 Pharmacology & Pharmacy, 2011, 2, 199-211 doi:10.4236/pp.2011.23029 Published Online July 2011 (http://www.scirp.org/journal/pp) Copyright © 2011 SciRes. PP 199 Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Updesh B. Lade*, Yogesh M. Amgaonkar, Rupesh V. Chikhale*, Dinesh M. Biyani, Milind J. Umekar Department of Pharmaceutics, Smt. Kishoritai Bhoyar College of Pharmacy, Sadashivrao Patil Shikshan Sanstha, New Kamptee, Nagpur (M.S), India. Email: *yamgaonkar123@gmail.com, rupeshchikhale7@gmail.com Received January 24th, 2011; revised March 30th, 2011; accepted May 28th, 2011. ABSTRACT Budesonide is a highly potent synthetic, nonhalogenated corticosteroid. The mechanism of action of corticosteroids in allergic rhinitis remains unknown, but may involve reductions in number of various mediator cells such as basophils, eosinophils, T-helper cells, mast cells, and neutrophils. In the nasal mucosa, nasal reactivity to allergens, and release of inflammatory mediators and proteolytic enzymes. Budesonide is very effective and quikly acting as it is rapidly and almost completely absorbed after oral administration, but has poor systemic availability (about 10%) due to extensive first-pass metabolism in the liver, mainly by the cytochrome P450 isoenzyme CYP3A4. The major metabolites, 6-β- hydroxybudesonide and 16-α-hydroxyprednisolone have less than 1% of the glucocorticoid activity of unchanged drug with a terminal half-life of about 2 - 4 hours. Polymeric films containing Eudragit RL 100: Eudragit RS: drug (7:3:1, 7: 2:1) and Ethyl cellulose: PVP: drug (7:3:1, 7:2:1) were selected for transdermal administration based on evaluation studies. These polymeric films were prepared by mercury substrate method employing PEG-400 as plasticizer. Two different penetration enhancers Urea and Dimethyl sulphoxide (DMSO) were employed in the study. The patches in each group were uniform in drug content, thickness. In Vitro drug permeation, moisture absorption and WVTR studies were carried out on these test patches. It was found that at all humidity condition the absorption increases which were linear to the moisture absorbed. In PVA and EUDRAGIT RL 100 patches the water vapor transmission rate was found to be higher at 75% RH, RT conditions. Therefore at both % RH, RT condition the PVA and EUDRAGIT RL 100 patches provide the best resistance to water vapor. Therefore, when applied to animals (in further studies) these patches may provide more occlusion to water vapor loss from skin thus making atmosphere beneath the skin more hu- mid that aid in drug permeation. Keywords: Budesonide, Transdermal Drug Delivery 1. Introduction Corticosteroids and their biologically active synthetic derivatives differ in their metabolic (glucocorticoid) and electrolyte-regulating (mineralocorticoid) activities. These agents are employed at physiological doses for replace- ment therapy when endogenous production is impaired. In addition, glucocorticoids potently suppress inflamma- tion, and their use in a variety of inflammatory, asthmatic conditions, skin disorders, rhinitis, inflammatory bowel disease, collagenous colitis and autoimmune diseases makes them among the most frequently prescribed classes of drugs [1]. Budesonide is a highly potent syn- thetic, nonhalogenated corticosteroid. It has high gluco- corticoid and weak mineralocorticoid activity [2]. Exact mechanism(s) of action of corticosteroids in allergic rhinitis remains unknown, but may involve reductions in the following: number of mediator cells (basophils, eosinophils, T-helper cells, mast cells, and neutrophils) in the nasal mucosa, nasal reactivity to allergens, and release of inflammatory mediators and proteolytic en- zymes [3]. Budesonide is rapidly and almost completely absorbed after oral administration, but has poor systemic availabil- ity (about 10%) due to extensive first-pass metabolism in the liver, mainly by the cytochrome P450 isoenzyme CYP3A4 [4]. The major metabolites, 6-β-hydroxybude- sonide and 16-α-hydroxyprednisolone have less than 1% of the glucocorticoid activity of unchanged drug with a terminal half-life of about 2 - 4 hours [4-6]. The polymeric film containing Eudragit RL 100: Eud- ragit RS: drug (7:3:1, 7:2:1) and Ethyl cellulose: PVP:  200 Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide drug (7:3:1, 7:2:1) were selected for transdermal admini- stration based on evaluation studies [7,8]. The polymeric films were prepared by mercury substrate method em- ploying PEG-400 as plasticizer. Two different penetra- tion enhancers Urea and Dimethyl sulphoxide (DMSO) were employed in the study. The dried polymeric film was evaluated using different parameters including thi- ckness uniformity, drug content of the film, in vitro drug release from films and in vitro skin permeation of drug, prior to their in vivo evaluation [8,9]. 2. Materials and Methods 2.1. Materials Budesonide (gift sample from M/s. Cipla Pharmaceuti- cals Ltd., Mumbai, India), Eudragit RL-100, Eudragit RS-100 (Röhm GmbH & Co. KG, Pharma Polymers, Darmstadt, Germany), Polyethylene glycol (PEG-400), Cellophane Membrane, Polyvinyl alcohol (PVA), Ethyl cellulose (14 cps), PVP (Mol. Wt. 40,000), Potassium dihydrogenorthophosphate, Potassium carbonate, Potas- sium nitrate, Sodium chloride, Sodium hydroxide, Urea, Dimethyl sulphoxides (DMSO), Glyceryl triacetate, all other chemicals used were of analytical grade and ob- tained commercially. 2.2. Animals The Swiss albino rats (170 - 190 gm) were obtained from National Chemical Labortary, Pune, India, and main- tained at 25 ± 1˚C for the study. The animals were housed in stainless metabolic cages and provided with standard diet and water ad libitum. Necessary approvals were obtained from CPCSEA India, for conducting the study. 