 Pharmacology & Pharmacy, 2011, 2, 212-225 doi:10.4236/pp.2011.23030 Published Online July 2011 (http://www.scirp.org/journal/pp) Copyright © 2011 SciRes. PP Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Renu Chadha1*, Sushma Gupta1, Natasha Pathak1, Geeta Shukla2, Dharamvir Singh Jain3, Raghuvir R. S. Pissurlenkar4, Evans C. Coutinho4 1University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India; 2Department of Microbiology, Panjab Uni- versity, Chandigarh, India; 3Department of Chemistry, Panjab University, Chandigarh, India; 4Department of Pharmaceutical Chem- istry, Bombay College of Pharmacy, Mumbai, India. Email: renukchadha@rediffmail.com Received April 10th, 2010; revised May 30th, 2011; accepted June 22nd, 2010. ABSTRACT The purpose of the present work is to improve the antimalarial activity of arteether through enhancing its solubility subsequently bioavailability by incorporating the drug into the cyclodextrins cavity. The effect of hydrophilic polyvinyl propylene (PVP) polymer on the complexation and solubilizing efficiencies of cyclodextrins (CDs ) is also elucidated. Inclusion of arteether molecule in solid state was evidenced by Differential scanning calorimeter (DSC), Powder X-ray diffractometery (PXRD), and in solution state by NMR and solution calorimetry. A 1:1 stoichiometry was proposed by the phase solubility studies both in presence and absence of PVP. The most plausibe mode of inclusion of arteether into the CD cavity is revealed by molecular modeling studies utalizing Fast Rigid Exhaustive Docking acronym. Solution calorimetry was used further to confirm 1:1 stiochiometry in presence or absence of PVP by determining the enthalpy of interaction between the drug and cyclodextrins. The inclusion of drug was found to be exothermic process accompa- nied by small positive value of entropy (ΔS˚). The methylated-β-CD showed the best ability to solublize arteether which is approximately at par with β-CD in the presence of PVP. Better complexation efficiency of β-CD in presence of PVP is also reflected by the higher numerical values of stability constant (K). Compelete eradication of the parasite from the blood and highest anti-malarial pharmacological activity was observed in the complexes of arteether with M- -CD while 83.7% was observed for ternary complexes of -CD in presence of PVP. Significance of Work: The encapsulation of the arteether by CDs has resulted in improvement of solubility, dissolu- tion and consequently the bioavailability leading to enhanced antimalarial activity of this poorly soluble antimalarial drug Keywords: Arteether, Cyclodextrins, Ternary Complexes, Complexation Efficiency, In Vivo Studies 1. Introduction Arteether, a semi synthetic derivative of artemisinine, is active constituent of the plant, Artemisia annua [1]. It is a blood scihozonticide and active against all stages of Plasmodium falciparum. The drug is also used for the treatment of cerebral malaria as well as for the chloro- quine resistant cases [2-5]. Unfortunately, it is water- insoluble (17 µg/ml at room temperature) [6] and the formulation causes difficulties to the biopharmaceutical scientist. Moreover, the therapeutic efficacy is greatly hampered due to its poor bioavailability as ~ 40% of the drug degrades in the stomach [7-8]. Unless a drug is de- livered to its target area at a rate and concentration that minimize side effects and maximize therapeutic effects, the drug is not beneficial to the patient and, though po- tentially useful, may be discarded. Moreover, from an economic point of view, low oral bioavailability results in wasting of a large portion of an oral dose and adds to the cost of drug therapy, especially when the drug is an expensive one [9,10]. Cyclodextrins (CDs), especially β- CD and its derivatives (Methyl-β-CD, and Hydroxypro- pyl- β-CD) have been established as potential candidates to alter physical, chemical and biological properties of these molecules through the formation of inclusion com- plexes [11-16]. However, due to several factors including  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies 213 toxicity, cost and bulk, and the amount of CD in the dosage form has to be reduced as far as possible. One of the approaches to achieve this is by enhancing the com- plexation efficiency of CDs by incorporating with wa- ter-soluble polymers [17-20]. Thus, the rationale of this study is to improve the solubility as well as therapeutic efficacy of arteether by encaging the drug in the hydrophobic cavity of cyclo- dextrin. The stability constant along with other thermo- dynamic parameters which are pre-requisite for formula- tion development are determined by solution calorimetry. These parameters are further used to find the relationship between noncovalent structure and free energy of bind- ing to include the roles of enthalpy and entropy of asso- ciation. The emphasis of the current study is laid to evaluate the efficacy of these complexes and on the in- vivo model to monitor their suitability in enhancing the bioavailability. Literature survey has revealed that not much data is avialable on the inclusion complexes of this poorly solu- ble drug. However, few reports are present on the NMR studies and molecular modeling studies of the prepared complexes. Thermodynamic parameters accompaning the inclusion as well as animal studies are also lacking [21-23]. 2. Experimental 2.1. Preparation of Binary Complexes Arteether (Ae) with β-CD, M-β-CD or HP-β-CD was mixed in 1:1 molar ratio by different methods as given below 2.1.1. Physical Mixing (PM) Physical mixtures were prepared by simple mixing of drug with the selected CD in mortar and to ensure uni- form mixing. The vials filled with the mixtures were subjected to vortex mixing for 5 min. 2.1.2. Kneading (KN) Drug and CD was blended together in mortar with water; a paste was obtained and this was kneaded for 90 min. The product was then dried under vacuum at 40˚C for 48 h and passed through a 150 µm mesh and stored in a glass vial in vaccum dessicator. 