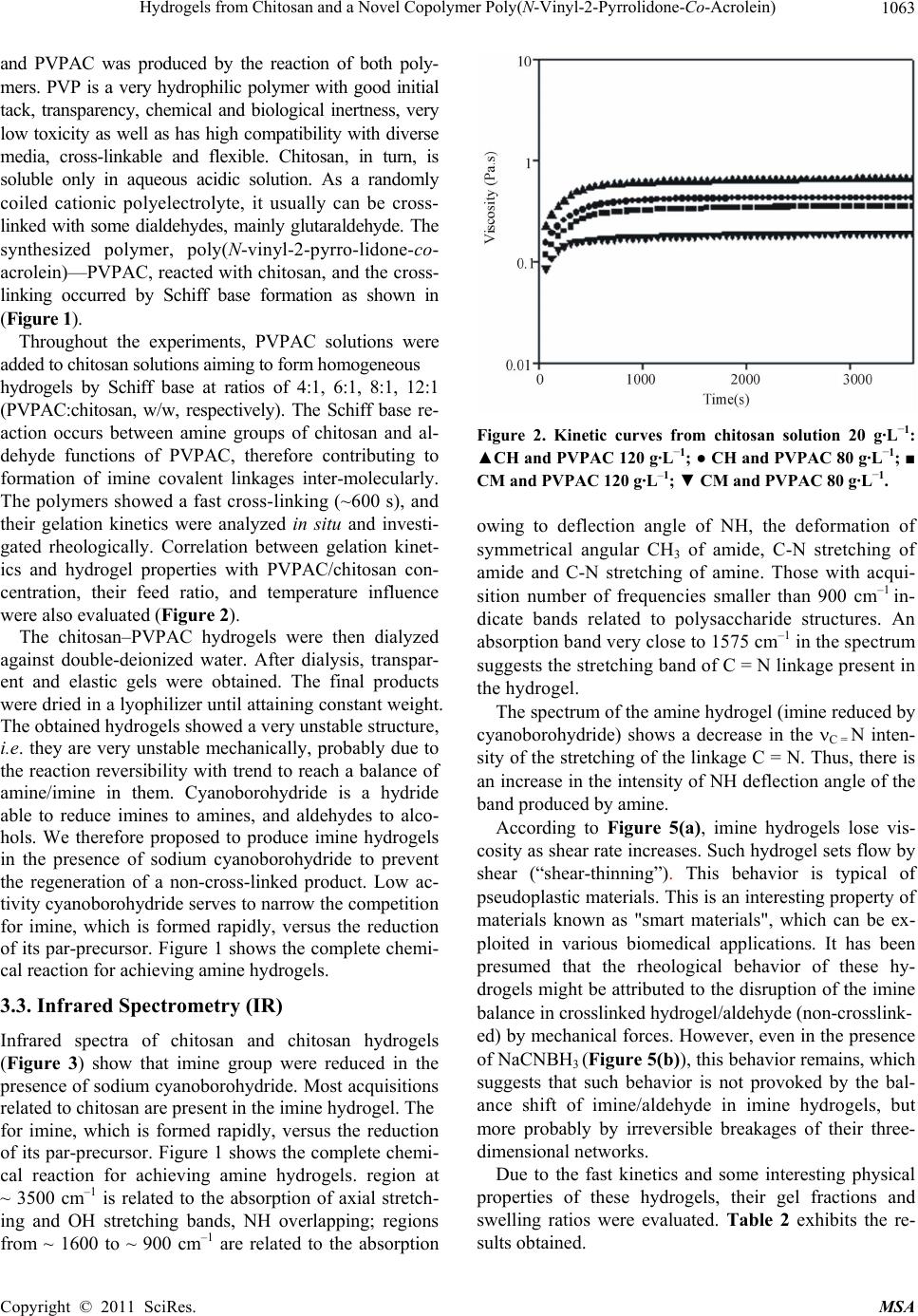 Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1063 and PVPAC was produced by the reaction of both poly- mers. PVP is a very hydrophilic polymer with good initial tack, transparency, chemical and biological inertness, very low toxicity as well as has high compatibility with diverse media, cross-linkable and flexible. Chitosan, in turn, is soluble only in aqueous acidic solution. As a randomly coiled cationic polyelectrolyte, it usually can be cross- linked with some dialdehydes, mainly glutaraldehyde. The synthesized polymer, poly(N-vinyl-2-pyrro-lidone-co- acrolein)—PVPAC, reacted with chitosan, and the cross- linking occurred by Schiff base formation as shown in (Figure 1). Throughout the experiments, PVPAC solutions were added to chitosan solutions aiming to form homogeneous hydrogels by Schiff base at ratios of 4:1, 6:1, 8:1, 12:1 (PVPAC:chitosan, w/w, respectively). The Schiff base re- action occurs between amine groups of chitosan and al- dehyde functions of PVPAC, therefore contributing to formation of imine covalent linkages inter-molecularly. The polymers showed a fast cross-linking (~600 s), and their gelation kinetics were analyzed in situ and investi- gated rheologically. Correlation between gelation kinet- ics and hydrogel properties with PVPAC/chitosan con- centration, their feed ratio, and temperature influence were also evaluated (Figure 2). The chitosan–PVPAC hydrogels were then dialyzed against double-deionized water. After dialysis, transpar- ent and elastic gels were obtained. The final products were dried in a lyophilizer until attaining constant weight. The obtained hydrogels showed a very unstable structure, i.e. they are very unstable mechanically, probably due to the reaction reversibility with trend to reach a balance of amine/imine in them. Cyanoborohydride is a hydride able to reduce imines to amines, and aldehydes to alco- hols. We therefore proposed to produce imine hydrogels in the presence of sodium cyanoborohydride to prevent the regeneration of a non-cross-linked product. Low ac- tivity cyanoborohydride serves to narrow the competition for imine, which is formed rapidly, versus the reduction of its par-precursor. Figure 1 shows the complete chemi- cal reaction for achieving amine hydrogels. 3.3. Infrared Spectrometry (IR) Infrared spectra of chitosan and chitosan hydrogels (Figure 3) show that imine group were reduced in the presence of sodium cyanoborohydride. Most acquisitions related to chitosan are present in the imine hydrogel. The for imine, which is formed rapidly, versus the reduction of its par-precursor. Figure 1 shows the complete chemi- cal reaction for achieving amine hydrogels. region at ~ 3500 cm–1 is related to the absorption of axial stretch- ing and OH stretching bands, NH overlapping; regions from ~ 1600 to ~ 900 cm–1 are related to the absorption Figure 2. Kinetic curves from chitosan solution 20 g·L–1: ▲CH and PVPAC 120 g·L–1; ● CH and PVPAC 80 g·L–1; ■ CM and PVPAC 120 g·L–1; ▼ CM and PVPAC 80 g·L–1. owing to deflection angle of NH, the deformation of symmetrical angular CH3 of amide, C-N stretching of amide and C-N stretching of amine. Those with acqui- sition number of frequencies smaller than 900 cm–1 in- dicate bands related to polysaccharide structures. An absorption band very close to 1575 cm–1 in the spectrum suggests the stretching band of C = N linkage present in the hydrogel. The spectrum of the amine hydrogel (imine reduced by cyanoborohydride) shows a decrease in the C = N inten- sity of the stretching of the linkage C = N. Thus, there is an increase in the intensity of NH deflection angle of the band produced by amine. According to Figure 5(a), imine hydrogels lose vis- cosity as shear rate increases. Such hydrogel sets flow by shear (“shear-thinning”). This behavior is typical of pseudoplastic materials. This is an interesting property of materials known as "smart materials", which can be