2.3. Preparation and Evaluation of Polymeric Films 2.3.1. Preparation of Transdermal Patch by Solvent Casting on Mercury Substrate [10,11] The transdermal patch was prepared by solvent evapora- tion technique on mercury substrate. Polymer solution was prepared in ethanol (10 ml) and to it budesonide was added. The plasticizer or permeation enhancer or the pore forming agent were added during patch casting. The solution was poured on glass rings placed on mercury surface and allowed to dry in air for 24 hours. Circular patches of 2 cm diameter (3.14 cm2) were cut from semi- dried patches and placed in desiccator with 0% Relative Humidity (RH). 2.3.2. Evaluation of patch 1) Measurement of thickness [11] Thickness was measured using micrometer screw gauge. Each patch was measured for thickness at five different points to ascertain thickness uniformity in patch. 2) Drug content [11] Accurately weighed patches were individually dissol- ved in minimum quantity of ethanol and volume was made up to 20 ml with pH 7.4 phosphate buffer contain- ing 2.5% ethanol. From this solution, 1 ml was trans- ferred to volumetric flask and volume was made up to 10 ml. The absorbance was recorded at 247 nm. The blank solution was prepared in the similar way except that the patches without drug were used. 3) Moisture absorption studies [12] The moisture absorption study was carried out at 43, 75, 93% RH, RT at 25 ± 1 °C. The pre-weighed samples of patches were kept under the humidity conditions and weighed after 24 hours. The increase in weight indicates the moisture absorption by samples. 4) Dissolution studies [13,14] The accurately weighed patches were fixed on glass discs of 2.5 cm diameter using standard glue. This as- sembly was kept in dissolution vessel such that the patch faced the dissolution media upwards. The dissolution media was 900 ml of pH 7.4 phosphate buffer containing 2.5% ethanol at 32 ± 0.5˚C. The speed of paddle was kept at 50 rpm. Samples were withdrawn at 1 hour inter- val till 12 hours and replaced with the media. The ab- sorbance was measured at 247 nm against the blank. 5) In vitro skin permeation studies [10] Cellophane membrane was used. The membrane was mounted between the donor and receptor compartment of Franz diffusion cell. The patch was kept in contact with cellophane membrane. The receptor compartment con- tained pH 7.4 phosphate buffer containing 2.5% ethanol. The assembly was kept on a magnetic stirrer and stirred at a speed of 200 rpm. The temperature of assembly was kept at 37 ± 1˚C. After each hour, 1ml of sample was withdrawn and replaced with same media up to 24 hours to study. 6) Effect of drugs loading and polymer concentra- tion on film [15] Films with different drug load and polymer concentra- tion were prepared and studied for their thickness, mois- ture absorption, and In vitro drug permeation. 7) Effect of penetration enhancers on film Two penetration enhancers namely urea, dimethylsul- phoxide (DMSO) were incorporated in different pro- portion 5 and 10%. The films were then evaluated for the properties as mentioned earlier. 8) Effect of plasticizers on film Two plasticizers namely polyethylene glycol-400, glyceryl triacetate were added in different concentration 5 and 10 % of polymer concentration. These films were then evaluated for the properties as mentioned earlier. C opyright © 2011 SciRes. PP 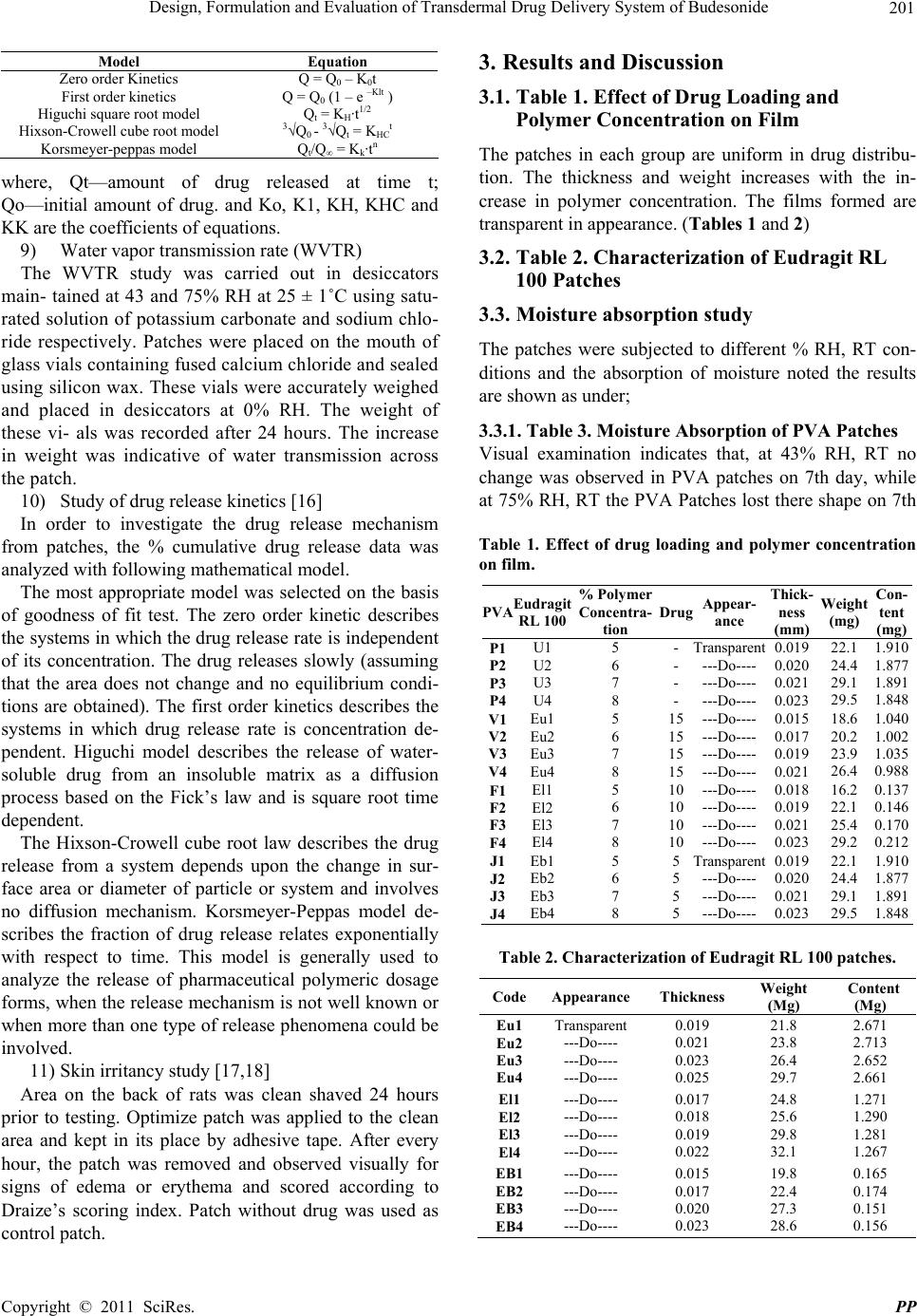 Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide 201 Model Equation Zero order Kinetics Q = Q0 – K0t First order kinetics Q = Q0 (1 – e –Klt ) Higuchi square root model Qt = KH·t1/2 Hixson-Crowell cube root model 3√Q0 - 3√Qt = KHCt Korsmeyer-peppas model Qt/Q∞ = Kk·tn where, Qt—amount of drug released at time t; Qo—initial amount of drug. and Ko, K1, KH, KHC and KK are the coefficients of equations. 9) Water vapor transmission rate (WVTR) The WVTR study was carried out in desiccators main- tained at 43 and 75% RH at 25 ± 1˚C using satu- rated solution of potassium carbonate and sodium chlo- ride respectively. Patches were placed on the mouth of glass vials containing fused calcium chloride and sealed using silicon wax. These vials were accurately weighed and placed in desiccators at 0% RH. The weight of these vi- als was recorded after 24 hours. The increase in weight was indicative of water transmission across the patch. 10) Study of drug release kinetics [16] In order to investigate the drug release mechanism from patches, the % cumulative drug release data was analyzed with following mathematical model. The most appropriate model was selected on the basis of goodness of fit test. The zero order kinetic describes the systems in which the drug release rate is independent of its concentration. The drug releases slowly (assuming that the area does not change and no equilibrium condi- tions are obtained). The first order kinetics describes the systems in which drug release rate is concentration de- pendent. Higuchi model describes the release of water- soluble drug from an insoluble matrix as a diffusion process based on the Fick’s law and is square root time dependent. The Hixson-Crowell cube root law describes the drug release from a system depends upon the change in sur- face area or diameter of particle or system and involves no diffusion mechanism. Korsmeyer-Peppas model de- scribes the fraction of drug release relates exponentially with respect to time. This model is generally used to analyze the release of pharmaceutical polymeric dosage forms, when the release mechanism is not well known or when more than one type of release phenomena could be involved. 