2.1.3. Freeze-Drying Method (LY) The required 1:1 stoichiometric quantity of drug was added to aqueous solution of the selected CD and was agitated on a magnetic stirrer for 24 hrs. The resulting solutions was frozen at (–80˚C) in deep freezer for over- night. This was then lyophilized under 17.2 m Torr for 48 hrs. The samples were transferred immediately into a vacuum desiccator and dried over silica gel under vac- uum for at least 24 hrs. 2.2. Preparation of Ternary Cyclodextrin Complexes 2.2.1. Physical Mixing (PM). Physical mixtures were prepared by simple mixing of drug, β-CD and 0.20% PVP in mortar to ensure uniform mixing. The vials filled with this were subjected to vor- tex mixing for 5 min. 2.2.2. Kneading (KN). Drug and β-CD were blended together in mortar with 0.20% of PVP for 90 min and a paste was obtained. The product was then dried under vacuum at 40˚C for 48 hrs and passed through a 150 µm mesh and stored in a glass vial in vacuum dessicator. 2.2.3. Lyophilized Suspension Method (LY Susp). Ternary complexes were prepared by dissolving the equimolar amounts of arteether and β-CD with 0.20% PVP in water. The resulting solution was stirred at room temperature for 48 hr. The solid residue was then sepa- rated by centrifugation at 15000 rpm for 15 minutes and upper liquid layer was filtered over a 0.45 Millipore filter paper. The filtrate was then lyophilized under 17.2 mTorr for 48 hrs and placed in vacuum desiccators. 2.2.4. Co evaporated (CoE) Solid System Method Equimolar amounts of arteether and β-CD systems with 0.20% PVP dissolved in water. The resulting solution was evaporated on a rota vapour at 60˚C to get a solid residue. The prepared system was dried in vacuum des- iccators. 3. Characterization Binary and ternary inclusion complexes of arteether were characterized in solid phase by DSC, PXRD, FT-IR, mass spectrometry and in solution phase by solution calorimetry and NMR 3.1. Phase Solubility Studies of Arteether with or without 0.2% PVP Phase solubility diagrams of arteether for both binary and ternary systems with various CDs in phosphate buffer (pH 6.8) were obtained according to Higuchi and Connors [24]. An excess amount of are was added to 100 ml buffer or CD buffered solutions with a concentra- tion of v/v (2 to150 mM) in 20 ml glass vials with or without a polymer (0.20% PVP). The suspensions were sealed and shaken in water-bath shaker MSW-275 (Macroscien- tific works, Delhi) at 37 ± 0.5˚C for 24 hrs to ensure equilibrium. After equilibration, aliquots of the super- natant were withdrawn, filtered through 0.45 µm milli- pore filter paper, and the arteether content was de- ter- mined spectrophotometerically at λ 230 nm (UV/VIS. Spectrophotometer (Perkin Elmer Lamda 15, USA). The Copyright © 2011 SciRes. PP  214 Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies presence of CDs did not interfere with the spectropho- tometric assay of the drug. 3.2. Mass Spectrometry ESI-MS studies were performed using a Q-ToF quadru- ple time of flight mass spectrometer (Waters) equipped with an electrospray source. The sample was introduced via a syringe pump at a flow rate of 5 µL/min. High flow rate nitrogen gas was employed as the nebulizing gas as well as the drying gas to aid desolvation. The sheath gas flow rate was 0.5 µL·min–1. After optimization of the MS parameters, the spray voltage was set to 2.5 kV in the positive mode, and the heated metal capillary tempera- ture was set at 80˚C. The mass scale was calibrated by using the standard calibration procedure and compounds provided by manufacturer. 3.3. Differential Scanning Calorimetry (DSC) DSC thermograms were obtained on DSC, (Q20, TA Instruments-Waters LLC, USA). The calorimeter was calibrated for temperature and heat flow accuracy using the melting of pure indium (mp 156.6˚C and ∆H of 25.45 J·g–1). The temperature range was from 50˚C - 350˚C with a heating rate of 10˚C per minute. 3.4. X-Ray Powder Diffraction Powder diffraction patterns were recorded on an X-ray diffractometer (XPERT-PRO, PANalytical, Netherlands, Holand) with Cu as tube anode the diffractograms were recorded under following conditions: voltage 40 kV, 35 mA, angular range 5 and fixed divergence slit. 3.5. Fourier Transform Infrared Spectrometry (FT-IR) The FT-IR spectra were obtained on FT-IR spectrometer, Mode spectrum RXI, Perkin Elmer, England over the range 400 - 4000 cm–1. Dry KBr (50 mg) was finely ground in an agate mortar and samples of drug and their complexes (1 - 2 mg) were subsequently added and mixed gently. A manual press was used to form the pel- lets. Analysis in solution state 3.6. 2D COESY, Proton Nuclear Magnetic Resonance (1H-NMR) and 13C NMR Spectroscopy 1H-NMR, 13C NMR and 2D COESY spectra in d6 DMSO of arteether and inclusion complexes were recorded with a Brucker AC 300˚C NMR spectrometer apparatus oper- ating at 300 MHz using tetramethylsilane as an internal standard. For 2D COESY experiments, samples were equilibrated for at least 24 hrs. 3.7. Molecular Modeling Studies 3.7.1. Computational Details The computational studies were carried out on a Linux Cluster (ROCKS 5.4). The structure preparation, simula- tions analysis were carried out with Maestro version 9.1, (Schrödinger LLC, New York, NY, 2010), while docking studies were carried out with Fast Rigid Exhaustive Docking acronym (FRED version 2.2.5, OpenEye Scien- tific Software, Santa Fe, USA [25,26]. The Molecular Dynamics simulations were performed using Desmond (version 2.4, DE Shaw Research, NY, USA). 3.7.2. Structure Preparation The 3D structures of β-cyclodextrin (β-CD) and arteether were retrieved from the Protein Data Bank [27] and PubChem (CID 72416). The geometries of the structures were optimized after the assignment of atom types and charges based on the OPLS 2005 force field in Schrö- dinger Suite. 