ex- ploited in various biomedical applications. It has been presumed that the rheological behavior of these hy- drogels might be attributed to the disruption of the imine balance in crosslinked hydrogel/aldehyde (non-crosslink- ed) by mechanical forces. However, even in the presence of NaCNBH3 (Figure 5(b)), this behavior remains, which suggests that such behavior is not provoked by the bal- ance shift of imine/aldehyde in imine hydrogels, but more probably by irreversible breakages of their three- dimensional networks. Due to the fast kinetics and some interesting physical properties of these hydrogels, their gel fractions and swelling ratios were evaluated. Table 2 exhibits the re- sults obtained. Copyright © 2011 SciRes. MSA 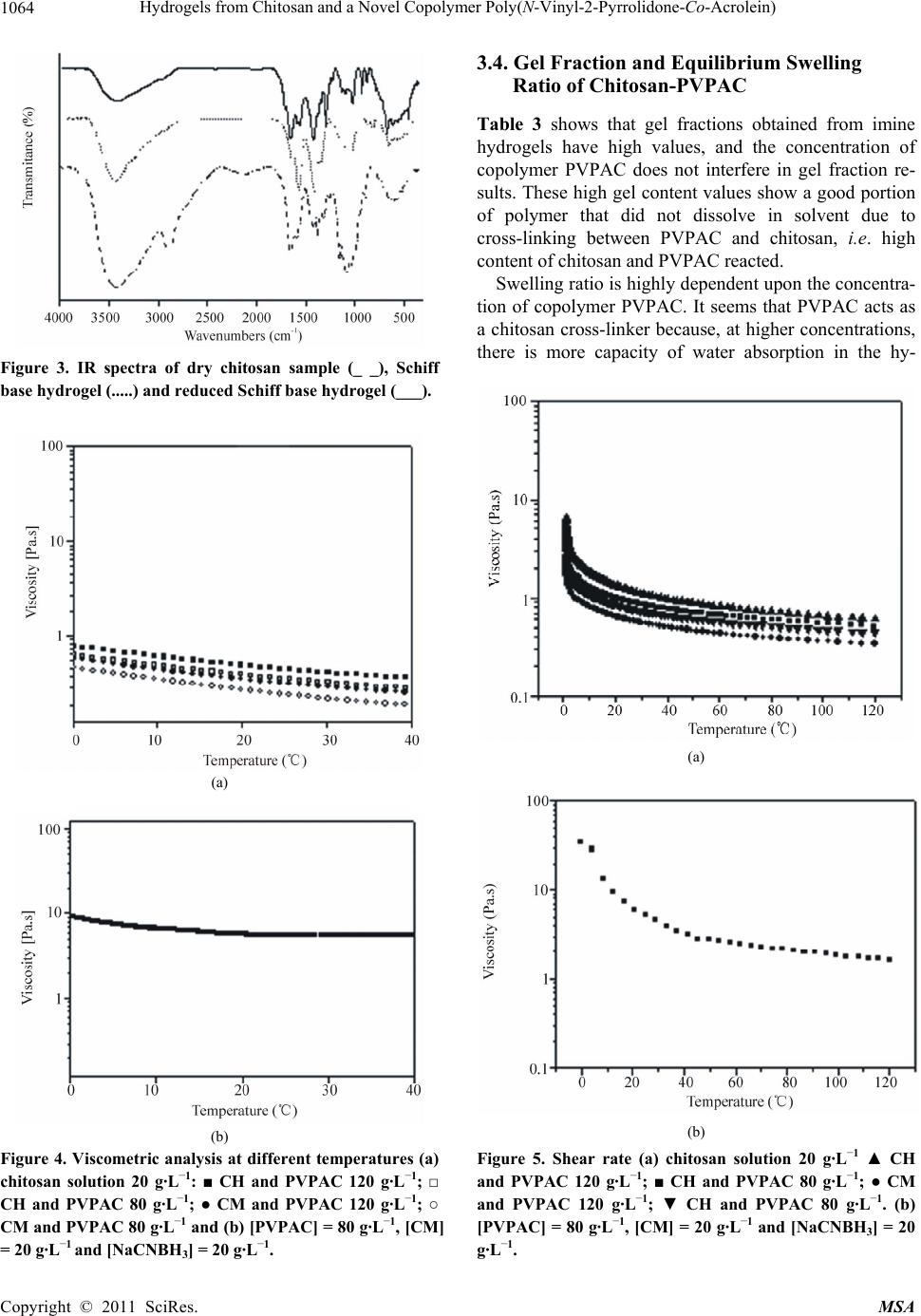 Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) Copyright © 2011 SciRes. MSA 1064 3.4. Gel Fraction and Equilibrium Swelling Ratio of Chitosan-PVPAC Table 3 shows that gel fractions obtained from imine hydrogels have high values, and the concentration of copolymer PVPAC does not interfere in gel fraction re- sults. These high gel content values show a good portion of polymer that did not dissolve in solvent due to cross-linking between PVPAC and chitosan, i.e. high content of chitosan and PVPAC reacted. Swelling ratio is highly dependent upon the concentra- tion of copolymer PVPAC. It seems that PVPAC acts as a chitosan cross-linker because, at higher concentrations, there is more capacity of water absorption in the hy- Figure 3. IR spectra of dry chitosan sample (_ _), Schiff base hydrogel (.....) and reduced Schiff base hydrogel (___). (a) (a) (b) (b) Figure 5. Shear rate (a) chitosan solution 20 g·L–1 ▲ CH and PVPAC 120 g·L–1; ■ CH and PVPAC 80 g·L–1; ● CM and PVPAC 120 g·L–1; ▼ CH and PVPAC 80 g·L–1. (b) [PVPAC] = 80 g·L–1, [CM] = 20 g·L–1 and [NaCNBH3] = 20 g·L–1. Figure 4. Viscometric analysis at different temperatures (a) chitosan solution 20 g·L–1: ■ CH and PVPAC 120 g·L–1; □ CH and PVPAC 80 g·L–1; ● CM and PVPAC 120 g·L–1; ○ CM and PVPAC 80 g·L–1 and (b) [PVPAC] = 80 g·L–1, [CM] = 20 g·L–1 and [NaCNBH3] = 20 g·L–1.  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1065 Table 3. Average gel fractions and equilibrium swelling ratios of hydrogels obtained from mixtures of chitosan and PVPAC solutions in diverse proportions. Samples taken in triplicate. CM CH Samples Average gel fraction (%) Average swelling ratioSamples Average gel fraction (%) Average swelling ratio 4:1 86 ± 15 31 ± 9 4:1 82 ± 2 76 ± 9 6:1 87 ± 10 25 ± 9 6:1 93 ± 4 49 ± 15 8:1 85 ± 9 32 ± 11 8:1 50 ± 6 44 ± 9 12:1 94 ± 3 30 ± 6 12:1 92 ± 3 31 ± 10 Table 4. Average gel fractions and equilibrium swelling ratios of hydrogels obtained directly from chitosan-PVP blend films and mixtures of chitosan and PVP co-solutions after submitted to UV254 nm irradiation for 1.5 h (films) and 4 h (solutions) at ca. 30˚C, under N2 atmosphere. Samples in triplicate. Films Hydrogel* Samples Average gel fraction (%)* Average equilibrium swelling ratio* Samples Average gel fraction (%)** Average equilibrium swelling ratio *** PVP 64 ± 6 97 ± 21 PVPac 63 ± 8 250 ± 50 CM5 74 ± 16 45 ± 8 CM5 76 ± 4 240 ± 30 CH5 80 ± 16 79 ± 4 CH5 88 ± 3 160 ± 30 CM50 79 ± 2 27 ± 5 CM20 70 ± 5 280 ± 30 CH50 88 ± 2 30 ± 3 CH20 51 ± 7 430 ± 120 CM95 93 ± 7 28 ± 8 CM30 26 ± 8 740 ± 210 CH95 94 ± 5 30 ± 2 CH30 41 ± 7 490 ± 120 PVPac: PVP dissolved in aqueous acid solution : 2 g of PVP : 98 g of 2% acetic acid-H2O (v/v) Numbers on the right of CM and CH represents weight % chitosan in dry hydrogel. * Films immersed in deionized water for 48 h at room conditions; ** After 24 h of Sohxlet extraction in deionized water, dried for 72 h at 70˚C in ventilated oven; *** After Sohxlet extraction, hydrogels kept im- mersed in deionized water for 48 h at room conditions. drogel probably influenced by the formed networks allied to the high hydrophilicity of the former. On the other side, the hydrogel produced with