11) Skin irritancy study [17,18] Area on the back of rats was clean shaved 24 hours prior to testing. Optimize patch was applied to the clean area and kept in its place by adhesive tape. After every hour, the patch was removed and observed visually for signs of edema or erythema and scored according to Draize’s scoring index. Patch without drug was used as control patch. 3. Results and Discussion 3.1. Table 1. Effect of Drug Loading and Polymer Concentration on Film The patches in each group are uniform in drug distribu- tion. The thickness and weight increases with the in- crease in polymer concentration. The films formed are transparent in appearance. (Tables 1 and 2) 3.2. Table 2. Characterization of Eudragit RL 100 Patches 3.3. Moisture absorption study The patches were subjected to different % RH, RT con- ditions and the absorption of moisture noted the results are shown as under; 3.3.1. Table 3. Moisture Absorption of PVA Patches Visual examination indicates that, at 43% RH, RT no change was observed in PVA patches on 7th day, while at 75% RH, RT the PVA Patches lost there shape on 7th Table 1. Effect of drug loading and polymer concentration on film. PVAEudragit RL 100 % Polymer Concentra- tion Drug Appear- ance Thick- ness (mm) Weight (mg) Con- tent (mg) P1 P2 P3 P4 U1 U2 U3 U4 5 6 7 8 - - - - Transparent ---Do---- ---Do---- ---Do---- 0.019 0.020 0.021 0.023 22.1 24.4 29.1 29.5 1.910 1.877 1.891 1.848 V1 V2 V3 V4 Eu1 Eu2 Eu3 Eu4 5 6 7 8 15 15 15 15 ---Do---- ---Do---- ---Do---- ---Do---- 0.015 0.017 0.019 0.021 18.6 20.2 23.9 26.4 1.040 1.002 1.035 0.988 F1 F2 F3 F4 El1 El2 El3 El4 5 6 7 8 10 10 10 10 ---Do---- ---Do---- ---Do---- ---Do---- 0.018 0.019 0.021 0.023 16.2 22.1 25.4 29.2 0.137 0.146 0.170 0.212 J1 J2 J3 J4 Eb1 Eb2 Eb3 Eb4 5 6 7 8 5 5 5 5 Transparent ---Do---- ---Do---- ---Do---- 0.019 0.020 0.021 0.023 22.1 24.4 29.1 29.5 1.910 1.877 1.891 1.848 Table 2. Characterization of Eudragit RL 100 patches. CodeAppearanceThickness Weight (Mg) Content (Mg) Eu1 Eu2 Eu3 Eu4 Transparent ---Do---- ---Do---- ---Do---- 0.019 0.021 0.023 0.025 21.8 23.8 26.4 29.7 2.671 2.713 2.652 2.661 El1 El2 El3 El4 ---Do---- ---Do---- ---Do---- ---Do---- 0.017 0.018 0.019 0.022 24.8 25.6 29.8 32.1 1.271 1.290 1.281 1.267 EB1 EB2 EB3 EB4 ---Do---- ---Do---- ---Do---- ---Do---- 0.015 0.017 0.020 0.023 19.8 22.4 27.3 28.6 0.165 0.174 0.151 0.156 Copyright © 2011 SciRes. PP  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Copyright © 2011 SciRes. PP 202 Table 3. Moisture absorption of PVA patches. 43% RH, RT Pot. Carbonate 75% RH, RT Sod. Chloride 93% RH, RT Pot. nitrate CODE Wt. of patch (mg) Moist. Absorp. (mg) % ABS. Wt. of patch (mg) Moist. Absorp. (mg) %ABS Wt. of patch (mg) Moist. Absorp. (mg) % ABS P1 P2 P3 P4 16.0 18.5 17.9 24.9 1.7 1.5 0.4 1.4 10.62 8.10 2.23 5.62 16.4 17.9 18.0 24.7 2.7 2.9 3.6 3.9 16.46 16.20 20.00 15.78 15.0 17.0 16.0 19.8 5.6 6.1 8.4 9.2 37.33 35.88 52.50 46.46 V1 V2 V3 V4 22.1 24.4 29.1 29.5 10.5 7.14 5.79 7.15 47.51 29.26 19.89 24.23 21.9 24.6 28.2 30.4 13.8 15.8 8.2 11.1 63.01 64.22 29.07 36.51 22.4 25.2 27.4 29.6 17.0 19.0 13.7 15.6 75.89 75.39 50.00 52.70 F1 F2 F3 F4 18.6 20.2 23.9 26.4 11.17 6.29 7.43 8.03 60.05 31.13 31.08 30.41 18.8 19.9 24.2 27.0 6.7 8.75 8.55 10.10 35.63 43.96 35.33 37.40 19.4 20.9 23.6 27.2 7.6 10.6 10.8 14.3 39.17 51.45 45.76 52.57 J1 J2 J3 J4 16.2 22.1 25.4 29.2 4.1 3.5 2.4 2.8 25.3 15.83 9.44 9.65 16.5 21.4 24.3 28.2 4.9 3.9 8.6 7.0 29.69 18.22 35.39 24.82 16.9 22.6 25.9 29.6 5.6 8.6 9.9 9.0 33.13 38.05 38.22 30.40 Table 4. Moisture absorption of Eudragit RL 100 patches. 43% RH, RT Pot. Carbonate 75% RH, RT Sod. Chloride 93% RH, RT Pot. nitrate CODE Wt. of patch (mg) Moist. Absorp. (mg) % ABS.Wt. of patch (mg)Moist. Absorp. (mg) %ABS Wt. of patch (mg) Moist. Absorp. (mg) % ABS U1 U2 U3 U4 15.9 17.7 18.8 20.1 0.3 2.0 1.5 0.8 1.88 11.29 7.97 3.98 15.3 17.9 18.1 20.7 0.3 1.4 1.3 0.5 1.96 7.82 7.18 2.41 15.6 17.4 18.9 20.4 0.6 2.0 1.2 1.6 3.84 11.49 6.34 7.84 EU1 EU2 EU3 EU4 21.8 23.8 26.4 29.7 0.4 2.9 0.7 1.8 1.83 12.18 2.65 6.06 22.2 23.6 27.1 29.9 1.3 0.5 0.8 2.8 5.85 2.11 2.95 9.36 21.3 23.2 26.8 30.1 1.4 3.3 12.5 0.6 6.57 14.22 9.32 1,99 EL1 EL2 EL3 EL4 24.8 25.6 29.8 32.1 1.2 1.4 0.6 1.6 4.83 5.46 2.01 4.98 24.3 25.1 29.3 31.8 0.8 0.6 1.9 1.3 3.29 2.39 6.48 4.08 21.7 26.1 28.6 32.7 2.5 3.5 2.6 2.8 11.52 13.40 9.09 7.95 EB1 EB2 EB3 EB4 19.8 22.4 27.3 28.6 0.9 1.0 1.4 0.8 4.54 4.46 5.12 2.79 19.6 21.9 27.8 28.3 0.4 0.6 1.1 0.7 2.04 2.73 3.95 2.47 20.1 22.9 27.0 28.9 3.0 1.4 1.5 1.8 14.92 6.11 5.55 6.22 day, however the patches were found to be less stable at 93% RH, RT on 5th day. 3.3.2. Table 4. Moisture Absorption of Eudragit RL 100 Patches For Eudragit RL100 patches at 43% RH, RT the Patches lost their shape on 7th day, while at 75% RH, RT the Patches lost their shape on 5th day and at 93% RH, RT patch were still more unstable and lost their shape at 4th day. Since the absorption pattern is not uniform in both the cases therefore no conclusion can be drawn regarding the stability of patch from the above data. At every % RH, RT condition the Eudragit RL 100 patches absorb less moisture than PVA patches, but PVA patches were found to be more stable than the Eudragit RL 100 patches. 