Paper Menu >>
Journal Menu >>
 Journal of Software Engineering and Applications, 2011, 4, 391-395 doi:10.4236/jsea.2011.47045 Published Online July 2011 (http://www.SciRP.org/journal/jsea) Copyright © 2011 SciRes. JSEA 391 Parkinson’s Disease Recognition Using Artificial Immune System* Badra Khellat Kihel, Mohamed Benyettou Laboratoire de Modélisation et d’Optimisation des Systèmes Industriels LAMOSI, Université des Sciences et de la Technologie d’Oran, Oran, Algeria. Email: {khellat_badra, med_benyettou}@yahoo.fr Received January 31st, 2011; revised March 25th, 2011; accepted April 20th, 2011. ABSTRACT This work deals the application of the artificial immune system to discrimina te between healthy and people with Park- inson’s disease (PWP). As the symptoms of Parkinson’s disease (PD) occur gradually and mostly targetin g the elderly people for whom physical visits to the clinic are inconvenient and costly, telemonitoring of the disease using measure- ments of dysphonia (vocal features ) has a vital role in its early diagnosis. Taking inspiration from natural immune sys- tems, we try to grab useful properties such as automatic recognition, memorization and adaptation. The developed al- gorithms have as a base the algorithm of training bio inspired CLONCLAS. The results obtained are satisfactory and show a great reliability o f the approach. Keywords: Parkinson’s Disease, Dysphonia Measures, Speech Analysis, Immune System, Clonal Selection Algorithm 1. Introduction Neurological disorders, including Parkinsons disease (PD), Alzheimers and epilepsy, affect profoundly the lives of patients and their families. Parkinsons disease affects over one million people in North America alone [1]. Moreover, an aging population means this number is expected to rise as studies suggest rapidly increasing prevalence rates after the age of 60 [1]. In addition to increased social isolation, the financial burden of PD is significant and is estimated to rise in the future [2]. Cur- rently there is no cure, although medication is available offering significant alleviation of sy mptoms , es peci ally at the early stages of the disease [3]. The goal of this study is to develop an application that identify persons having Parkinson’s disease using bio-in- spired approach : artificial immune system (AIS). 2. The Artificial Immune System An artificial immune system (AIS) is a category of algo- rithm inspired by the principles and the operations of the natural immune system (NIS) of vertebrate. [4] The artificial immune system uses three basic algo- rithms: negative selection clonale selection immune network In this study, we apply the clonal selection algorithm. The Natural Clonal Selection As mentioned in (Figure 1), when a new antigen pene- trates in the body, the immunizing answer passes by the following stages (principles of the clonal selection) [5]: At the beginning, the concentration of the antigen is so weak that only innate immunity is activated. As the antigen is new so, no B cell is enough specific to bind with; As the antigen develops, its concentration becomes enough high to activate the least specific cells B; Once B cells activated, th ey will multip ly to p roduce a great number of clones. Each clone is a B cell ide- ntical to the cell which produces it. The number of clones is proportional to the affinity of the connec- tion B cell-antigen; To increase the specificity of the antibodies and the effectiveness of the immunizing answer; the clones enter a phase of hyper changes, thus modifying the structure of their receivers (antibody). As the changes are random, the cells obtained (known as mature) can become more specific or less specific; When the concentration of the antigen decreases (bec- * This work was supported by LAMOSI laboratory. 