chitosan of medium molecu- lar weight (CM), differently of that with chitosan of high molecular weight (CH), didn’t show dependence on PVPAC concentration in the swelling test. That can be related to the fact that the swelling limit had already reached the maximum with CM. The results obtained for the Schiff base hydrogels were compared with those of hydrogels attained from films of chitosan–PVP blends prepared by casting on plastic molds as well as mixtures of 2% polymer co-solutions at different polymer ratios on a dry basis, packed and sealed in quartz tubes. The samples in triplicate were submitted to the same UV radiation dose and reaction time in a UV chamber with an N2 atmosphere. Table 3 gives a sum- mary of the effect of the formulation compositions on average gel fractions and swelling ratios. These hydrogels are likely semi-interpenetrating networks (SIPNs), capa- ble of keeping chitosan and PVP non-cross-linked mole- cules entrapped within the networks, increasing hence the gel fractions. Their values also corroborate the assump- tion of the existence of a strong interaction between both polymers, which form miscible blends [77,78], as well as the formation of probable SIPNs induced by UV cross-linking, in which the cross-linked part is probably constituted of PVP. Attempts to cross-link films or solu- tions made of pure chitosan without any photoinitiator or chemical modification of this polymer haven’t afforded convincing results with this process in our experiments under the conditions described here. It has been verified that, in attainment of hydrogels from mixtures of 2% polymer co-solutions, the gel frac- tions increase as PVP content increases in the mixtures, confirming, therefore, that this polymer effectively is more prone to cross-link than chitosan in these experi- ments. Nevertheless, there is a limitation on directly ob- taining hydrogels from mixtures of diluted acid aqueous solutions of chitosan and PVP after being irradiated by UV light, when such mixtures contain amounts above 30% of chitosan (w/w) on a dry basis. This fact may be owing to a similar reactivity observed by Zhao et al. [79] in attainment of hydrogels from blend of carboxy- methyl-chitosan and PVP using EB-radiation. Hence, chitosan oligomers and other likely products derived from its degradation by UV irradiation would be acting as scavengers of free radicals and would interfere with the cross-linking of PVP, leading to products insuffi- ciently cross-linked to form hydrogels. 3.5. Gel Fractions and Swelling Ratios of Chitosan-PVP Mixtures See Table 4 4. Conclusions 4.1. Hydrogels from Poly(N-vinyl-2-co-Acrolein) and Chitosan Reaction The novel copolymer of N-vinyl-2-pyrrolidone and ac- Copyright © 2011 SciRes. MSA  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1066 rolein reacted with chitosan and produced a hydrogel through imine bonds. A reduction of imine to amines with sodium cyanoborohydride has shown to be a good alternative to stabilize the structure of the hydrogel. It was demonstrated that the dynamic equilibrium linkage between the polymers can be a controllable parameter as well as an interesting property that can be exploited in further experiments. Moreover, the hydrogel has a poten- tial uses as wound dressings and systems for controlled release of drugs, because of the compatibility of PVP with the body fluids, added to the properties of the chi- tosan as linker and binder [80-82], fungicide, and it has a good permeability to gas, immunogenic compatibility, low toxicity and good bioabsorption. Attainment of hy- drogels from Schiff base by reaction of poly(N-vinyl-2-co- acrolein) and chitosan proved to be an effective method- ology for the production of hydrogels. The resultant re- action products exhibit high values of gel content and swelling ratio, and provide a material that maintains some characteristics of both hydrogels from PVP and chitosan. Another advantage of this process is that they may also be produced from more concentrated solutions of initial polymers. 