3.7.3. Docking Studies The β-CD molecule was subsequently imported into the program FRED-RECEPTOR (version 2.2.5) for docking. First the active site box where the ligand or guest is ex- pected to bind is defined and the shape of the active site is described by two shape potential contours, referred to as the inner and outer contour. It is essential that the docked host guest poses fit within the shape of the outer contour and ensures that the center of at least one heavy atom of any docked pose touches the inner contour. Docking succeeds the grid generation protocol in the second step. The docking is a rigid body process involv- ing only rotational and transitional motions of the guest molecule inside the host cavity. The arteether: β-CD configurations of the guest molecule inside the host are rated by the intrinsic scoring function Chemgauss 3. The predominant configurations are stored for further analy- sis. 3.7.4. MD Simulations Before simulation the complex of Ae-β-CD was solvated with TIP3P waters [28] to form a water shell 10 Å thick around the Ae-β-CD complex. The solvated host guest system was simulated for a period of 5 ns with the ‘NPT relaxprotoco l’ in Desmond. The protocol involves an initial minimization of the solvent with the solute re- strained. The minimization is followed by short MD simulations of 12 - 24 ps in sequential NVT and NPT ensembles with the Langevin thermostat and barostat [29]. The temperature was maintained by coupling to an external 300 K bath based on the Langevin algorithm [29]. The pressure was isotropically restrained to 1 bar with the Langevin barostat. High-frequency vibrations were removed by applying the SHAKE algorithm [30] C opyright © 2011 SciRes. PP  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies 215 by constraining all bonds to their equilibrium values. Initial velocities were generated randomly from a Max- well distribution at 300 K in accordance with the masses assigned to the atoms. The trajectories and corresponding energies were sampled every 5 ps. No constraints were applied on the β-CD-arteether system during the simula- tions, so as to avoid introduction of any artifacts in the ligand conformation in the binding site. 3.8. Microcalorimetric Study Isoperibol solution calorimetry (ISC) (Calorimetry Sci- ence Corporation, UTAH, USA) model 4300 was used for thermal measurements. The calorimeter consists of a constant temperature bath held at 37˚C (± 0.005˚C) and heater assembly. The drug was filled into batch adaptor of volume 0.9 ml, sealed on both sides with ‘O’ rings and cover glass. The batch adaptor holding the drug was in- serted into the Dewar flask containing buffer (25 ml). The combined unit was then lowered in the calorimeter bath. The glass stirrer was rotated at 100 revolutions/min. and was allowed to equilibrate for 90 min. The ampoule was shattered automatically by means of plunger and temperature change noted. The performance of the sys- tem was checked using KCl, which has known enthalpy of solution and a good agreement (± 0.03 kJ/mol), was found with published value 3.9. Dissolution Study: The dissolution studies of the arteether and its binary and ternary complexes were performed in 900 ml of phos- phate buffer (pH 6.8 using) USP (12) apparatus at pre- equilibrated temperature 37 ± 0.5˚C and at a stirring rate of 50 rpm. Drug and its inclusion complexes each con- taining 100 mg of drug were filled in hard gelatin cap- sules. Samples was withdrawn at different intervals for a period of 6 hr and analyzed spectrophotometrically at λ 230 nm. 3.10. In Vivo Studies: Evaluation of Pharmacological Antimalarial Activity of Arteether, Its Binary and Ternary Complexes in Mice Four to five weeks old BALB/c mice (25 - 30 g) were procured and maintained in the Central Animal House. They were provided with standard pellet diet and water ad libtum. Experiments were performed as per guidelines of Control and Supervision on Experiments on Animals (CPC-SEA) committee. The experimental protocol was approved by Institutional Animal Ethics Committee (A. I. E. C.). Plasmodium berghei (NK 65) strain was used for evaluation of antimalarial activity in vivo studies and was maintained in the mice. All the mice belonging to control group were challenged with 106 P. berghei infected RBCs intraperitonial (i/p). After challenge mean percent para- sitaemia, percent activities of various complexes of ar- teether along with animal survivality were monitored. Mean percent parasitaemia was calculated for each group on every alternate day upto 30 days by tail blood smear, fixed in methanol and stained in Giemsa stain by count- ing at least 500 cells. Mean percent parasiteamia = infected RBCs × 100/Total No. of RBCs Animals were divided into 6 groups and each group comprised of 6 animals (n = 6). These were treated with single dose therapy (6 mg/kg of arteether) two times a day on 1 day of PI for 7 days to monitor the efficacy and potency of prepared lyophilized binary and ternary com- plexes. Each animal were treated with arteether equal to 100 µl. 1) Control group—treated with 0.5% carboxymethyl cellulose (CMC) suspension; 2) Standard group—administered arteether in 0.5% CMC suspension; 3) Test group 1—treated with binary Ae-β-CD com- plex in 0.5% CMC suspension; 4) Test group 2—treated with binary Ae-M-β-CD complex in 0.5% CMC suspension; 5) Test group 3—treated with binary Ae-HP-β-CD complex in 0.5% CMC suspension; 6) Test group 4—treated with ternary Ae-β-CD-PVP complex in 0.5% CMC suspension; 3.11. Statistical Analysis Data was expressed as mean ± S. D. and parasitaemia as well as in vitro drug release of the arteether and its bi- nary and ternary inclusion complexes were statistically assessed by one-way ANOVA followed by Turkey’s test using Jandel sigma stat 2.0 version. Differences were considered significant at P < 0.05. 