3.4. In Vitro Drug Permeation through Cellophane Membrane The in-vitro permeation of PVA patches was studied by using cellophane membrane, results obtained are as shown under; 3.4.1. Table 5. Drug Permeation from PVA Patches (15 mg) 3.4.2. Table 6. Drug Permeation from PVA Patches (10 mg) 3.4.3. Table 7. Drug Permeation from PVA Patches (5 mg) 3.4.4. Figure 1. Effect of PVA Concentration with Constant Drug Concentration (15 mg) on Drug Permeation  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide 203 Table 5. Drug permeation from PVA patches (15 mg). (%) Percent permeated Time in (hrs.) CODE 1 2 3 4 5 6 12 24 V1 4.11 4.98 6.21 7.53 8.85 10.33 11.3912.98 V2 4.39 5.30 6.54 7.79 9.06 10.72 11.9413.48 V3 4.99 6.16 7.48 8.73 10.15 11.58 12.9715.05 V4 4.19 5.14 6.36 7.64 8.98 10.26 11.3712.89 Table 6. Drug permeation from PVA patches (10 mg). (%) Percent permeated Time in (hrs.) CODE 1 2 3 4 5 6 12 24 F1 3.82 5.22 6.27 7.31 8.29 9.26 10.7212.06 F2 4.19 5.61 7.19 8.32 9.54 10.70 12.1413.43 F3 4.79 5.88 7.56 8.94 10.18 11.71 13.1414.86 F4 3.63 5.11 5.93 7.14 8.16 9.36 11.0612.41 Table 7. Drug permeation from PVA patche s (5 mg). (%) Percent permeated Time in (hrs.) CODE 1 2 3 4 5 6 12 24 J1 3.42 4.81 5.83 6.85 7.82 8.77 10.2111.53 J2 3.79 5.20 6.76 7.87 9.07 10.21 11.6312.91 J3 4.40 5.46 7.13 8.49 9.71 11.21 12.6314.32 J4 3.23 4.71 5.48 6.68 8.48 9.84 11.3412.57 Figure 1. Effect of PVA concentration with constant drug concentration (15 mg) on drug permeation. 3.4.5. Figure 2. Effect of PVA Concentration with Constant Drug Concentration (10 mg) on Drug Permeation 3.4.6. Figure 3. Effect of PVA Concentration with Constant Drug Co ncent r a tion (5 mg) on Drug Permeation The in vitro drug permeation in all the cases increase up to 7% w/w polymer concentration while at 8% it de- creases with constant drug load, the permeation of drug increases with increase in polymer content up to 7% w/w and thereafter it decrease. With constant polymer con- centration, higher drug load gives higher permeation of drug. Thus the maximum percent permeation in V, F, Figure 2. Effect of PVA concentration with constant drug concentration (10 mg) on drug permeation. Figure 3. Effect of PVA concentration with constant drug concentration (5 mg) on drug permeation. and J series was observed with 7% w/w polymer concen- tration and it was 15.05%, 14.86%, 14.32% respectively as represented by Figure 1, 2 and 3 for PVA concentra- tion. 3.5. In Vitro Drug Permeation through Cellophane Membrane The drug permeation through cellophane membrane was studied, results obtained are as shown under; 3.5.1. Table 8. Drug Permeation from EUDRAGIT RL 100 Patches (15 mg) 3.5.2. Figure 4. Effect of EUDRAGIT RL 100 conc. with Constant Drug Concentration (15 mg) on Drug Permeation 3.5.3. Table 9. Drug Permeation from EUDRAGIT RL 100 Patches (10 mg) 3.5.4. Figure 5. Effect of EUDRAGIT RL 100 conc. with Constant Drug Concentration (10 mg) on Drug Permeation Copyright © 2011 SciRes. PP  204 Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Table 8. Drug permeation from EUDRAGIT RL 100 patches (15 mg). (%) Percent permeated Time in (hrs) CODE 1 2 3 4 5 6 12 24 EU1 5.76 6.61 7.83 8.74 10.25 11.49 13.0614.60 EU2 6.23 7.46 8.55 9.85 10.85 12.13 13.4615.44 EU3 5.28 6.54 7.92 8.89 10.48 11.48 13.1514.72 EU4 5.45 6.41 7.54 8.56 9.87 11.11 12.3713.92 Figure 4. Effect of EUDRAGIT RL 100 conc. with constant drug concentration (15 mg) on drug perm e ation. Figure 5. Effect of EUDRAGIT RL 100 conc. with constant drug concentration (10 mg) on drug perm e ation. Table 9. Drug permeation from EUDRAGIT RL 100 patches (10 mg). (%) Percent permeated Time in (hrs) CODE 1 2 3 4 5 6 12 24 EL1 4.28 5.25 6.44 8.05 9.21 10.68 11.8413.22 EL2 5.21 5.94 6.97 8.56 9.69 11.05 12.315.39 EL3 3.76 5.54 6.56 7.94 9.32 10.67 11.9213.63 EL4 4.09 5.32 6.33 7.65 8.84 10.16 11.5013.06 3.5.5. Table 10. Drug Permeation from EUDRAGIT RL 100 (5 mg) 3.5.6. Figure 6. Effect of EUDRAGIT RL 100 conc. with Constant Drug Concentration (5 mg) on Drug Permeation The in vitro drug permeation in all the cases increase upto 6% w/w polymer concentration & there after it decreases. Higher drug load gives higher permeation of drug. thus the maximum percent permeation in EU2, EL2, EB2 series was observed with 6% w/w polymer Table 10. Drug Permeation from EUDRAGIT RL 100 (5 mg). (%) Percent permeated Time in (hrs) CODE 1 2 3 4 5 6 12 24 EB13.895.116.017.60 8.74 10.66 11.8213.21 EB24.825.536.548.11 9.22 11.26 13.1515.20 EB33.375.136.137.49 8.86 10.42 11.6613.60 EB43.704.915.907.20 8.37 9.76 11.4212.67 Figure 6. Effect of EUDRAGIT RL 100 conc. with constant drug concentration (5 mg) on dr ug permeation. concentration and it was 15.05%, 14.86%, 14.32% res- pectively after 24 hrs. PVA 7% w/w gives maximum drug permeation while EUDRAGIT RL 100 at 6% w/w gives maximum permeation of drug. The maximum % permeation with PVA was 15.05 while with EUDRAGIT RL 100 it was 15.44%. For same drug content, the per- meation through EUDRAGIT RL100 was better and higher than with PVA. Also with constant polymer con- centration the drug permeation by EUDRAGIT RL100 was higher than PVA. Therefore, with EUDRAGIT RL 100 better permea- tion may be obtained with less polymer requirement in in-vitro studies, therefore, EUDRAGIT RL 100 was better polymer than PVA. 3.6. Effect of Penetration Enhancer on Film The compositions of various films and patches obtained were examined and characterized for the parameter are shown as below; 3.6.1. Table 11. Compositions of Patches with Different Penetration Enhancers. 