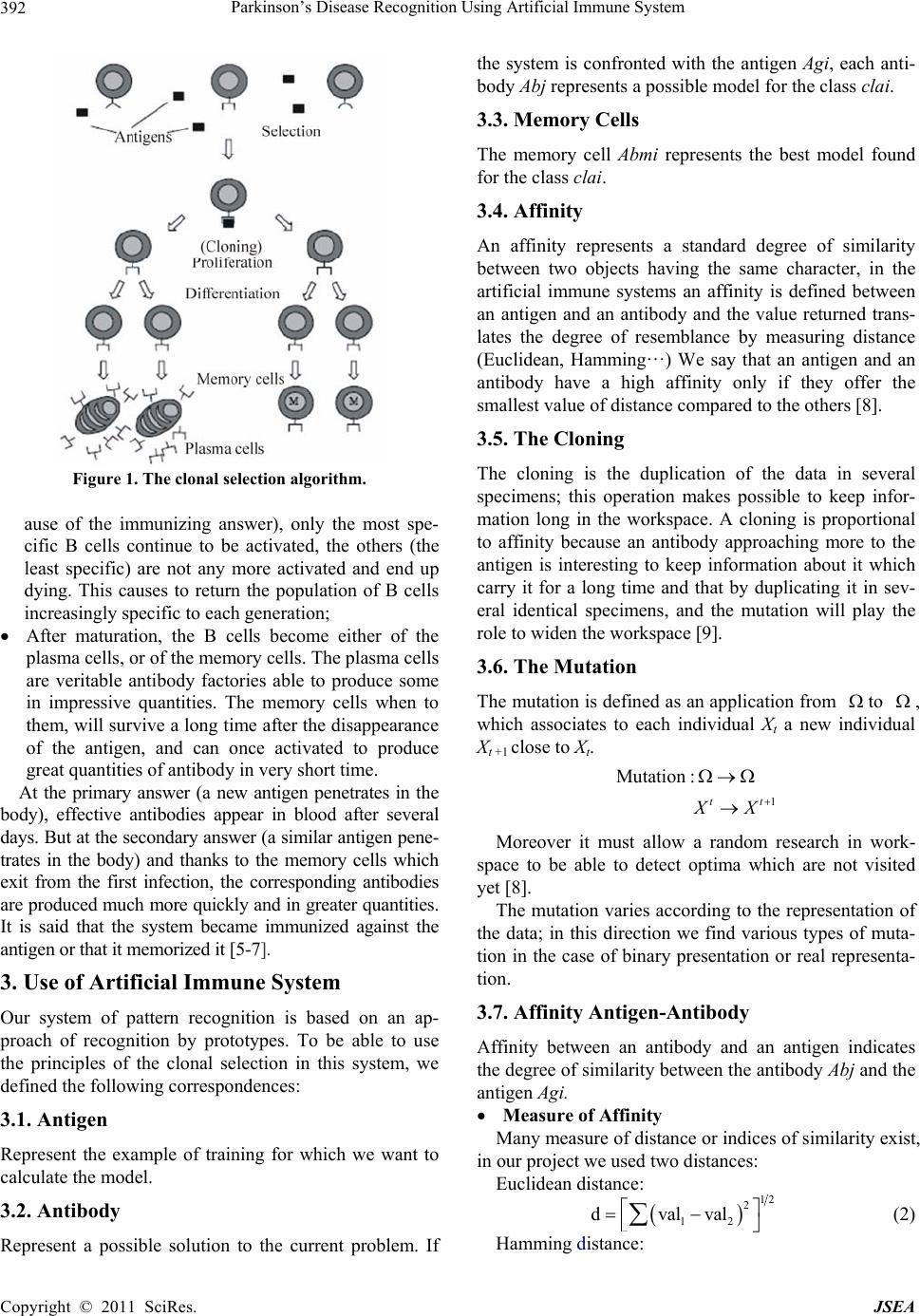 Parkinson’s Disease Recognition Using Artificial Immune System 392 Figure 1. The clonal selection algorithm. ause of the immunizing answer), only the most spe- cific B cells continue to be activated, the others (the least specific) are not any more activated and end up dying. This causes to return the population of B cells increasingly specific to each generation; After maturation, the B cells become either of the plasma cells, or of the memory cells. The plasma cells are veritable antibody factories able to produce some in impressive quantities. The memory cells when to them, will survive a long time after the disappearance of the antigen, and can once activated to produce great quantities of antibody in very short time. At the primary answer (a new antigen penetrates in the body), effective antibodies appear in blood after several days. But at the secondary answer (a similar antigen pene- trates in the body) and thanks to the memory cells which exit from the first infection, the corresponding antibodies are produced much more quickly and in greater quantities. It is said that the system became immunized against the antigen or that it memorized it [5-7]. 3. Use of Artificial Immune System Our system of pattern recognition is based on an ap- proach of recognition by prototypes. To be able to use the principles of the clonal selection in this system, we defined the following correspondences: 3.1. Antigen Represent the example of training for which we want to calculate the model. 3.2. Antibody Represent a possible solution to the current problem. If the system is confronted with the antigen Agi, each anti- body Abj represents a possible model for the class clai. 3.3. Memory Cells The memory cell Abmi represents the best model found for the class clai. 3.4. Affinity An affinity represents a standard degree of similarity between two objects having the same character, in the artificial immune systems an affinity is defined between an antigen and an antibody and the value returned trans- lates the degree of resemblance by measuring distance (Euclidean, Hamming…) We say that an antigen and an antibody have a high affinity only if they offer the smallest value of distance compared to the others [8]. 3.5. The Cloning The cloning is the duplication of the data in several specimens; this operation makes possible to keep infor- mation long in the workspace. A cloning is proportional to affinity because an antibody approaching more to the antigen is interesting to keep information about it which carry it for a long time and that by duplicating it in sev- eral identical specimens, and the mutation will play the role to widen the workspace [9]. 3.6. The Mutation The mutation is defined as an application from to , which associates to each individual Xt a new individual Xt +1 close to Xt. 1 Mutation: tt X X Moreover it must allow a random research in work- space to be able to detect optima which are not visited yet [8]. The mutation varies according to the representation of the data; in this direction we find various types of muta- tion in the case of binary presentation or real representa- tion. 3.7. Affinity Antigen-Antibody Affinity between an antibody and an antigen indicates the degree of similarity be tween the antib ody Abj and the antigen Agi. Measure of Af f ini ty Many measure of distance or indices of similarity exist, in our project we used two distances: Euclidean distance: 12 2 12 dvalval (2) Hamming distance: Copyright © 2011 SciRes. JSEA  Parkinson’s Disease Recognition Using Artificial Immune System Copyright © 2011 SciRes. JSEA 393 1 dvalva 2 l (3) Affinity = –d (4) Number of clone Formulate We will calculate the number of clones in our algo- rithm of CLONCLAS using: 22 round B*affiniteaffinite ij jj We applied a range of dysphonia measures which have been successfully used in similar problems aimed at separating healthy controls and PWP [1]. We used the classical dysphonia measures (Table 1), which include quantifying fundamental frequency perturbations (jitter), amplitude perturbations (shimmer), and signal to noise ratios (harmonics to noise ratio). We used the “MDVP” prefix to associate the measures which are equivalent to the results of the Kay Pentax Multi-Dimensional Voice Program. All measures are summarized in Table 1. [3] number ofclones (5) where B is the cloning parameter. 