4. Results and Discussion Equilibrium Phase Solubility Studies: The equilibrium phase solubility of the arteether in- creased in a linear fashion as a function of -CD, M- - CD and HP- -CD concentration and followed an AL-type system. The linear host-guest correlation with slope less than one suggested the formation of first order soluble complexes (Figure 1). The increment in the solubility of drug depends upon inclusion ability of cyclodextrin molecules with the solu- bilization strength increasing in the order: β-CD < HP-β-CD < M-β-CD. Selection of Third Component: To select the most ap- propriate third component for enhancing the solublizing efficiency of CDs several water-soluble polymers viz. PVP, hydroxylpropyl methyl cellulose (HPMC), poly- Copyright © 2011 SciRes. PP 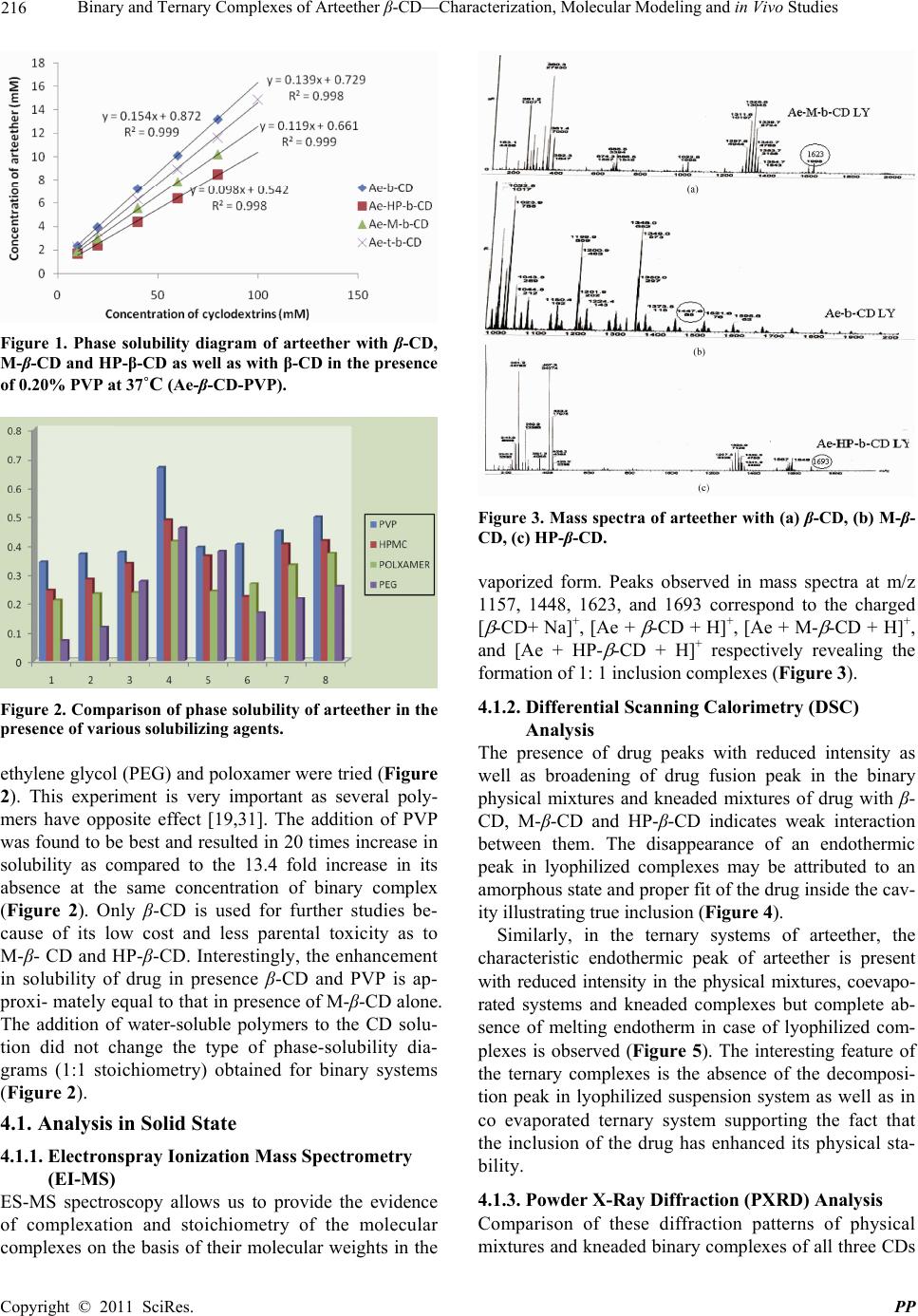 216 Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Figure 1. Phase solubility diagram of arteether with β-CD, M-β-CD and HP-β-CD as well as with β-CD in the presence of 0.20% PVP at 37˚C (Ae-β-CD-PVP). Figure 2. Comparison of phase solubility of arteether in the presence of various solubilizing agents. ethylene glycol (PEG) and poloxamer were tried (Figure 2). This experiment is very important as several poly- mers have opposite effect [19,31]. The addition of PVP was found to be best and resulted in 20 times increase in solubility as compared to the 13.4 fold increase in its absence at the same concentration of binary complex (Figure 2). Only β-CD is used for further studies be- cause of its low cost and less parental toxicity as to M-β- CD and HP-β-CD. Interestingly, the enhancement in solubility of drug in presence β-CD and PVP is ap- proxi- mately equal to that in presence of M-β-CD alone. The addition of water-soluble polymers to the CD solu- tion did not change the type of phase-solubility dia- grams (1:1 stoichiometry) obtained for binary systems (Figure 2). 4.1. Analysis in Solid State 4.1.1. Electronspray Ionization Mass Spectrometry (EI-MS) ES-MS spectroscopy allows us to provide the evidence of complexation and stoichiometry of the molecular complexes on the basis of their molecular weights in the Figure 3. Mass spectra of arteether with (a) β-CD, (b) M-β- CD, (c) HP-β-CD. vaporized form. Peaks observed in mass spectra at m/z 1157, 1448, 1623, and 1693 correspond to the charged [ -CD+ Na]+, [Ae + -CD + H]+, [Ae + M- -CD + H]+, and [Ae + HP- -CD + H]+ respectively revealing the formation of 1: 1 inclusion complexes (Figure 3). 4.1.2. Differential Scanning Calorimetry (DSC) Analysis The presence of drug peaks with reduced intensity as well as broadening of drug fusion peak in the binary physical mixtures and kneaded mixtures of drug with β- CD, M-β-CD and HP-β-CD indicates weak interaction between them. The disappearance of an endothermic peak in lyophilized complexes may be attributed to an amorphous state and proper fit of the drug inside the cav- ity illustrating true inclusion (Figure 4). Similarly, in the ternary systems of arteether, the characteristic endothermic peak of arteether is present with reduced intensity in the physical mixtures, coevapo- rated systems and kneaded complexes but complete ab- sence of melting endotherm in case of lyophilized com- plexes is observed (Figure 5). The interesting feature of the ternary complexes is the absence of the decomposi- tion peak in lyophilized suspension system as well as in co evaporated ternary system supporting the fact that the inclusion of the drug has enhanced its physical sta- bility. 4.1.3. Powder X-Ray Diffraction (PXRD) Analysis Comparison of these diffraction patterns of physical mixtures and kneaded binary complexes of all three CDs C opyright © 2011 SciRes. PP  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies 217 Figure 4. DSC thermograms of (a) Arteether (b) Ae- -CD- PM (c) Ae- -CD-KN (d) Ae- -CD LY (e) Ae-M- -CD-PM (f) Ae-M- -CD-KN (g) Ae-M- -CD-LY (h) Ae-HP- -CD-PM (i) Ae-HP- -CD-KN (j) Ae-HP- -CD-LY. Figure 5. DSC thermograms of arteether with (a) Ae-β-CD– PVP-PM (b) Ae-β-CD-PVP-CoE (c) Ae-β-CD-PVP-KN (d) Ae-β-CD-PVP-LY. with those of pure components shows a reduced number of signals, with remarkably lowered intensity indicating incomplete inclusion phenomenon (Figure 6). The evi- dence of complete disappearance of drug peaks in the PXRD diffraction pattern of lyophilized complexes indi- cates the entrapment of drug inside the CD cavity with the formation of true inclusion complex with amorphous nature except β-CD which shows diffused diffraction pattern. The ternary systems showed complete amorphous halo Figure 6. XRPD pattren of a) Arteether (b) Ae- -CD-PM (c) Ae- -CD-KN (d) Ae- -CD LY (e) Ae-M- -CD-PM (f) Ae- M- -CD-KN (g) Ae-M- -CD-LY (h) Ae-HP- -CD-PM (i) Ae-HP- -CD-KN (j) Ae-HP- -CD-LY. Figure 7. XRPD pattren of arteether with (a) Ar-β-CD– PVP-PM (b) Ar-β-CD-PVP-CoE (c) Ar-β-CD-PVP-KN (d) Ar-β-CD-PVP-LY. and is attributed to a complete interaction between all the three components. Some diffraction peaks with lower intensity are still detectable in the ternary complex withβ-CD which may be attributed to a crystalline nature f β-CD (Figure 7). o Copyright © 2011 SciRes. PP  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Copyright © 2011 SciRes. PP 218 Figure 8. FTIR spectra of (a) Arteether (b) Ae- -CD-PM (c) Ae--CD-KN (d) Ae- -CD LY (e) Ae-M- -CD-PM (f) Ae-M- - CD-KN (g) Ae-M- -CD-LY (h) Ae-HP- -CD-PM (i) Ae-HP- -CD-KN (j) Ae-HP- -CD-LY. 4.1.4. Fourier Transform Infrared Spectroscopy (FT-IR) FT-IR could not give much useful information as the spectra of complexes of β-CD, M-β-CD and HP-β-CD were found quite similar to their pure CDs because of the coincidently absorption of the both the host and guest molecule in most of the special regions coincidently ab- sorption in most of spectral region. Bands of the included part of the guest molecule are masked by the bands of the spectrum of CDs. However, the small shifts in characte- ristic bands of drug at 1450 cm–1, 1376 cm–1, and 1036 cm–1 undoubtedly confirm the presence of drug in all the complexes (Figure 8-9). 4.2. Analysis of Binary and Ternary Systems in Solution Phase 4.2.1. Proton NMR Spectroscopy Proton magnetic resonance spectroscopy plays a vital role in predicting the exact geometry of complex and also to characterize the binding mode. A downfield shift is observed in the cycloheptane protons H-d, H-g, H-h, H-m and H-n of drug molecule (Table 1) indicating the insertion of the whole drug molecule inside the cavity Figure 9. FTIR spectra of (a) Ar-β-CD-PVP-PM (b) Ar-β- CD-PVP-CoE (c) Ar-β-CD-PVP-KN (d) Ar-β-CD-PVP-LY.  