3.6.2. Table 1 2. Ch aracterization of PVA & EUDRAGIT RL 100 with Diff erent Penetration Enhancers In both the cases the patches formed are uniform with respect to drug content also with increase in the amount of penetration enhancer the weight of the patch also increase linearly .The thickness was also to be uniform throughout the patch. C opyright © 2011 SciRes. PP  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Copyright © 2011 SciRes. PP 205 Table 11. Compositions of patches with different penetra- tion enhancers. CODE Polymer (mg) Drug (mg) Urea (mg) DMSO (mg) S1/M1 PVA/Eudragit RL100 - 700 5 35 -- S2/M2 PVA/Eudragit RL100 - 700 5 70 -- T1/R1 PVA/Eudragit RL100 - 700 5 -- 35 T2/R2 PVA/Eudragit RL100 - 700 5 -- 70 Table 12. Characterization of PVA & EUDRAGIT RL 100 with different penetration enhancers. CODE AppearanceThickness (mm) Weight (mg)Drug con- tent (mg) S1 Transparent 0.018 26.3 0.302 M1 Transparent 0.017 24.3 0.472 S2 Transparent 0.020 29.1 0.453 M2 Transparent 0.018 27.9 0.879 T1 Transparent 0.017 28.2 0.226 R1 Transparent 0.019 26.2 0.335 T2 Transparent 0.019 32.1 0.430 R2 Transparent 0.021 29.4 0.789 Table 13. Moisture Absorption by PVA and EUDRAGIT RL 100 Patches with Different Penetration Enhancers. 43% RH, RT 75% RH, RT 93% RH, RT CODE Wt. of Patch (Mg) Moist. Ab- sor. (Mg)% Absorption Wt. of Patch (Mg) Moist. Ab- sor. (Mg)%Absorption Wt. of Patch (Mg) Moist. Ab- sor. (Mg) % Absorption S1 26.1 4.5 17.24 27.2 11.9 43.75 28.0 15.0 53.57 S2 28.7 6.2 21.60 28.5 14.1 49.47 28.3 16.8 59.36 T1 28.0 3.1 11.07 28.3 7.4 26.14 27.9 11.2 40.14 T2 31.7 7.1 22.39 31.6 11.8 37.34 32.0 14.6 45.62 M1 24.6 2.1 8.53 24.8 3.9 15.72 24.2 4.8 19.83 M2 27.4 3.6 13.13 27.8 5.4 19.42 27.9 6.7 24.01 R1 26.4 1.8 6.8 26.6 2.6 9.77 26.8 3.5 13.05 R2 29.2 2.9 9.93 29.4 3.7 12.58 29.5 5.1 17.22 3.7. Moisture Absorption 3.7.1. Table 13. Moisture Absorption by PVA and EUDRAGIT RL 100 Patches with Different Penetration Enhancers At 43% RH, RT, the moisture absorption by PVA pa- tches is comparable with patch without the enhancers i.e. J3. The moisture absorption increases with increase in enhancer content. DMSO 10% w/w gives greater ab- sorption than other i.e. 22.39%. It is found that 75% RH, RT the absorption pattern is higher than at 43% RH, RT. At 75% RH, RT maximum absorption is shown by urea 10% w/w with 49.47% absorption & at 95% RH, RT with 59.36%. All humidity condition the absorption in- creases with increase in enhancer content. As the humid- ity increases, there increase in moisture absorption and this increase linear. However, at 43% RH, RT all the patches retain their shape at the end of 7th day, At 75% RH, RT the urea patches lose their shape on 7th day, while DMSO patches a little sticky to touch. At 95% RH, RT the urea patches lose their shape on 4th day while DMSO patches still sticky & lose their shape on 6th day. It is found that around 43% RH, RT, the moisture ab- sorption by EUDRAGIT RL 100 patches is comparable with patch without the enhancers i.e. EB3 urea 10% w/w absorb moisture more than any other. When the condi- tions were of 75% RH, RT and at 95% RH, RT the ab- sorption pattern was found to be higher than at 43% RH, RT At 75% RH, RT maximum absorption is shown by urea 10% w/w with 19.42% absorption & at 95% RH, RT with 24.015. Around all the humidity condition the absorption increases with increase in enhancer content. As the humidity increases, there increase in moisture absorption and this increase linear. At 43% RH, RT all the patches retain their shape at the end of 7th day, At 75% RH, RT the urea patches lose their shape on 6th day, while DMSO patches a little sticky to touch. At 95% RH, RT the urea patches lose their shape on 3rd day while DMSO patches still sticky & lose their shape on 6th day. Therefore amongst the enhancers, the patches of UREA & DMSO are stable for 7 days under different humidity condition. 3.8. In Vitro Drug Permeation through Cellophane Membrane The patches were then subjected to in vitro drug permea- tion through cellophane membrane and the results ob- tained are indicated as below; 3.8.1. Table 14. Drug Permeation from PVA Patches 5 (Mg) with Different Urea Concentration 3.8.2. Figure 7. Effect on Drug Permeation from PVA Patches 5 (Mg) with Different Urea Concentration 3.8.3. Table 15. Drug Permeation from PVA Patches 5 (Mg) with Different DMSO Concentration 3.8.4. Figure 8. Effect on Drug Permeation from PVA Patches 5 (Mg) wi th Different DMSO Concentration The result obtained is compared with patches without 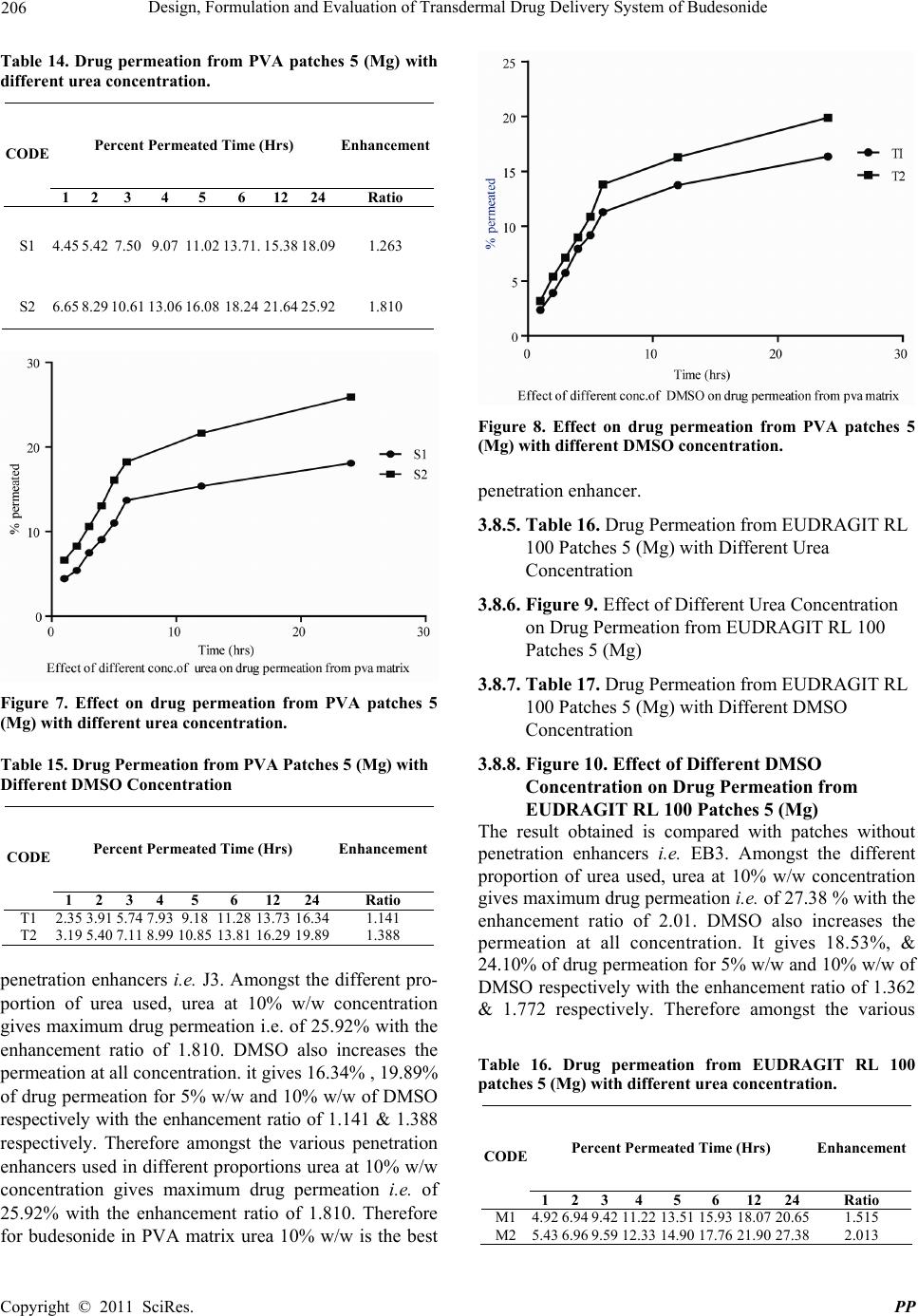 206 Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Table 14. Drug permeation from PVA patches 5 (Mg) with different urea concentration. Percent Permeated Time (Hrs) Enhancement CODE 1 2 3 4 5 6 12 24 Ratio S1 4.45 5.42 7.50 9.07 11.02 13.71. 15.38 18.09 1.263 S2 6.65 8.29 10.61 13.06 16.08 18.24 21.64 25.92 1.810 Figure 7. Effect on drug permeation from PVA patches 5 (Mg) with different urea concentration. Table 15. Drug Permeation from PVA Patches 5 (Mg) with Different DMSO Concentration Percent Permeated Time (Hrs) Enhancement CODE 1 2 3 4 5 6 12 24 Ratio T1 2.35 3.91 5.74 7.93 9.18 11.28 13.73 16.34 1.141 T2 3.19 5.40 7.11 8.99 10.85 13.81 16.29 19.89 1.388 penetration enhancers i.e. J3. Amongst the different pro- portion of urea used, urea at 10% w/w concentration gives maximum drug permeation i.e. of 25.92% with the enhancement ratio of 1.810. DMSO also increases the permeation at all concentration. it gives 16.34% , 19.89% of drug permeation for 5% w/w and 10% w/w of DMSO respectively with the enhancement ratio of 1.141 & 1.388 respectively. Therefore amongst the various penetration enhancers used in different proportions urea at 10% w/w concentration gives maximum drug permeation i.e. of 25.92% with the enhancement ratio of 1.810. Therefore for budesonide in PVA matrix urea 10% w/w is the best Figure 8. Effect on drug permeation from PVA patches 5 (Mg) with different DMSO concentration. penetration enhancer. 