4.2. Hydrogels from Mixtures of Aqueous Acidic Co-Solutions of Chitosan and PVP In relation to the hydrogels obtained from chitosan-PVP films or directly from mixtures of aqueous acidic co-solutions of both polymers in diverse proportions un- der UV radiation for inducing cross-linking, their resul- tant gel fractions and swelling ratios have also shown that these hydrogels are promising materials for several applications, for example, as biomaterials. 5. Acknowledgements The authors would like to thank the Fundação de Amparo a Pesquisa do Estado de São Paulo e FAPESP and the Conselho Nacional de Desenvolvimento Científico e Tecnológico e CNPq, for financial support REFERENCES [1] O. Wichterle and D. Lim, “Hydrophilic Gels for Biologi- cal Use,” Nature, Vol. 185, 1960, pp. 117-118. doi:10.1038/185117a0 [2] B. Baroli, “Hydrogels for Tissue Engineering and Deliv- ery of Tissue Inducing Substances,” Journal of Pharma- ceutical Sciences, Vol. 96, No. 9, 2007, pp. 2197-2223. doi:10.1002/jps.20873 [3] A. Patel and K. Mequanint, “Novel Physically Cros- slinked Polyurethane-block-Poly(Vinyl Pyrrolidone) Hy- drogel Biomaterials,” Macromolecular Bioscience, Vol. 7, No. 5, 2007, pp. 727-737. doi:10.1002/mabi.200600272 [4] J. Thomas, A. Lowman, M. Marcolongo, “Novel Associ- ated Hydrogels for Nucleus Pulposus Replacement,” Journal of Biomedical Materials Research, Vol. 67A, No. 4, 2003, pp. 1329-1337. doi:10.1002/jbm.a.10119 [5] F. J.-K. Suh and H. W. T. Matthew, “Application of Chi- tosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review,” Biomaterials, Vol. 21, No. 24, 2000, pp. 2589-2598. doi:10.1016/S0142-9612(00)00126-5 [6] J. Rosiak, J. Olejniczak, W. Pekala, “Fast Reaction of Irradiated Polymers - I. Crosslinking and Degradation of Polyvinylpyrrolidone,” Radiation Physics and Chemistry, Vol. 36, No. 6, 1990, pp. 747-755. [7] Z. Ajji, I. Othman and J. M. Rosiak, “Production of Hy- drogel Wound Dressings Using Gamma Radiation,” Nu- clear Instruments & Methods in Physics Research, Sec- tion B: Beam Interactions with Materials and Atoms, Vol. 229, No. 3-4, 2005, pp. 375-380. doi:10.1016/j.nimb.2004.12.135 [8] Y. Qiu and K. Park, “Environment-Sensitive Hydrogels for Drug Delivery,” Advanced Drug Delivery Reviews, Vol. 53, 2001, pp. 321-339. doi:10.1016/S0169-409X(01)00203-4 [9] C.-C. Lin and A. T. Metters, “Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling,” Advanced Drug Delivery Reviews, Vol. 58, No. 12-13, 2006, pp. 1379-1408. doi:10.1016/j.addr.2006.09.004 [10] G. U. Ostrovidova, A. V. Makeev and M. M. Shamtsian, “Polyfunctional Film Coatings for Medical Use,” Materi- als Science and Engineering C, Vol. 23, 2003, pp. 545-550. [11] C. S. Satish, K. P. Satish and H. G. Shivakumar, “Hy- drogels as Controlled Drug Delivery Systems: Synthesis, Crosslinking, Water and Drug Transport Mechanism,” Indian Journal of Pharmaceutical Science, Vol. 68, No. 2, 2006, pp. 133-140. doi:10.4103/0250-474X.25706 [12] S.A. Dergunov, I. K. Nam, G. A. Mun, Z. S. Nurkeeva, E. M. Shaikhutdinov, “Radiation Synthesis and Characteri- zation of Stimuli-Sensitive Chitosan polyvinylpyrrolidone Hydrogels,” Radiation Physics and Chemistry, Vol. 72, No. 5, 2005, pp. 619-623. doi:10.1016/j.radphyschem.2004.03.011 [13] N. A. Peppas, Y. Huang, M. Torres-Lugo, J. H. Ward, J. Zhang, “Physicochemical Foundations and Structural De- sign of Hydrogels in Medicine and Biology,” Annual Re- view of Biomedical Engineering, Vol. 2, 2000, pp. 9-29. doi:10.1146/annurev.bioeng.2.1.9 [14] N. A. Peppas, Y. Huang, M. Torres-Lugo, J. H. Ward, J. Zhang, “Physicochemical Foundations and Structural De- sign of Hydrogels in Medicine and Biology,” Annual Re- view of Biomedical Engineering, Vol. 2, 2000, pp. 9-29. doi:10.1146/annurev.bioeng.2.1.9 [15] S. O. Rogero, S. M. Malmonge, A. B. Lugao, T. I. Ikeda, L. Miyamaru, A. S. Cruz, “Biocompatibility Study of Polymeric Biomaterials,” Artificial