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies 219 Table 1. Variation of 1H chemical shifts before and after inclusion ∆δ = δ (complex) – δ (Free). Arteether δdrug δae-b-cd, ae-M-b-cd, ae-HP- b-cd complexes (ppm) Δδae-b-cd, ae-M-b-cd, ae-HP- b-cd complexes (ppm) H-a 4.7545 4.6279, 4.685, 4.6383 –0.1266, –0.0695, –0.01162 H-b 5.5163 5.7255, 5.3974, 5.4213 0.2092, –0.1189, –0.095 H-c 3.5223 3.3756, 3.3918, 3.3835 –0.1467, –0.1305, –0.1388 H-d 2.1766 2.1853, 2.1681, 2.0437 0.0087, 0.02787, 0.01297 H-e 1.244 1.1519, 1.147, 1.1574 0.0921, –0.097, –0.00866 H-f, j 1.355 1.347, 1.34832, 1.3538 –0.00802, –0.0068, –0.01336 H-g 2.428 2.4014, 2.4051, 2.4023 0.00266, 0.0229, 0.0257 H-h 1.781 1.799, 1.796, 1.697 0.018, 0.015, –0.084 H-i 1.5938 1.5525, 1.6039, 1.5575 –0.04132, 0.0101, –0.03627 H-k 2.5073 2.5066, 2.5072, 2.5072 –0.0007, –0.0001, –0.0001 H-l 0.8958 0.8905, 0.8928, 0.8445 –0.00525, –0.003, –0.00545 H-m 0.8390 0.8400, 0.8423, 0.8949 0.00105, 0.0033, 0.009 H-n 1.2865 1.2933, 1.2944, 1.2946 0.0068, 0.0079, 0.0099 H-o 3.0218 3.056, 3.0328, 3.085 –0.0342, –0.011, –0.0632 Figure 10. (a) The chemical structure of arteether (b) Inclu- sion mode of drug into cyclodextrin cavity. except ether linkage which is protruding outside the cavity. Insertion was favored towards the cycloheptane ring of the artemisinin derivatives due to its narrower dimension (2.89 A˚) as compared to the opposite end of the drug molecule, consisting of two cyclohexane rings (6.9 A˚) (Figure 10). Upfield shift in H-e, H-b, H-f protons of the molecule is due to the variation in the local polarity. In two-di- mensional (2D) COESY spectra, the appearance of cross peaks (Figure 11) between H-5 protons of CD and H-b and H-n protons support our proposed inclusion mode involving insertion of cycloheptane ring with endoper- oxide bridge deep into the cavity. Besides this, cross peaks are also present between H-3 protons of CD and H-a proton of oxygen containing cycloheaxane ring of drug. 4.2.2. Molecular Modeling Studies Arteether was docked into the cavity of β-CD using FRED which places the guest into the active cavity using a rotational and translational motion resulting into rigid conformations. The best configurations are identified using the intrinsic scoring function Chemgauss 3. During docking, it was observed that the arteether molecules oriented itself inside the β-CD cavity in two different ways. In case-1, the ethoxy fragment protrudes into the Figure 11. 1H COESY of arteether with (a) β-CD, (b) M-β- CD and (c) HP-β-CD. Copyright © 2011 SciRes. PP 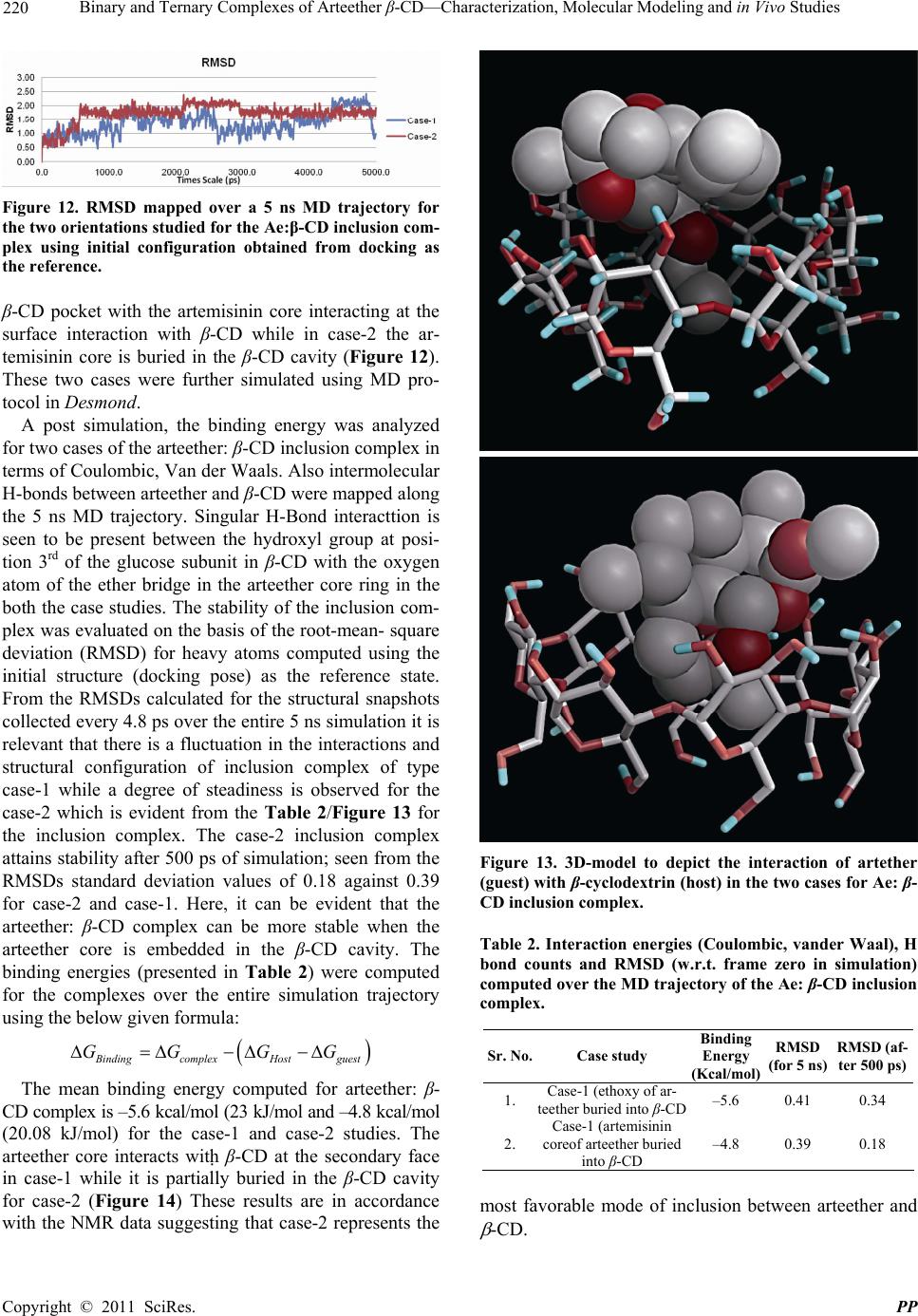 220 Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Figure 12. RMSD mapped over a 5 ns MD trajectory for the two orientations studied for the Ae:β-CD inclusion com- plex using initial configuration obtained from docking as the reference. β-CD pocket with the artemisinin core interacting at the surface interaction with β-CD while in case-2 the ar- temisinin core is buried in the β-CD cavity (Figure 12). These two cases were further simulated using MD pro- tocol in Desmond. A post simulation, the binding energy was analyzed for two cases of the arteether: β-CD inclusion complex in terms of Coulombic, Van der Waals. Also intermolecular H-bonds between arteether and β-CD were mapped along the 5 ns MD trajectory. Singular H-Bond interacttion is seen to be present between the hydroxyl group at posi- tion 3rd of the glucose subunit in β-CD with the oxygen atom of the ether bridge in the arteether core ring in the both the case studies. The stability of the inclusion com- plex was evaluated on the basis of the root-mean- square deviation (RMSD) for heavy atoms computed using the initial structure (docking pose) as the reference state. From the RMSDs calculated for the structural snapshots collected every 4.8 ps over the entire 5 ns simulation it is relevant that there is a fluctuation in the interactions and structural configuration of inclusion complex of type case-1 while a degree of steadiness is observed for the case-2 which is evident from the Table 2/Figure 13 for the inclusion complex. The case-2 inclusion complex attains stability after 500 ps of simulation; seen from the RMSDs standard deviation values of 0.18 against 0.39 for case-2 and case-1. Here, it can be evident that the arteether: β-CD complex can be more stable when the arteether core is embedded in the β-CD cavity. The binding energies (presented in Table 2) were computed for the complexes over the entire simulation trajectory using the below given formula: indingcomplexHost guest GG GG The mean binding energy computed for arteether: β- CD complex is –5.6 kcal/mol (23 kJ/mol and –4.8 kcal/mol (20.08 kJ/mol) for the case-1 and case-2 studies. The arteether core interacts with β-CD at the secondary face in case-1 while it is partially buried in the β-CD cavity for case-2 (Figure 14) These results are in accordance with the NMR data suggesting that case-2 represents the Figure 13. 