3.8.5. Table 16. Drug Permeation from EUDRAGIT RL 100 Patches 5 (Mg) with Different Urea Concentration 3.8.6. Figure 9. Effect of Different Urea Concentration on Drug Permeation from EUDRAGIT RL 100 Patches 5 (Mg) 3.8.7. Table 17. Drug Permeation from EUDRAGIT RL 100 Patches 5 (Mg) with Different DMSO Concentration 3.8.8. Figure 10. Effect of Different DMSO Concentration on Drug Permeation from EUDRAGIT RL 100 Patches 5 (M g) The result obtained is compared with patches without penetration enhancers i.e. EB3. Amongst the different proportion of urea used, urea at 10% w/w concentration gives maximum drug permeation i.e. of 27.38 % with the enhancement ratio of 2.01. DMSO also increases the permeation at all concentration. It gives 18.53%, & 24.10% of drug permeation for 5% w/w and 10% w/w of DMSO respectively with the enhancement ratio of 1.362 & 1.772 respectively. Therefore amongst the various Table 16. Drug permeation from EUDRAGIT RL 100 patches 5 (Mg) with different urea concentration. Percent Permeated Time (Hrs) Enhancement CODE 123456 12 24 Ratio M14.926.949.4211.22 13.51 15.93 18.07 20.65 1.515 M25.436.969.5912.33 14.90 17.76 21.90 27.38 2.013 C opyright © 2011 SciRes. PP 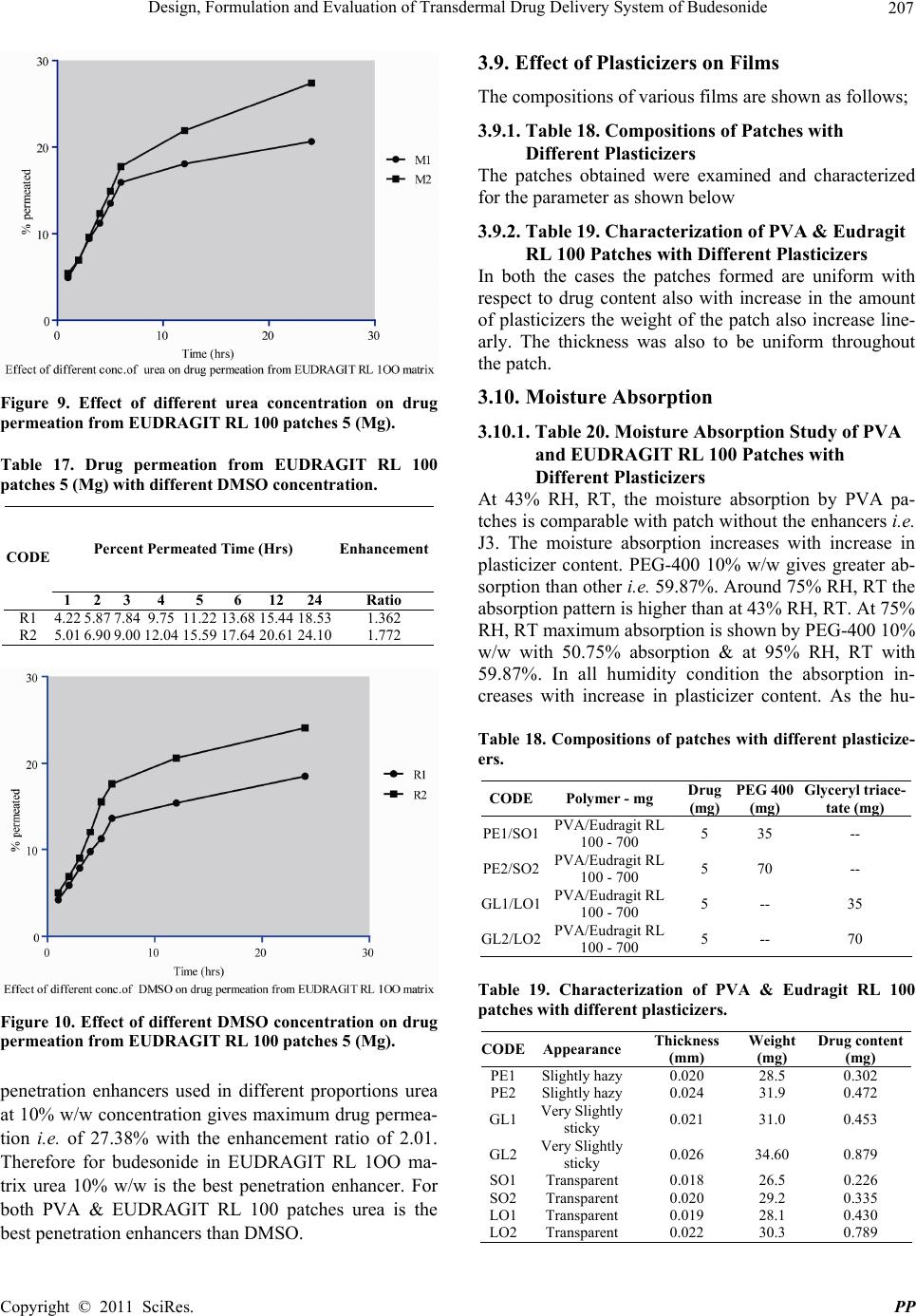 Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide 207 Figure 9. Effect of different urea concentration on drug permeation from EUDRAGIT RL 100 patches 5 (Mg). Table 17. Drug permeation from EUDRAGIT RL 100 patches 5 (Mg) with different DMSO concentration. Percent Permeated Time (Hrs) Enhancement CODE 1 2 3 4 5 612 24 Ratio R1 4.22 5.87 7.84 9.75 11.22 13.68 15.44 18.53 1.362 R2 5.01 6.90 9.00 12.04 15.59 17.64 20.61 24.10 1.772 Figure 10. Effect of different DMSO concentration on drug permeation from EUDRAGIT RL 100 patches 5 (Mg). penetration enhancers used in different proportions urea at 10% w/w concentration gives maximum drug permea- tion i.e. of 27.38% with the enhancement ratio of 2.01. Therefore for budesonide in EUDRAGIT RL 1OO ma- trix urea 10% w/w is the best penetration enhancer. For both PVA & EUDRAGIT RL 100 patches urea is the best penetration enhancers than DMSO. 3.9. Effect of Plasticizers on Films The compositions of various films are shown as follows; 3.9.1. Table 18. Compositions of Patches with Different Plasticizers The patches obtained were examined and characterized for the parameter as shown below 3.9.2. Table 19. Characterizati on of P VA & Eudragit RL 100 Patches with Different Plasticizers In both the cases the patches formed are uniform with respect to drug content also with increase in the amount of plasticizers the weight of the patch also increase line- arly. The thickness was also to be uniform throughout the patch. 3.10. Moisture Absorption 3.10.1. Table 20 . Moi sture Absorption Study of PVA and EUDRAGIT RL 100 Pa tches with Different Plasticizers At 43% RH, RT, the moisture absorption by PVA pa- tches is comparable with patch without the enhancers i.e. J3. The moisture absorption increases with increase in plasticizer content. PEG-400 10% w/w gives greater ab- sorption than other i.e. 59.87%. Around 75% RH, RT the absorption pattern is higher than at 43% RH, RT. At 75% RH, RT maximum absorption is shown by PEG-400 10% w/w with 50.75% absorption & at 95% RH, RT with 59.87%. In all humidity condition the absorption in- creases with increase in plasticizer content. As the hu- Table 18. Compositions of patches with different plasticize- ers. CODEPolymer - mg Drug (mg) PEG 400 (mg) Glyceryl triace- tate (mg) PE1/SO1 PVA/Eudragit RL 100 - 700 5 35 -- PE2/SO2 PVA/Eudragit RL 100 - 700 5 70 -- GL1/LO1 PVA/Eudragit RL 100 - 700 5 -- 35 GL2/LO2 PVA/Eudragit RL 100 - 700 5 -- 70 Table 19. Characterization of PVA & Eudragit RL 100 patches with different plasticizers. CODEAppearance Thickness (mm) Weight (mg) Drug content (mg) PE1Slightly hazy 0.020 28.5 0.302 PE2Slightly hazy 0.024 31.9 0.472 GL1 Very Slightly sticky 0.021 31.0 0.453 GL2 Very Slightly sticky 0.026 34.60 0.879 SO1Transparent 0.018 26.5 0.226 SO2Transparent 0.020 29.2 0.335 LO1Transparent 0.019 28.1 0.430 LO2Transparent 0.022 30.3 0.789 Copyright © 2011 SciRes. PP  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Copyright © 2011 SciRes. PP 208 Table 20. Moisture absorption study of PVA and EUDRAGIT RL 100 patches with different plasticizers. 43% RH, RT 75% RH, RT 93% RH, RT CODE Wt. of Patch (mg) Moist. Absorb. (mg) % absorp- tion Wt. o f Patch (mg) Moist. Absorb. (mg) % absorp- tion Wt. of Patch (mg) Moist. Absorb. (mg) % absorp- tion PE1 28.7 5.1 17.77 28.9 12.8 44.29 28.5 15.4 54.03 PE2 32.4 7.4 22.83 33.1 16.8 50.75 32.9 19.7 59.87 GL1 31.4 4.1 13.05 30.8 8.2 26.62 30.9 13.2 42.71 GL2 34.6 7.2 20.80 34.3 13.5 39.35 34.1 17.2 50.43 SO1 26.2 2.2 8.39 26.7 3.4 12.37 26.5 6.9 26.03 SO2 29.8 3.5 11.74 29.1 6.8 23.36 29.7 9.7 32.65 LO1 28.4 3.5 12.32 29.1 6.9 23.71 28.6 10.8 37.76 LO2 30.2 4.8 15.89 29.8 7.6 25.50 30.6 11.2 36.60 midity increases, there increase in moisture absorption and this increase linear. At 43% RH, RT all the patches retain their shape at the end of 7th day, At 75% RH, RT the PEG-400 patches lose their shape on 7th day, while glyceryl triacetate patches a little sticky to touch. At 95% RH, RT the PEG- 400 patches lose their shape on 5th day while glyceryl triacetate patches still sticky & lose their shape on 6th day. At 43% RH, RT, the moisture absorption by EUDRAGIT RL 100 patches is comparable with patch without the enhancers i.e. EB3. Glyceryl triacetate 10% w/w absorbs moisture