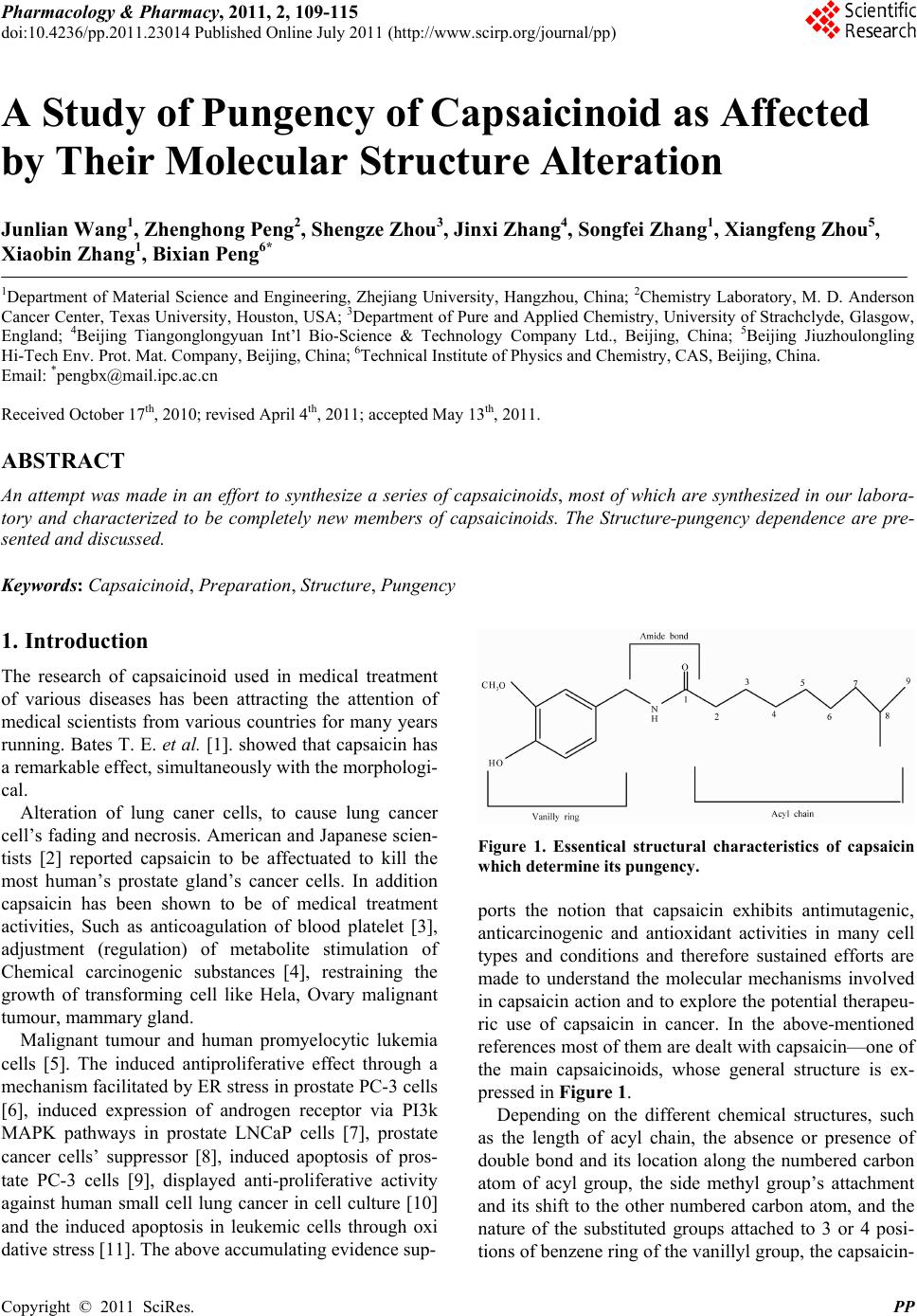
 Pharmacology & Pharmacy, 2011, 2, 109-115 doi:10.4236/pp.2011.23014 Published Online July 2011 (http://www.scirp.org/journal/pp) Copyright © 2011 SciRes. PP 109 A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration Junlian Wang1, Zhenghong Peng2, Shengze Zhou3, Jinxi Zhang4, Songfei Zhang1, Xiangfeng Zhou5, Xiaobin Zhang1, Bixian Peng6* 1Department of Material Science and Engineering, Zhejiang University, Hangzhou, China; 2Chemistry Laboratory, M. D. Anderson Cancer Center, Texas University, Houston, USA; 3Department of Pure and Applied Chemistry, University of Strachclyde, Glasgow, England; 4Beijing Tiangonglongyuan Int’l Bio-Science & Technology Company Ltd., Beijing, China; 5Beijing Jiuzhoulongling Hi-Tech Env. Prot. Mat. Company, Beijing, China; 6Technical Institute of Physics and Chemistry, CAS, Beijing, China. Email: *pengbx@mail.ipc.ac.cn Received October 17th, 2010; revised April 4th, 2011; accepted May 13th, 2011. ABSTRACT An attempt was made in an effort to synthesize a series of capsaicinoids, most of which are synthesized in our labora- tory and characterized to be completely new members of capsaicinoids. The Structure-pungency dependence are pre- sented and discussed. Keywords: Capsaicinoid, Preparation, Structure, Pungency 1. Introduction The research of capsaicinoid used in medical treatment of various diseases has been attracting the attention of medical scientists from various countries for many years running. Bates T. E. et al. [1]. showed that capsaicin has a remarkable effect, simultaneously with the morphologi- cal. Alteration of lung caner cells, to cause lung cancer cell’s fading and necrosis. American and Japanese scien- tists [2] reported capsaicin to be affectuated to kill the most human’s prostate gland’s cancer cells. In addition capsaicin has been shown to be of medical treatment activities, Such as anticoagulation of blood platelet [3], adjustment (regulation) of metabolite stimulation of Chemical carcinogenic substances [4], restraining the growth of transforming cell like Hela, Ovary malignant tumour, mammary gland. Malignant tumour and human promyelocytic lukemia cells [5]. The induced antiproliferative effect through a mechanism facilitated by ER stress in prostate PC-3 cells [6], induced expression of androgen receptor via PI3k MAPK pathways in prostate LNCaP cells [7], prostate cancer cells’ suppressor [8], induced apoptosis of pros- tate PC-3 cells [9], displayed anti-proliferative activity against human small cell lung cancer in cell culture [10] and the induced apoptosis in leukemic cells through oxi dative stress [11]. The above accumulating evidence sup- Figure 1. Essentical structural characteristics of capsaicin which determine its pungency . ports the notion that capsaicin exhibits antimutagenic, anticarcinogenic and antioxidant activities in many cell types and conditions and therefore sustained efforts are made to understand the molecular mechanisms involved in capsaicin action and to explore the potential therapeu- ric use of capsaicin in cancer. In the above-mentioned references most of them are dealt with capsaicin—one of the main capsaicinoids, whose general structure is ex- pressed in Figure 1. Depending on the different chemical structures, such as the length of acyl chain, the absence or presence of double bond and its location along the numbered carbon atom of acyl group, the