3D-model to depict the interaction of artether (guest) with β-cyclodextrin (host) in the two cases for Ae: β- CD inclusion complex. Table 2. Interaction energies (Coulombic, vander Waal), H bond counts and RMSD (w.r.t. frame zero in simulation) computed over the MD trajectory of the Ae: β-CD inclusion complex. Sr. No.Case study Binding Energy (Kcal/mol) RMSD (for 5 ns) RMSD (af- ter 500 ps) 1. Case-1 (ethoxy of ar- teether buried into β-CD –5.6 0.41 0.34 2. Case-1 (artemisinin coreof arteether buried into β-CD –4.8 0.39 0.18 most favorable mode of inclusion between arteether and -CD. C opyright © 2011 SciRes. PP 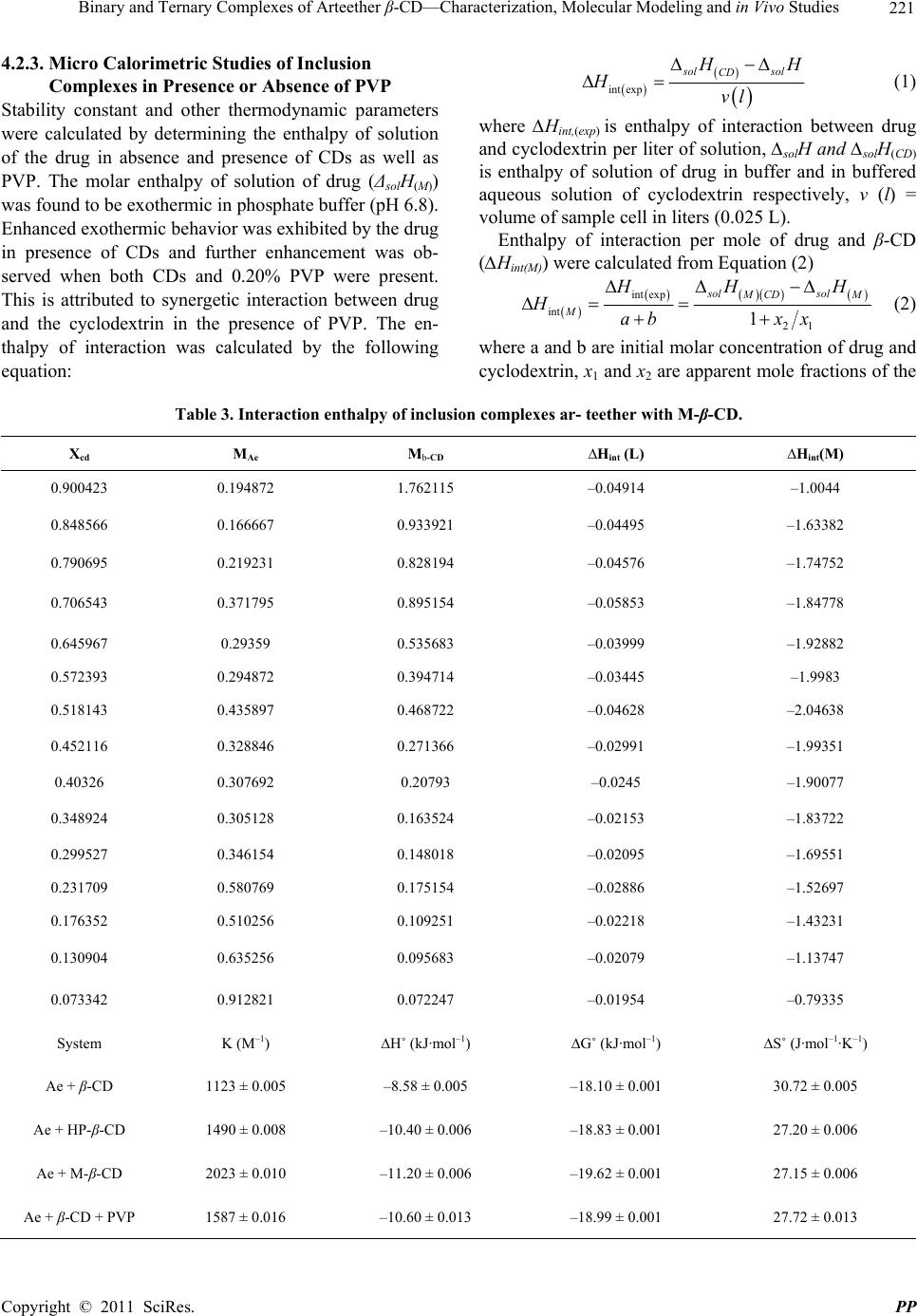 Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Copyright © 2011 SciRes. PP 221 4.2.3. Micro Calorimetric Studies of Inclusion Complexes in Presence or Absence of PVP int exp sol sol CD H Hvl (1) Stability constant and other thermodynamic parameters were calculated by determining the enthalpy of solution of the drug in absence and presence of CDs as well as PVP. The molar enthalpy of solution of drug (ΔsolH(M)) was found to be exothermic in phosphate buffer (pH 6.8). Enhanced exothermic behavior was exhibited by the drug in presence of CDs and further enhancement was ob- served when both CDs and 0.20% PVP were present. This is attributed to synergetic interaction between drug and the cyclodextrin in the presence of PVP. The en- thalpy of interaction was calculated by the following equation: where ∆Hint,(exp) is enthalpy of interaction between drug and cyclodextrin per liter of solution, ∆solH and ∆solH(CD) is enthalpy of solution of drug in buffer and in buffered aqueous solution of cyclodextrin respectively, v (l) = volume of sample cell in liters (0.025 L). Enthalpy of interaction per mole of drug and β-CD (∆Hint(M)) were calculated from Equation (2) int exp int 21 1 sol sol MCD M M HH Hab xx H (2) where a and b are initial molar concentration of drug and cyclodextrin, x1 and x2 are apparent mole fractions of the Table 3. Interaction enthalpy of inclusion complexes ar- teether with M-β-CD. Xcd M Ae Mb-CD ∆Hint (L) ∆Hint(M) 0.900423 0.194872 1.762115 –0.04914 –1.0044 0.848566 0.166667 0.933921 –0.04495 –1.63382 0.790695 0.219231 0.828194 –0.04576 –1.74752 0.706543 0.371795 0.895154 –0.05853 –1.84778 0.645967 0.29359 0.535683 –0.03999 –1.92882 0.572393 0.294872 0.394714 –0.03445 –1.9983 0.518143 0.435897 0.468722 –0.04628 –2.04638 0.452116 0.328846 0.271366 –0.02991 –1.99351 0.40326 0.307692 0.20793 –0.0245 –1.90077 0.348924 0.305128 0.163524 –0.02153 –1.83722 0.299527 0.346154 0.148018 –0.02095 –1.69551 0.231709 0.580769 0.175154 –0.02886 –1.52697 0.176352 0.510256 0.109251 –0.02218 –1.43231 0.130904 0.635256 0.095683 –0.02079 –1.13747 0.073342 0.912821 0.072247 –0.01954 –0.79335 System K (M–1) ∆H˚ (kJ·mol–1) ∆G˚ (kJ·mol–1) ∆S˚ (J·mol–1·K–1) Ae + β-CD 1123 ± 0.005 –8.58 ± 0.005 –18.10 ± 0.001 30.72 ± 0.005 Ae + HP-β-CD 1490 ± 0.008 –10.40 ± 0.006 –18.83 ± 0.001 27.20 ± 0.006 Ae + M-β-CD 2023 ± 0.010 –11.20 ± 0.006 –19.62 ± 0.001 27.15 ± 0.006 Ae + β-CD + PVP 1587 ± 0.016 –10.60 ± 0.013 –18.99 ± 0.001 27.72 ± 0.013  222 Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Figure 14. Dissolution study of arteether (a) binary systems and (b) ternary systems. drug and cyclodextrin ignoring the concentration of buff- ers. The detailed calorimetric data for arteether and M-β-CD are given in Table 3. The stoichiometry of the complex was ascertained util- izing continuous variation method (Job’s plot) [32] by plotting (∆Hint(M)) versus (x2) (Figure 15). It can be seen that the minimum occurs at x2 = 0.5. This indicates that complex has 1:1 stoichiometry and supports its determi- nation by other techniques. Similarly the enthalpy of interaction for ternary system was calculated by subtracting the enthalpy of solution of drug in presence of cyclodextrin and 0.20% PVP from that in pure buffer (ΔsolH(M)). The binding parameters were then calculated using Equations (1) and (2). Now the thermodynamic constants are calculated as- suming the following equilibria. CD + arteether ↔ CD: arteethe (3) The experimentally calculated enthalpy of interaction (ΔHint(exp)) is proportional to the product of molar con- centration of CD: arteether complex (c) in the solution at equilibrium and enthalpy of binding per mole of drug (ΔH˚) ΔHint(exp) = ΔH˚ × c (4) The binding constant K and enthalpy of binding (Hº) for both binary and ternary systems were computed from the experimentally determined enthalpy of interaction (∆Hint(exp)). The calculations were done by our computer program utilizing an iterative non-linear least square re- gression method to minimize the value of Σ(∆Hint(exp) – ∆Hint(calc))2 and are given in Table 4. The values of free energy of inclusion (G˚) and en- tropy of inclusion (S˚) were calculated from the fol- lowing equations and given in Table 4. G˚ = – RTlnK (5) S˚ = (H˚ – G˚)/T (6) The experimentallly determined ∆G˚ (–18.10 kJ/mol) is closer to the theoretically calculated binding energy for case-2 (20.08 kJ/mol). These results clearly indicate that in case-2 where the arteether core is embedded in the β-CD cavity is more stable as to case-1 where arteether core interacts on the surface of the CD cavity. Our NMR data is also supporting this mode of inclusion. The magnitude of K reflects optimum value for the in- clusion of guest molecule inside the cavity of the cyclo- dextrin molecule for both binary and ternary systems (Table 4). The absolute value of K increases in the order β-CD < HP-β-CD < M-β-CD. This shows that substi- tutent groups for assisting in binding by lengthening the cavity and thus increasing the hydrophobicity. Methyla- tion also makes the environment more hydrophobic and allows for increased adaptability of CD towards the guest through enhanced flexibility. Enthalpy of binding (H˚) is negative in all the cases reflecting an exothermic in- teraction. The entrapment of drug molecule into the CD cavity results in Van der Waal’s interaction between the hydrophobic part of drug and walls of the CD cavity, leading to enthalphic gain, but also induces the rear- rangement of water molecules or loss of solvation of the guest, affording an entropic gain. The enhancement of K upon addition of the PVP was observed which indicates that the inclusion is facilitated in presence of third component. This may be attributed to the establishment of different interactions with CDs and drug molecules such as hydrophobic bonds, Van der Waals forces, or hydrogen bonds [18,33]. Polymers are known to interact with the outer sur- faceof CDs and with drug-CD complexes, forming co- complexes or aggregates that show higher stability con- stants (Kc) values than those for the binary drug-CD system [34]. They increase the complexation efficiency, and therefore a smaller amount of CD can be used in the preparation of the complex [17]. 