more than any other. At 75% RH, RT and at 95% RH, RT the absorption pattern is higher than at 43% RH, RT At 75% RH, RT maximum absorp- tion is shown by glyceryl triacetate 10% w/w with 25.50% absorption & at 95% RH, RT with 36.60%. At all humidity condition the absorption increases with in- crease in enhancer content. As the humidity increases, there increase in moisture absorption and this increase linear. At 43% RH, RT all the PEG-400 patches retain their shape at the end of 7th day, while glyceryl triacetate patches lost their shape on 6th day. At 75% RH, RT the PEG-400 patches lose their shape on 6th day, while glyc- eryl triacetate patches lost their shape on 5th day. At 95% RH, RT the PEG-400 patches lose their shape on 4th day while glyceryl triacetate patches still sticky & lose their shape on 3rd day. Therefore amongst the plas- ticizers, the patches of PEG-400 & glyceryl triacetate are stable for 7 days under different humidity condition, and selected for final formulation of EUDRAGIT RL 100 & PVA respectively. 3.11. In Vitro Drug Permeation 3.11.1. Table 21. Drug Permeation from PVA Patches with Different PEG-400 and Glyceryl Triacetate Concentration 3.11.2. Table 22. Drug Permeation from Eudragit RL 100 Patches with Different PEG-400 and Glyc- eryl Triacetate Concentration 3.11.3. Figure 11. Effect of Different Conc. of PEG-400 on Drug Permeation from PVA Matrix 3.11.4. Figure 12. Effect of Different Conc. of Glyceryl Triacetate on Drug Permeation from PVA Matrix 3.11.5. Figure 13. Effect of Different Conc. of PEG-400 on Drug Permeation from EUDRAGIT RL 100 Matrix 3.11.6. Figure 14. Effect of Different Conc. of Glyceryl Triacetate on Drug Permeation from EUDRAGIT RL 100 Matrix The results are compared with patches without plasticiz- ers i.e. J3. The PVA patches with 5% & 10% w/w of PEG-400, the permeation increased from 16.50% to 19.96%. Similarly with glyceryl triacetate the permeation increased from 17.56% to 23.76 % respectively thus the glyceryl triacetate with 10% gives maximum permeation hence it is selected for final formulation. The results are compared with patches without plasti- cizers i.e. EB3. The EUDRAGIT RL 100 patches with Table 21. Drug permeation from PVA patches with differ- ent PEG-400 and Glyceryl triacetate conce nt r ation. Percent permeated Time in (hrs.) CODE 1 2 3 4 5 6 12 24 PE1 2.394.525.968.20 9.75 11.21 13.8116.50 PE2 3.145.126.829.15 11.30 14.38 17.0719.96 GL1 3.284.946.918.87 10.49 12.64 14.7817.56 GL2 5.017.139.0211.26 13.78 16.40 19.7823.76 Table 22. Drug permeation from Eudragit RL 100 patches with different PEG-400 and Glyceryl triacetate concentra- tion. Percent permeated Time in (hrs.) CODE 1 2 3 4 5 6 12 24 SO1 4.226.068.4610.68 12.71 15.05 17.5320.94 SO2 5.016.298.7911.07 13.72 16.39 20.1924.33 LO1 4.175.647.839.41 11.38 13.89 16.6519.42 LO2 4.826.569.0710.99 13.46 16.30 18.9722.54  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide 209 Figure 11. Effect of different Conc. of PEG-400 on drug permeation from PVA matrix. Figure 12. Effect of different Conc. of Glyceryl Triacetate on drug permeation from PVA matrix. Figure 13. Effect of different Conc. of PEG-400 on drug permeation from EUDRAGIT RL 100 matrix. 5% & 10% w/w of PEG-400, the permeation increased from 20.94% to 24.33% .Similarly with glyceryl triaceta- te the permeation increased from 19.42% to 22.54% resp. thus the PEG-400 with 10% gives maximum permeation Figure 14. Effect of different Conc. of GLYCERYL TRI- ACETATE on drug permeation from EUDRAGIT RL 100 Matrix. hence it is selected for final formulation. 3.12. Final Preparation 3.12.1. Preparation and Evaluation of Patch Using Optimum Concentration Composition of various film prepared is shown in Table 23. 3.12.1.1. Table 23. Composition of Films of PVA and EUDRAGIT RL 100 The patch obtained was studied and their characterization was done and is listed in following Table 24. 3.12.1.2. Table 24. Characterization of PVA and EUDRAGIT RL 100 Patch The patch in each group is uniform in drug content and thickness through the patch. And further In Vitro drug permeation, moisture absorption and WVTR studies were carried out. 3.12.2. Moisture Absorption: Table 25. Moisture Absorption Study of Final PVA and EUDRAGIT RL 100 Table 23. Composition of films of PVA and EUDRAGIT RL 100. CODEPOLYMER (mg)DRUG (Mg) PLASTISIZER (Mg) ENHANCER (Mg) FP-1PVA-700 5 GLY.TRI-70 UREA-70 FE-1 EUDRAGIT RL 100 - 700 5 PEG400- 70 UREA-70 Table 24. Characterization of PVA and EUDRAGIT RL 100 patch. CODEAPPEARANCETHICKNESS WEIGHT (Mg) DRUG CON- TENT (Mg) FP-1Transparent 0.019 33.4 1.971 FE-1Transparent 0.018 31.6 2.174 Copyright © 2011 SciRes. PP  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide Copyright © 2011 SciRes. PP 210 Table 25. Moisture absorption study of final PVA and EUDRAGIT RL 100. 43% RH, RT 75% RH, RT 93% RH, RT CODE Wt. of Patch (mg) Moist. Absorb. (mg) % absorp- tion Wt. o f Patch (mg) Moist. Absorb. (mg) % absorp- tion Wt. of Patch (mg) Moist. Absorb. (mg) % absorp- tion FP-1 34.4 9.8 28.48 34.9 17.9 51.28 34.1 21.3 62.46 FE-1 31.9 6.7 21.01 31.7 12.8 40.37 32.1 15.9 49.53 Table 26. WVTR of PVA and Eudragit RL-100 patches. 43% RH, RT 75% RH, RT CODE Wt. of Patch (mg) Moist. Absorb. (mg) % absor ptionWt. of Patch (mg) M oi st. Absorb. (mg) % absorption FP-1 0.0021 0.020 0.217 0.0151 0.022 0.882 FE-1 0.0391 0.017 12.76 0.145 0.018 18.86 Table 28. In-Vivo drug permeation from EUDRAGIT RL 100 patches. 