Organs, Vol. 27, No. 5, 2003, pp. 424-427. Copyright © 2011 SciRes. MSA  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1067 doi:10.1046/j.1525-1594.2003.07249.x [16] B. V. Robinson, “PVP: A Critical Review of the Kinetics and Toxicology of Polyvinylprrolidone (Povidone),” Lewis Publishers, Chelsea MI., 1990, p. 232. [17] A. J. C. Brant, “Preparação e Caracterização de Hidrogéis a Partir de misturas de Soluções de Quitosana e Po- li(N-vinil-2-pirrolidona),” Ph.D. dissertation, Chemistry Institute, University of São Paulo, Brazil, 2008. [18] D. L. A. De Faria, H. A. C. Gil, A. A. A. De Queiroz, “The Interaction between Polyvinylpyrrolidone and I2 as Probed by Raman Spectroscopy,” Journal of Molecular Structure, Vol. 478, No. 1-3, 1999, pp. 93-98. doi:10.1016/S0022-2860(98)00755-8 [19] K. Raghunath Rao, K. Panduranga, B. Nagarajan, K. Tho- mas Joseph, “Grafting of Poly (vinylpyrrolidinone) onto Gelatin and Its Application as Synthetic Plasma Ex- pander,” European Polymer Journal, Vol. 21, No. 2, 1985, pp. 195-199. doi:10.1016/0014-3057(85)90053-9 [20] Y. Jiao, Z. Liu, S. Ding, L. Li, C. Zhou, “Preparation of Biodegradable Crosslinking Agents and Application in PVP Hydrogel,” Journal of Applied Polymer Science, Vol. 101, No. 3, 2006, pp. 1515-1521. doi:10.1002/app.22291 [21] J. M. Rosiak, “Radiation Formation of Hydrogels for Drug Delivery,” Journal of Controlled Release, Vol. 31, 1994, pp. 9-19. doi:10.1016/0168-3659(94)90246-1 [22] J. Thomas, A. Lowman and M. Marcolongo, “Novel As- sociated Hydrogels for Nucleus Pulposus Replacement,” Journal of Biomedical Materials Research, Vol. 67A, No. 4, 2003, pp. 1329-1337. doi:10.1002/jbm.a.10119 [23] M. T. Razzak, Zainuddin, Erizal, S. P. Dewi, H. Lely, E. Taty and Sukirno, “The Characterization of Dressing Component Materials and Radiation Formation of PVA– PVP Hydrogel,” Radiation Physics and Chemistry, Vol. 55, No. 2, 1999, pp. 153-165. doi:10.1016/S0969-806X(98)00320-X [24] H. K. Can, “Synthesis of Persulfate Containing Poly (N - vinyl - 2 - Pyrrolidone) (PVP) Hydrogels in Aqueous So- lutions by γ -Induced Radiation,” Radiation Physics and Chemistry, Vol. 72, No. 6, 2005, pp. 703-710. doi:10.1016/j.radphyschem.2004.04.028 [25] L. C. Lopérgolo, A. B. Lugão and L. H. Catalani, “Direct UV Photocrosslinking of Poly (N-Vinyl-2-Pyrrolidone) (PVP) to Produce Hydrogels,” Polymer, Vol. 44, 2003, pp. 6217-6222. doi:10.1016/S0032-3861(03)00686-4 [26] C. F. Brunius and E. Ranucci, “Grafting of x-Functionalized PVP Oligomers onto Dextran: A Novel Route to Biodegradable and Biocompatible Polymers,” Micromole Rapid Community, Vol. 22, 2001, pp. 1474- 1480. - doi:10.1002/1521-3927(20011201)22:18<1474::AID-MA RC1474>3.0.CO;2-7 [27] Y. C. Nho, K. R. Park, “Preparation and Properties of PVA/PVP Hydrogels Containing Chitosan by Radiation,” Journal of Applied Polymer Science, Vol. 85, 2002, pp. 1787-1794. doi:10.1002/app.10812 [28] M. C. Terence, S. B. Faldini, L. F. Miranda, “Estudo da Funcionalização do Poli(n-vinil-2-pirrrolidona) Com ma- leato de Dietila,” 17º CBECIMat - Congresso Brasileiro de Engenharia e Ciência dos Materiais, 15 a 19 de No- vembro de 2006, Foz do Iguaçu, Brasil. [29] S. K. Bajpai and S. S. Saggu, “Water Uptake Behavior of Poly (methacrylamide-co-N-vinyl-2-pyrrolidone- co- ita- conic acid) as pH-Sensitive Hydrogels: Part I.,” Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, Vol. 43, 2006, pp. 1135-1150. doi:10.1080/10601320600735116 [30] A. Harun, M. Basri, M. B. Ahmad and A. B. Salleh, “Enantioselective Esterification Reaction Using Immobi- lized Candida Ugosa Lipase on Poly (N-Vinyl-2-Pyrrolid- One-Co-Styrene) Hydrogel,” Journal of Applied Polymer Science, Vol. 92, No. 5, 2004, pp. 3381-3386. [31] S. K. Bajpai, Dubey, Seema, “In Vitro Dissolution Stud- ies for Release of Vitamin B12 from Poly (N-vinyl-2-py- rrolidone-co-acrylic Acid) Hydrogels,” Reactive & Func- tional Polymers, Vol. 62, No. 1, 2005, pp. 93-104. [32] C.-H. Chen, F.