4.2.4. Dissolution Studies Rapid dissolution is the characteristic behavior of inclu- sion complexes but the comparative release of active material was strongly affected by the method of formula- tion. In binary systems, the lyophilized system exhibited the best dissolution properties and was followed by kneaded complex and physical mixtures (Figure 15). Moreover, highest dissolution rate was found for M-β- CD complex followed by that of HP-β-CD and β-CD. The much-enhanced dissolution rate observed in ter- nary system is due to (1) the enhancement of the com- plexation and solubilization efficiencies of β-CD (2) the stronger drug amorphization and better inclusion caused y the combined action of β-CD and the hydrophilic b C opyright © 2011 SciRes. PP  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies 223 Table 4. Thermodynamic parameters of inclusion complex of arteether with β-CD in presence of PVP. S. No. Groups Treatment Mean % Parasitaemia* on day 8th PI % mortality (n = 6, t = 40 days) 1 Control group 0.5% CMC solution 49.23 ± 5.34 100 2 Standard group Arteether ~ (5 mg/kg) 38.48 ± 3.21 66.7 3 Test group 1 Ae-β-CD ~ (6mg/kg of arteether) 24.46 ± 3.03 50 4 Test group 2 Ae-M-β-CD ~ (6mg/kg of arteether)0.009 ± 0.0001 0 5 Test group 3 Ae-HP-β-CD ~ (6 mg/kg of arteether)8.95 ± 1.21 33.3 6 Test group 4 Ae-β-CD-PVP ~ (6 mg/kg of arteether)4.75± 0.034 16.7 Figure 15. Plot of ΔsolHint(M) versus mole fraction (x2) of arteether with β-CD, M-β-CD, HP-β-CD and As-β-CD-PVP. Figure 16. Antimalarial activity of β-CD lyophilized com- plexes of arteether in P. berghei infected mice “(n = 6)” as compared to drug. polymers (Figure 13). Therefore, the lyophilized binary and ternary complexes with the highest dissolution rate are most suitable for the animal studies. 4.2.5. In Vivo Antimalarial Activity of Arteether and Its Binary and Ternary Inclusion Complexes Suspensions containing arteether, binary and ternary in- clusion complexes were tested with respect to para- sitemia progression and survival period. It was observed that arteether alone (Standard Group) is insufficient to prevent the mortality but significantly prolonged their survival period (day 14 - 19) compared to control (day 9). Test Group 1, Test Group 2 and Test Group 3 treated mice died between 15 - 25 days, 20 - 26 and 27 - 35 days, respectively (Table 5), whereas Test Group 4 (Ternary lyophilized system) resulted in a 83.3% survival of in- fected mice even after 30 days. The percent mortality rate with arteether alone, β-CD complex, HP-β-CD com- plex and ternary complexes of β-CD were 50%, 66.7%, 83.3% and respectively. However, M-β-CD (Test Group 3) complexes have resulted in complete clearance of the parasite from peripheral blood. Significantly less (P < 0.001) mean percent parasitaemia is observed in the Test Group 3 (0.009 ± 0.0001) compared to all test groups. ANOVA have also shown significant (P < 0.05) antima- larial activity of all binary and ternary complexes as to arteether (Figure 16). 4.2.6. Conclusions The present work proved the suitability of PVP as auxil- iary substance in enhancing the complexation efficiency of β-CD towards arteether. The free energy of binding and inclusion mode of arteether is determined experi- mentally as well as by theoretically mean binding energy by molecular modeling studies. Higher numerical values of K for M-β-CD complexes and ternary complexes of β- CD in presence of PVP are accompained by increment the in vitro dissolution rate and dissolution efficiency. However, 100% survival rate and complete eradication of parasite from was observed for only for Methylated β- CD complexes. 5. Acknowledgements The financial assistance provided by Indian Council of Medical research (ICMR; BMS-45/29/2006), New Delhi, India and Instrumentation assistance by Department of Science Technology (DST), New Delhi is gratefully ac- knowledged. The computational facilities supported by the Department of Biotechnology (DBT; BT/TF-8/BRB/ 2009) and Department of Science and Technology (DST; SR/FST/LSI-163/2003), New Delhi are gratefully ac- knowledged. 6. References [1] D. L. Klaymann, “Qinghaosu, an Antimalarial from China,” Science, Vol. 228, No. 4703, 1985, pp. 1049- 1055. doi:10.1126/science.3887571 [2] R. G. Patel, A. C. Shah and J. H. Patel, “An Arteether Injection for Treatment of Malaria,” WIPO Patent Appli- cation WO/2010/082219, IN2009/000757, 22 July 2010. Copyright © 2011 SciRes. PP  224 Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies [3] G. A. Balint, “Artemisnin and Its Derivatives an Impor- tant New Class of Antimalarial Agents,” Pharmacology & Therapeutics, Vol. 90, No. 2-3, May-June 2001, pp. 261- 265. doi:10.1016/S0163-7258(01)00140-1 [4] Report of a Joint CTD/DMP/TDR, “The Use of Artemis- inin and Its Derivatives as Antimalarial Drugs,” World Q3 Health Organization, Malaria Unit Division of Con- trol of Tropical Diseases, World Health Organization 1998, Geneva. (WHO, WHO/MAL/98/1086). http://www.doh.gov.za/issues/malaria/red_reference/case _management/cm2.PDF. [5] R. Ferone, “Folate Metabolism in Malaria,” Bulletin - World Health Organization, Vol. 55, 1977, pp. 291-298. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2366725/ pdf/bullwho00446-0156.pdf [6] A. K. Krishna and D. R. Flanagan, “Micellar Solubilisa- tion of a New Antimalarial Drug, β-Arteether,” Journal of Pharmaceutical Sciences, Vol. 78, No. 7, July 1989, pp. 574-576. doi:10.1002/jps.2600780713 [7] A. J. Lin and R. E. Miller, “Antimalarial Activity of New Dihydroartemisinin Derivatives. 