3.12.3. Water Vapor Transmi ssi on Ra te Study (WVTR): Table 26. WVTR of PVA and Eudragit RL-100 Patches Percent permeated Time ( hrs) CODE 1 2 3 4 5 6 12 24 FE-110.9414.25 17.60 20.85 24.26 27.71 32.07 37.25 At all humidity condition the absorption increases. As At all humidity condition the absorption increases. As the humidity increases, there is a increase in moisture ab- sorption and this increases linearly. The patch FP-1 gives maximum absorption at 93% RH, RT i.e. 62.46 than any other. At 43% RH, RT the PVA patches retain their shape at the end of 7th day, At 75% RH, RT the PVA patches lose their shape on 7th day, At 95% RH, RT the PVA patches lose their shape on 5th day. 3.12.4. Drug Permeation through Cellophane Membrane 3.12.4.1. Table 27. In-Vitro Drug Permeation from EUDRAGIT RL 100 Patches 3.12.4.2. Table 28. In-Vivo Drug Permeation from EUDRAGIT RL 100 Patches 3.12.4.3. Figure 15. Drug Permeation of Patch FP-1 3.12.4.4. Figure16. Drug Permeation of Patch Fe-1 At all humidity condition the absorption increases. As the humidity increases, there increase in moisture ab- sorption and this increase linearly. The patch FE-1 gives maximum absorption at 93% RH, RT i.e. 49.53% than any other. At 43% RH, RT the EUDRAGIT RL 100 Figure 15. Drug Permeation of Patch FP-1. Table 27. In-Vitro drug permeation from EUDRAGIT RL 100 patches. Percent permeated Time ( hrs) CODE 1 2 3 4 5 6 12 24 FE-1 10.94 14.25 17.60 20.85 24.26 27.71 32.07 37.25 Figure 16. Drug Permeation of Patch Fe-1.  Design, Formulation and Evaluation of Transdermal Drug Delivery System of Budesonide 211 patches lost their shape at the end of 7th day, At 75% RH, RT the EUDRAGIT RL 100 patches lose their shape on 6th day, At 95% RH, RT the EUDRAGIT RL 100 patches lose their shape on 4th day. In PVA and EUDRAGIT RL 100 patches the water vapor transmis- sion rate was found to be higher at 75% RH, RT condi- tions. Therefore at both % RH, RT condition the PVA and EUDRAGIT RL 100 patches provides the best resis- tance to water vapor. Therefore, when applied to animals (in further studies) these patches may provide more oc- clusion to water vapor loss from skin thus making at- mosphere beneath the skin more humid that aid in drug permeation. 4. References [1] B. J. Unde, “Pharmacotherapy of Asthma,” In: L. L. Brunton, J. S. Lazo and K. L. Parker, Eds., Goodman & Gilman’s, The Pharmacological Basis of Therapeutics, Mcgraw-Hill Medical Publishing Division, New York, 2006, pp. 897-934. [2] C. M. Spencer and D. McTavish, “Budesonide: A Review of Its Pharmacological Properties and Therapeutic Effi- cacy in Inflammatory Bowel Disease,” Drugs, Vol. 50, No. 5, 1995, pp. 854-872. doi:10.2165/00003495-199550050-00006 [3] R. N. Brogden and D. McTavish, “Budesonide: An Up- dated Review of Its Pharmacological Properties, and Therapeutic Efficacy in Asthma and Rhinitis,” Drugs, Vol. 44, 1998, pp. 375-407. doi:10.2165/00003495-199244030-00007 [4] G. Jeffrey, et al., “Have Done Budesonide Enema for the Treatment of Active, Distal Ulcerative Colitis and Procti- tis: A Dose Ranging Study,” Current Therapy, Vol. 22, 2005, pp. 23-27. [5] N. Mygind and T. J. H. Clark, “Topical Steroid Treat- ment for Asthma and Rhinitis,” B. Tindall Inc., London, 1980, pp. 89, 91,152,159,172. [6] K. Masuyama, et al., “Glucocorticosteroid (Fluticasone propionate) Inhibits Cells Expressing Cytokine mRNA for Interleukin-4 in the Nasal Mucosa in Allergen-In- duced Rhinitis,” Immunology, Vol. 82, 1994, pp. 192- 199. [7] N. S. Chandrashekar and R. H. S. Rani, “Design, Fabrica- tion and Calibration of Modified Franz Diffusion Cell for Transdermal Diffusion Studies,” International Journal of Pharmaceutical Excipients, October-Novem- ber 2005, pp. 104-106. [8] J. Eliassaf, “Detection of Small Quantity of Poly(Vinyl Alcohol) in Poly(Vinyl Chloride) Resins,” Polymer Let- ters, Vol. 16, 1972, pp. 225-235. [9] S. P. Gupta and S. K. Jain, “Development of Matrix- Membrane Transdermal Drug Delivery System for At- enolol,” Drug Delivery, Vol. 11, No. 5, 2004, pp. 281- 286. doi:10.1080/10717540490493943 [10] P. R. P. Verma and S. S. Iyer, “Transdermal Delivery of Propranolol Using Mixed Grades of Eudragit: Design and in Vitro and in Vivo Evaluation,” Drug Development and Industrial Pharmacy, Vol. 26, No. 4, 2000, pp. 471-476. doi:10.1081/DDC-100101257 [11] R. Krishna and J. K. Pandit, “Transdermal Delivery of Propranolol,” Industrial Pharmacy, Vol. 20, No. 15, 1994, pp. 2459- 2465. [12] P. Arora and B. Mukherjee, “Design, Development, Physicochemical and in Vitro and in Vivo Evaluation of Transdermal Patches Containing Diclofenac Diethylam- monium Salt,” Journal of Pharmaceutical Sciences, Vol. 91, 2002, pp. 2076-2089. doi:10.1002/jps.10200 [13] United States Pharmacopoeia, “Physical Tests <711> Dissolution,” Vol. 24, 2000, pp. 1941. [14] M. Siewert, J. Dressman, C. K. Brown and V. P. Shah, “FIP/AAPS Guidelines to Dissolution/in Vitro Release Testing of Novel/Special Dosage Forms,” AAP Spharm- scitech, Vol. 4, No. 1, 2003, pp. 1-10. [15] C. Valenta and B. G. Auner, “The Use of Polymers for Dermal and Transdermal Delivery,” European Journal of Pharmaceutical Sciences, Vol. 58, No. 2, 2004, pp. 279- 289. [16] P. Costa, “Modeling and Comparison of Dissolution Pro- files,” European Journal of Pharmaceutical Sciences, Vol. 13, No. 2, 2001, pp. 123-133. doi:10.1016/S0928-0987(01)00095-1 [17] J. Singh and H. I. Maibach, “Irritancy of Topical Chemi- cals and Transdermal Delivery Systems,” Drug Pharma- ceutical Sciences, Vol. 1, 2001, pp. 281-296. [18] A. C. Calpena, E. Escribano and H. San Martin, “Influ- ence of Formulation on the in Vitro Transdermal Penetra- tion of Sodium Diclofenac,” Arzneimittle Forschung Drug Research, Vol. 49, No. 11, 1999, pp. 1012-1017. Copyright © 2011 SciRes. PP
|