-Y. Wang, C.-F. Mao, C.-H. Yang, “Stud- ies of Chitosan. I. Preparation and Characterization of Chitosan/Poly(Vinyl Alcohol) Blend Films,” Journal of Applied Polymer Science, Vol. 105, No. 3, 2007, pp. 1086-1092. doi:10.1016/j.reactfunctpolym.2004.09.004 [33] J. V. Cauich-Rodriguez, S. Deb and R. Smith, “Dynamic Mechanical Characterization of Hydrogel Blends of Poly (Vinyl Alcohol-Vinyl Acetate) with Poly (acrylic acid) or Poly (Vinylpyrrolidone),” Journal of Materials Science: Materials in Medicine, Vol. 7, No. 6, 1996, pp. 349-353. doi:10.1007/BF00154547 [34] M. Şen and E. N. Avci, “Radiation Synthesis of Poly (N-vinyl-2-pyrrolidone)-kcarrageenan Hydrogels and Their Use in Wound Dressing Applications. I. Prelimi- nary Laboratory Tests,” Journal of Biomedical Materials Research, Part A, Vol. 74, 2005, pp. 187-196. [35] J. R. Khurma, D. R. Rohindra and A. V. Nand, “Swelling and Thermal Characteristics of Genipin Crosslinked Chi- tosan and Poly (Vinyl Pyrrolidone) Hydrogels,” Polymer Bulletin, Vol. 54, No. 3, 2005, pp. 195-204. doi:10.1007/s00289-005-0375-4 [36] S. Şenel, M. J. Kremer, S. Kas, P. W. Wertz, A. A. Hincal, C. A. Squier, “Enhancing Effect of Chitosan on Peptide Drug Delivery across Buccal Mucosa,” Biomaterials, Vol. 21, No. 20, 2000, pp. 2067-2071. [37] B. Garcia, R. P. da Silva, D. L. Costa, M. Raffin, R. N. M. Nervo da Silva, “Evaluation of Gels Obtained from Ace- tylation of Chitosan in a Heterogeneous Medium,” Química Nova, Vol. 31, No. 3, 2008, pp. 486-492. [38] S. P. Campana-Filho, D. de Britto, E. Curti, F. R. Abreu, M. B. Cardoso, M. V. Battisti, P. C. Sim, R. C. Goy, R. Signini, R. L. Lavall, “Extraction, Structure and Proper- ties of α- and β- chitin,” Química Nova, Vol. 30, No. 3, 2007, pp. 644-650. [39] R. N. Tharanathan and F. S. Kittur, “Chitin—the Undis- Copyright © 2011 SciRes. MSA  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) 1068 puted Biomolecule of Great Potential,” Critical Reviews in Food Science and Nutrition, Vol. 43, 2003, pp. 61-87. doi:10.1080/10408690390826455 [40] P. R. Marreco, P. da Luz Moreira, S. C. Genari, A. M. Moraes, “Effects of Different Sterilization Methods on the Morphology, Mechanical Properties, and Cytotoxicity of Chitosan Membranes Used as Wound Dressings,” Journal of Biomedical Materials Research, Part B: Ap- plied Biomaterials, Vol. 71B, No. 2, 2004, pp. 268-277. doi:10.1002/jbm.b.30081 [41] T. Minagawa, Y. Okamura, Y. Shigemasa, S. Minami and Y. Okamoto, “Effects of Molecular Weight and Deacety- lation Degree of Chitin/Chitosan on Wound Healing,” Carbohydrate Polymers, Vol. 67, No. 4, 2007, pp. 640-644. [42] S. C. Tan, E. Khor, T. K. Tan, S. M. Wong, “The Degree of Deacetylation of Chitosan: Advocating the First De- rivative UV-spectrophotometry Method of Determina- tion,” Talanta, Vol. 45, No. 4, 1998, pp. 713-719. doi:10.1016/S0039-9140(97)00288-9 [43] T. A. Khan, K. K. Peh, H. S. Ching, “Reporting Degree of Deacetylation Values of Chitosan: The Influence of Analytical Methods,” Journal of Pharmacy & Pharma- ceutical Sciences, Vol. 5, 2002, pp. 205-212. [44] Y. Shigemasa and S. Minami, “Applications of Chitin and Chitosan for Biomaterials,” Biotechnology & Genetic En- gineering Reviews, Vol. 13, 1996, p. 383 [45] G. Crini and P. M. Badot, “Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature,” Progress in Po- lymer Science, Vol. 33, No. 4, 2008, pp. 399-447. doi:10.1016/j.progpolymsci.2007.11.001 [46] R. H. Chen and H. D. Hwa, “Effect of Molecular Weight of cChitosan with the Same Degree of Deacetylation on the Thermal, Mechanical, and Permeability Properties of the Prepared Membrane,” Carbohydrate Polymers, Vol. 29, No. 4, 1996, pp. 353-358. doi:10.1016/S0144-8617(96)00007-0 [47] R. Xing, S. Liu, Z. Guo, H. Yu, P. Wang, C. Li, Z. Li, P. Li, “Relevance of Molecular Weight of Chitosan and its Derivatives and Their Antioxidant Activities in Vitro,” Bioorganic & Medicinal Chemistry, Vol. 13, 2005, pp. 1573-1577. doi:10.1016/j.bmc.2004.12.022 [48] S. Zivanovic, C. C. Basurto, S. Chi, P. M. Davidson, J. Weiss, “Molecular Weight of Chitosan Influences An- timicrobial Activity in Oil-in-water Emulsions,” Journal of Food Protection, Vol. 67, No. 5, 2004, pp. 952-959. [49] M. Mucha, S. Ludwiczak, M. Kawinska, “Kinetics of Water Sorption by Chitosan and Its Blends with Poly (vinyl alcohol),” Carbohydrate Polymers, Vol. 62, No. 1, 2005, pp. 42-49. doi:10.1016/j.carbpol.2005.07.008 [50] J. Z. Knaul, M. R. Kasaai, V. T. Bui, K. A. M. Creber, “Characterization of Deacetylated Chitosan and Chitosan Molecular Weight Review,” Canadian Journal of Chem- istry, Vol. 76, 1998, pp. 1699-1706. [51] J. K. Hwang and H. H. Shin, “Rheological Properties of Chitosan Solutions,” Korea-Australia Rheology Journal, Vol. 12, No. 3-4, 2000, pp. 175-179. [52] K. M. N. C. Canella and R. B. Garcia, “Caracterização de Quitosana por Cromatografia de Permeação em Gel-Influê- ncia do Método de Preparação e do Solvente,” Química Nova, Vol. 24, 2001, pp. 13-17. [53] G. Bertha, H. Dautzenberg and M. G. Petera, “Physico- chemical Characterization of Chitosans Varying in De- gree of Acetylation,” Carbohydrate Polymers, Vol. 36, 1998, pp. 205-216. doi:10.1016/S0144-8617(98)00029-0 [54] R. A. A. Muzzarelli, C. Lovch and M. Emanuelli, “The Molecular Weight of Chitosans Studied by Laser Light- scattering,” Carbohydrate Research, Vol. 164, 1987, pp. 433-442. [55] A. C. Wu, M. Bough, A. Wayne, E. C. Conrad, K. E. Jr. Alden, “Determination of Molecular - Weight Distribu- tion of Chitosan by High-Performance Liquid Chroma- tography,” Journal of Chromatography, Vol. 128, No. 1, 1976, pp. enk 87-99. [56] E. B. Denkbaş and R. M. Ottenbrite, “Perspectives on: Chitosan Drug Delivery Systems Based on Their Geome- tries,” Journal of Bioactive and Compatible Polymers, Vol. 21, No. 4, 2006, pp. 351-368. doi:10.1177/0883911506066930 [57] C. G. L. Khoo, S. Frantzich, A. Rosinski, M. Sjoestrom, J. Hoogstraate, “Oral Gingival Delivery Systems from Chi- tosan Blends with Hydrophilic Polymers,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 55, No. 1, 2003, pp. 47-56. doi:10.1016/S0939-6411(02)00155-8 [58] P. Mallika, A. Himabindu and D. Shailaja, “Modification of Chitosan towards a Biomaterial with Improved Phys- icochemical Properties,” Journal of Applied Polymer Sci- ence, Vol. 101, No. 1, 2006, pp. 63-69. doi:10.1002/app.22893 [59] S. Hirano, M. Zhang, B. G. Chung and S. K. Kim, “The N-acylation of Chitosan Fibre and the N-deacetylation of Chitin Fibre and Chitin-Cellulose Blended Fibre at a Sol- id State,” Carbohydrate Polymer, Vol. 41, No. 2, 2000, pp. 175-179. doi:10.1016/S0144-8617(99)00081-8 [60] H. Sashiwa, Y. Shigemasa and R. Roy, “Homogeneous N, O-acylation of Chitosan in Dimethyl Sulfoxide with Cy- clic Acid Anhydrides,” Chemistry Letters, Vol. 29, No. 10, 2000, pp. 1186-1187. doi:10.1246/cl.2000.1186 [61] K. Kurita, H. Ikeda, M. Shimojoh and J. Yang, “N-Phthaloylated Chitosan as an Essential Precursor for Controlled Chemical Modifications of Chitosan: Synthe- sis and Evaluation,” Polymer Journal, Vol. 39, 2007, pp. 945-952. doi:10.1295/polymj.PJ2007032 [62] J. Yin, K. Luo, X. Chen and V. V. Khutoryanskiy, “Mis- cibility Studies of the Blends of Chitosan its Some Cellu- lose Ethers,” Carbohydrate Polymers, Vol. 63, No. 2, 2006, pp. 238-244. doi:10.1016/j.carbpol.2005.08.041 [63] M. Coleman, J. Graf and P. Painter, “Specific Interactions and the Miscibility in Polymer Blends,” Technomic, Copyright © 2011 SciRes. MSA  Hydrogels from Chitosan and a Novel Copolymer Poly(N-Vinyl-2-Pyrrolidone-Co-Acrolein) Copyright © 2011 SciRes. MSA 1069 Lancaster, PA, USA, 1991. [64] M. M. Feldstein, A. Roos, C. Chevallier, C. Creton and E. E. Dormidontova, “Relation of Glass Transition Tem- perature to The Hydrogen Bonding Degree and Energy in Poly(N-Vinyl Pyrrolidone) Blends with Hydroxyl-Conta- ining Plasticizers: 3, Analysis of Two Glass Transition Temperatures Featured for PVP Solutions in Liquid