6. Alpha-Alkylbenzyic Ethers,” Journal of Medicinal Chemistry, Vol. 38, No. 5, March 1995, pp. 764-770. doi:10.1021/jm00005a004 [8] Q. G. Li, L. L. Fleckenstein, K. Masonic, M. H. Heiffer and T. G. Brewer, “The Pharmacokinetics and Bioavail- ability of Dihydroartemisinin, Arteether, Artemether, Ar- tesunic Acid and Artelinic Acid in Rats,” Journal of Pharmacy and Pharmacology, Vol. 50, No. 2, February 1998, pp. 173-182. doi:10.1111/j.2042-7158.1998.tb06173.x [9] B. J. Aungst, “Novel Formulation Strategies for Improv- ing oral Bioavailability of Drugs with Poor Membrane Permeation or Presystemic Metabolism,” Journal of Pharmaceutical Sciences, Vol. 82, No. 10, October 1993, pp. 979-987. doi:10.1002/jps.2600821002 [10] H. Fridriksdottir, T. Loftsson and E. Stefansson, “Formu- lation and Testing of Methazolamide Cyclodextrin Eye Drop Solutions,” Journal of Controlled Release, Vol. 44, No. 1, February 1997, pp. 95-99. doi:10.1016/S0168-3659(96)01506-4 [11] D. Duchene and D. Wouessidjewe,” Pharmaceutical and Medicinal Applications of Cyclodextrins,” In: S. Dumi- triu, Ed., Polysaccharides in Medical Applications, Mar- cel Dekker, New York, 1996, pp. 575-602. [12] K. Uekama, F. Hirayama and T. Irie, “Cyclodextrin Drug Carrier Systems,” Chemical Reviews, Vol. 98, No. 5, July 1998, pp. 2045-2076. [13] T. Loftsson and M. E. Brewster, “Pharmaceutical Appli- cations of Cyclodextrins, I: Drug Solubilization and Sta- bilization,” Journal of Pharmaceutical Sciences, Vol. 85, No. 10, 1996, pp. 1017-1025. doi:10.1021/js950534b [14] R. A. Rajewski and V.J. Stella, “Pharmaceutical Applica- tions of Cyclodextrins, II: in Vivo Drug Delivery,” Jour- nal of Pharmaceutical Sciences, Vol. 85, No. 11, 1996, pp. 1142- 1169. doi:10.1021/js960075u [15] E. M. Del Valle, “Cyclodextrins and Their Uses: A Re- view,” Process Biochemistry, Vol. 39, No. 9, 2004, pp. 1033-1046. doi:10.1016/S0032-9592(03)00258-9 [16] T. Loftsson, H. Fridriksdottir and B. J. Olafsdottir, “Solubilization and Stabilization of Drugs through Cyclodextrin Complexation,” Acta Pharmaceuitca Nor- dica, Vol. 3, 1991, pp. 215-217. [17] T. Loftsson, H. Fridriksdottir, A. M. Sigurdadottir and H. Ueda, “The Effect of Water Soluble Polymers on Drug Cyclodextrin Complexation,” International Journal of Pharmaceutics, Vol. 110, 1994, pp. 169-177. doi:10.1016/0378-5173(94)90155-4 [18] T. Loftsson, H. Fridriksdottir, A. M. Sigurdadottir and T. K. Gudmundsdottir, “The Effect of Water Soluble Poly- mers on the Aqueous Solubility of Drugs,” International Journal of Pharmaceutics, Vol. 127, 1996, pp. 293-296. doi:10.1016/0378-5173(95)04207-5 [19] T. Loftsson, “Increasing the Cyclodextrin Complexation of Drugs and Drug Bioavailability through Addition of Watersoluble Polymers,” Pharmazie, Vol. 53, No. 1, 1998, pp. 733-740. [20] A. C. C. Asbahr, L. Franco, A. Barison, C. W. P. Silva, L. N. C. Rodrigues and H. G. Ferraz, “Binary and Ternary Inclusion Complexes of Finasteride in HPbCD and Polymers: Preparation and Characterization,” Bioorganic & Medicinal Chemistry, Vol. 17, No. 7, April, 2009, pp. 2718-2723. doi:10.1016/j.bmc.2009.02.044 [21] M. Bayomi, “Characterisation of Arteether Interactions with β-Cyclodextrin and Hydroxy-β—Cyclodextrin,” Saudi Pharmaceutical Journal, Saudi Pharmaceutical Society, Vol. 10, No. 1-2, 2002, pp. 36-43. [22] A. C. Illapakurthy, Y. A. Sabins, B. A. Avery, M. A. Avery and C. M. Wyandt, “Interaction of Artemisnin and Its Related Compounds with Hydroxypropyl-β-Cyclo- dextrin in Solution State: Experimental and Molecu- lar-Modeling Studies,” Journal of Pharmaceutical Sci- ence, Vol. 92, No. 3, March 2003, pp. 649-655. doi:10.1002/jps.10319 [23] P.-V. Jacqueline, G. Margriet, “Inclusion Complexes of Artemisinin or Derivatives with Cyclodextrins,” Patent No. WO/2004075921, International Application No. PCT/ EP2004/001851, September 2004. [24] M. McGann, H. Almond, A. Nicholls, J. A. Grant and F. Brown, “Gaussian. Docking Functions,” Biopolymers, Vol. 68, No. 1, January 2003, pp. 76-90. doi:10.1002/bip.10207 [25] G. B. McGaughey, R. P. Sheridan, C. I. Bayly, J. C. Cul- berson, C. Kreatsoulas, S. Lindsley, V. Maiorov, J.-F. Truchon and W. D. Cornell, “Comparison of Topological, Shape, and Docking Methods in Virtual Screening,” Journal of Chemical Information and Modeling, Vol. 47, No. 4, June 2007, pp. 1504-1519. doi:10.1021/ci700052x [26] H. M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov and P. E. Bourne, “The Protein Data Bank,” Nucleic Acids Research, Vol. 28, 2000, pp. 235-242. doi:10.1093/nar/28.1.235 [27] P. Mark, L. Nilsson, “Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K,” Jour- C opyright © 2011 SciRes. PP  Binary and Ternary Complexes of Arteether β-CD—Characterization, Molecular Modeling and in Vivo Studies Copyright © 2011 SciRes. PP 225 nal of Physical Chemistry A, Vol. 105, No. 43, October 2001, pp. 9954-9960. doi:10.1021/jp003020w [28] D. Quigley and M. I. J, Probert, “Constant Pressure Lange- vin Dynamics,” Theory and Application, Vol. 169, September 2004, pp. 322-325. [29] J. P. Ryckaert, G. Ciccotti and H. J. C. Berendsen, “Nu- meri- cal Integration of the Cartesian Equations of Mo- tion of a System with Constraints: Molecular Dynamics of n-al- kanes,” Journal of Computational Physics, Vol. 23, No. 3, March 1977, pp. 327-341. doi:10.1016/0021-9991(77)90098-5 [30] T. Higuchi and K. A. Connors, “Phase Solubility Tech- niques,” Advanced Analytical Chemistry of Instrumenta- tion, Vol. 4, 1965, pp. 117-212. [31] M. Valero, J. Tejedor and L. J. Rodriguez, “Encapsula- tion of Nabumetone by Means of -Drug: (β-Cyclodex- trin)2: Polyvinylpyrrolidone Ternary Complex Forma- tion,” Journal of luminescence, Vol. 126, No. 2, October 2007, pp. 297-302. doi:10.1016/j.jlumin.2006.07.028 [32] P. Job, “Recherches sur la formation de complexes min- eraux en solution et sur leur stabilite,” Annual Chemistry, Vol. 9, 1928, pp. 1132-114. [33] A. R. Patel and P. R. Vavia, “Effect of Hydrophilic Polymers on Solubilization of Fenofibrate by Cyclodex- trin Complexation,” Journal of Inclusion Phenomenon and Macrocyclic Chemistry, Vol. 56, 2006, pp. 247-251. doi:10.1007/s10847-006-9091-4 [34] L. Ribeiro, T. Loftsson, D. Ferreira and F. Veiga, “Inves- tigation and Physicochemical Characterization of Vinpo- cetine-Sulfobutyl Ether β-Cyclodextrin Binary and Ter- nary Complexes,” Chemistry Pharmaceutical Bulletin, Vol. 51, 2003, p. 914. doi:10.1248/cpb.51.914
|