6. Results and Discussion 4. Clonclas Train At the end of the training we have a population of mem- ory cells. This population will be used to classify the unknown forms, for that we used several algorithms of classification: Our algorithm uses the principles of the artificial clonal selection to generate memory cells in the training step (Figure 2): 5. Methods Classification by Measuring Affinity Parkinson’s Dataset For the first strategy we chose to classify the unknown forms by using a measurement of distance (Euclidean or distance of Ham ming). The dataset was created at the University of Oxford, in collaboration with the National Centre for Voice and Speech, Denver, Colorado [1] and has been made avai- lable online very recently, in June 2008. [1,4]. The principle of this classification is given as follows: In entry we have the memory cells obtained by the training (Abm) and a form to classify F; The data explored in this paper was obtained from the Oxford Parkinson's Disease Detection Dataset, composed of a range of biomedical voice measurements from 31 male and female subjects, 23 were clinically diagnosed with PD [1]. Each subject provided an average of six phonations of the vo wel /a/ (yieldin g 192 samples in total) [2]. The main aim of processing the data is to discriminate healthy people from those with PD, according to the “status” attribute which is set to non-PD for healthy and PD for people with Parkinson’s disease, which is a two-decision classi ficat i on pr o bl em [6,7]. For any memory cell Abmj from Abm , to calculate affinity Aff between Abmj and F: To find the memory cell Abmj such as AffJ is largest; To assign the new form to the same class of Abmj. Results are in (Table 2). For improving the results obtained we standardized the data by limiting them in the interval [0-1]. We will obtain (Table 3). The evaluation of any system of recognition is to de- terminate the rate of recognition which represents the probability with which the system can identify if a person has or not Parkinson’s disease. The vocal disturbances are caused for roughly 90% of patients suffering from the Parkinson’s disease (PD). Consequently, telediagnostic of PD by using measure- ments of dysphonia will relieve the clinical monitoring of old people and will increase the chances of its diagnosis early. The aim of this work is to extract clin ica lly usefu l infor- mation fro m the sustained v owel pho nations , the resul ts of the dysphonia measures for each phonation forma feature vector which is then used as input in a regression setting. 7. Comparative Study We have compared our results with other studies as in (Table 4). According to the results of these methods, we remark that the rates of recognition are better in the AIS approach. 8. Conclusions The experiments established in our study enable us to extract several characteristics from the artificial immune systems. We could also remark that with this new me- Figure 2. Clonclas train.  