Poly(Ethylene Glycol),” Polymer, Vol. 44, 2003, 1819- 1834. [65] M. T. Qurashi, H. S. Blair and S. J. Alien, “Studies on Modified Chitosan Membranes. II. Dialysis of Low Mo- lecular Weight Metabolites,” Journal of Applied Polymer Science, Vol. 46, No. 2, 1992, pp. 263-269. doi:10.1002/app.1992.070460207 [66] L. Fang and S. H. Goh, “Miscible Chitosan/Tertiary Am- ide Polymer Blends,” Journal of Applied Polymer Science, Vol. 76, 2000, pp. 1785-1790. [67] C. G. T. Neto, J. A. Giacometti, A. E. Job, F. C. Ferreira, J. L. C. Fonseca and M. R. Pereira, “Thermal, Analysis of Chitosan Based Networks,” Carbohydrate Polymers, Vol. 62, No. 2, 2005, pp. 97-103. doi:10.1016/j.carbpol.2005.02.022 [68] J. A. G. Barros, “Reticulação da Poli(N-vinil-2-pirrolido- na) e Copolímeros por Processos Químicos,” Ph.D. dis- sertation, Chemistry Institute, University of São Paulo, São Paulo, 2007. doi:10.1002/macp.1985.021860815 [69] J. G. Domszy and G. A. F. Roberts, “Evaluation of Infra- redspectroscopic Techniques for Analyzing Chitosan,” Makromol Chemie, Vol. 186, 1985, pp. 1671-1677. doi:10.1002/macp.1985.021860815 [70] M. Lavertu, Z. Xia, A. N. Serregi, M. Berrada, A. Rodr- gues, D. Wang, M. D. Buschmann and A. Gupta, “A Va- lidated 1H NMR Method for the Determination of the Degree of Deacetylation of Chitosan,” Journal of Phar- maceutical and Biomedical Analysis, Vol. 32, 2003, pp. 1149-1158. doi:10.1016/S0731-7085(03)00155-9 [71] T. A. Khan, K. K. Peh and H. S. Ching, “Reporting De- gree of Deacetilation Values of Chitosan: The Influence of Analytical Methods,” Jounal of Pharmaceutical Sci- ence, Vol. 5, No. 3, 2002, pp. 205-212. [72] T. Sannan, K. Kurita, K. Ogura and Y. Iwakura, “Studies on Chitin: 7. IR Spectroscopic Determination of Degree of Deacetylation,” Polymer, Vol. 19, 1978, pp. 458-459. [73] S. Sabnis and L. H. Block, “Improved Infrared Spectro- scopic Method of Analysis of Degree of N-Deacetylation of Chitosan,” Polymer Bulletin, Vol. 39, 1997, pp. 67-71. doi:10.1016/0032-3861(78)90256-2 [74] H. S, Blair, J. G. Guthrie, T. K. Law and P. Turkington, “Chitosan and Modified Chitosan Membranes I. Prepara- tion and Characterisation,” Journal of Applied Polymer Science, Vol. 35, 1987, pp. 641-656. doi:10.1002/app.1987.070330226 [75] K. Sweidan, A.-M. Jaber, N. Al-jbour, R. Obaidat, M. Al-Remawi and A. Badwan, “Further Investigation on the Degree of Deacetylation of Chitosan Determined by Po- tentiometric Titration,”Journal of Excipients and Food Chemicals, Vol. 2, No. 1, 2011, pp. 16-25. [76] A. Hirai, H. Odani and A. Nakajima, “Determination of Degree of Deacetylation of Chitosan by 1H NMR Spec- troscopy,” Polymer Bulletin, Vol. 26, No. 1, 1991, pp. 87-94. doi:10.1007/BF00299352 [77] K. Sakurai, T. Maegawa and T. Takahashi, “Glass Transi- tion Temperature of Chitosan and Miscibility of Chito- san/Poly (N-vinyl pyrrolidone) Blends,” Polymer, Vol. 41, No. 19, 2000, pp. 7051-7056. doi:10.1016/S0032-3861(00)00067-7 [78] M. F. Zeng, X. D. Sun, Y. Wang, et al., “Correlations between the Free-Volume Properties and the Miscibility of Chitosan/Polar Polymers Blend Membranes,” Radia- tion Physics and Chemistry, Vol. 77, 2008, pp. 1062- 1068. doi:10.1016/j.radphyschem.2008.01.008 [79] L. Zhao, H. Mitomo, N. Nagasawa, F. Yoshii and T. Kume, “Radiation Synthesis and Characteristic of the Hydrogels Based on Carboxymethylated Chitin Deriva- tives,” Carbohydrate Polymers, Vol. 51, 2003, pp. 169-175. doi:10.1016/S0144-8617(02)00210-2 [80] W. G. Malette, H. J. Quigley, R. D. Gaines, D. Johnson and W. G. Rainer, “Chitosan: A New Hemosta tic,” An- nals of Thoracic Surgery, Vol. 36, 1983, pp. 55-58. doi:10.1016/S0003-4975(10)60649-2 [81] A. A. Craveiro, A. C. Craveiro and D. C. Queiroz, “Qui- tosana—A Fibra do Futuro,” Padetec, 1999, p.124. [82] F. Shahidi, J. K. V. Arachchi and Y. J. Jeon, “Food Ap- plications of Chitin and Chitosans,” Trends in Food Sci- ence & Technology, Vol. 10, 1999, pp. 37-51. doi:10.1016/S0924-2244(99)00017-5 |