Parkinson’s Disease Recognition Using Artificial Immune System 394 Table 1. Description ofvocales mesurement used. DESRIPTION ATTRIBUT MIN MAX MOY Averege vocal fundamental freq MDVP: Fo(Hz) 88.33 260.11 154.23 Max vocal fundamental freq MDVP: Fhi(Hz) 102.15 592.03 197.11 Min vocal fundamental freq frequency MDVP: Flo(Hz) 65.48 239.17 116.33 Several measures of variation in fundamental frequency MDVP: Jitter(%) 0.002 0.033 0.006 MDVP: Jitter(Abs) 7E-06 26E-05 4.4E-05 MDVP: RAP 0.001 0.021 0.003 MDVP: PPQ 0.001 0.020 0.003 Jitter: DDP 0.002 0.064 0.010 Several measures of variation in amplitude MDVP: Shimmer 0.01 0.119 0.03 MDVP: Shimmer(dB)0.085 1.302 0.282 Shimmer: APQ3 0.005 0.056 0.016 Shimmer: APQ5 0.006 0.079 0.018 MDVP: APQ 0.007 0.138 0.024 Shimmer: DDA 0.014 0.169 0.047 Two measures of ratio of noise to tonal components in the voice NHR 0.001 0.315 0.025 HNR 8.441 33.047 21.886 Two nonlinear dynamical complexity measures RPDE 0.257 0.685 0.499 D2 1.423 3.671 2.328 Signal fractal scaling exponent DFA 0.574 0.825 0.718 Three nonlinear measures of fundamental frequency variation Spread1 -7.965 -2.434 -5.684 Spread2 0.006 0.450 0.227 PPE 0.045 0.527 0.207 Table 2. Results of clonclas algorithm. ALGORITHME AFFINITY Train Test Clonclas euclidean 100% 88.54% Clonclas hamming 100% 87.50% Table 3. Results of clonclas algorithm after standardization. Affinity Train Test pd npd euclidean 100% 92.70% 94.44% 87.50% hamming 100% 90.63% 91.67% 87.50% Table 4. Results for differente techniques. Techniques Train Test ClonClas 100% 92.70% Leaveoneindividual-out [4] - 81.53% Bootstrap resampling [1] - 91.40% Leaveoneindividual-out [1] - 65.13% PNN-IS [6] 81.73% 79.78% PNN-MCS [6] 81.48% 80.92% PNN-HS [6] 81.74% 81.28% PNN: Probabilistic Neural Network; IS: Incremental Search. MCS: Monte Carlo Search; HS: Hybrid search. thod we will save much time in the phase of training (compared to the networks of neurons, etc.) and we no- tice as well as the process of training inspired of the bio- logical phenomenon allows thanks to the phenomenon vaccination to learn and memorize only by the use of a population of train of a small size (optimization of the base of train). The results obtained were very encouraging and opens the doors of research towards the hybridization of the immune system algorithms with other approach such as Neural Network or genet i c algori t hm. REFERENCES [1] M. A. Little, et al., “Suitability of Dysphonia Measur- ments for Telemonitoring of Parkinson Disease,” IEEE Transactions on Biomedical Engineering, Vol. 56, No. 4, 2009, pp. 1015-1022. [2] K. Revett, et al., “Feature Selection in Parkinson’s Dis- ease: A Rough Sets Approach,” Proceedings of the Inter- national Multiconference on Computer Science and In- formation Technology, Mragowo, 12-14 October 2009, pp. 425-428. [3] N. Singh, V. Pillay and Y. E. Choonara, “Advances in the Treatment of Parkinson’s Disease,” Progress in Neurobi- ology, Vol. 81, No. 1, 2007, pp. 29-44. doi:10.1016/j.pneurobio.2006.11.009 [4] J. A. White and S. M. Garrett, “Improved Pattern Recog- nition with Artificial Clonal Selection?” Proceedings of the 2nd International Conference on Artificial Immune Systems, Edinburgh, 1-3 September 2003, pp. 181-193. [5] A. H. Deneche, “Approche bio Inspirées Pour la Reco- nnaissance de Formes,” Thèse de Magistère, Université Mentouri de Constantine, Algeria, 2006. [6] A. Tsanas, et al., “Enhanced Classical Dysphonia Meas- ures and Sparse Regression for Telemonitoring of Park- inson’s Disease Progression,” 2011. http://people.maths.ox.ac.uk/tsanas/Preprints/ICASSP201 0.pdf [7] C. O. Sakar and O. Kursun, “Telediagnosis of Parkinson’s Copyright © 2011 SciRes. JSEA  Parkinson’s Disease Recognition Using Artificial Immune System395 Disease Using Measurements of Dysphonia,” Journal of Medical Systems, Vol. 34, No. 4, 2009, pp. 591-599. [8] B. K. Kihel and M. Benyettou, “Identification Biomé- trique des Individus par Leurs Empreintes Digitales par l’AIS,” The 2009 IEEE International Symposium on Parallel and Distributed Proceeding with Applications, Chengdu and Jiuzhai Valley, 9-11 August 2009, p. 3. [9] N. Neggaz and A. Benyettou, “Les Algorithmes Evolu- tionnaires Appliqués à la Classification Phonétique,” Mémoire de Projet de Fin d’Etudes, Université des Sciences et de la Technologie d’Oran, 2004. Copyright © 2011 SciRes. JSEA |