side methyl group’s attachment and its shift to the other numbered carbon atom, and the nature of the substituted groups attached to 3 or 4 posi- tions of ben zene ring of the v anillyl group, the capsaicin-  110 A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration noids may differ and display greatly in their pungency, probably hence the susceptible curative dose and the finally optimized treatment effect. In present study an attempt was made in an effort to synthesize a series of capsaicinoids, some of which (No. 2, 3 and 7 in Table 1 .) had been shown [12-14] to be known—either extracted and separated from the natural pepper or prepared by artificial synthesis from chemical laboratoryes, the others of which are synthesized in our laboratory and charac- terized to be completely new members of capsaicinoids. The Structure-pungency dependence are presented and discussed. 2. Experiment 2.1. Synthesis The synthesis of 2 main intermediates, one is vanillyla- mine designated as I-1 in Equation (1), the other is cor- responding acid chloride designated as I-2 in Equation (1) and used for capsaicinoid-making is conducted in a simi- lar manner as described in our newly approved patent [15] with some modification in choice of appropriate bromine —substituted alkanes as starting raw material by reacting the corresponding acid chlorides (in dry ether) with a suspension of dry vanillylamine in dry ether under an atmosphere of nitrogen as indicated by the following Equation (1) being example for the capsaicin analogues whose molecular have a double bond in the acyl chain. In case of hydrocapsaicin analogues an unique differ- ence is to use the corresponding saturated fatty acid chlo- ride to replace the unsaturated ones. The structure for- mula characterized by element analysis. IR. HNMR are listed in Appendix 1. 2.2. Pungency Determination [16] Scoville’s sensory assessment method is widely acknow- ledged domestically and internationally to be used to determine the pungency of capsaicinoid. The basic principle of this method is based on 1) the first step is to prepare and purify a capsaicinoid (extract or artificial product), 2) an extract or product is weighed accurately (in general 0.050 g), 3) To dilute a solution of weighed sample with sucrose aquatic solution continu- ously and stepwise by multifold to an appropriate ex- treme degree by which the pungency of a capsaicinoid can be just tasted by sensory organs of individual’s group. 4) the experimentally found diluted limit (extreme dilu- tability) is considered to be scoville Index, expressed as Scoville Heat Unit (simply SHU). The pungency of all the capsaicinoids synthesized in present study are deter- mined in Nutrition and Food Safety Detection Centre of Hunan Agricultural University, Changsha city, Hunan, China. 3. Results and Discussion All the chemical structural formula of capsaicinoids are listed in Table 1. The experimental data needed to char- acterize individual capsaicinoid such as melting point, elemental analysis HNMR.CNMR, are summarized in Appendix 1. The determined value of capsaicinoid’s pungency expressed as SHU are collected in the last column of Table 1 for systematic comparisonas well. 3.1. Synthesis From data listed in Appendix 1, it is shown that the ex- perimentally found data of elemental analysis for each capsaicinoid is coincided with the calculated ones for C.H.N content (%) within an allowed deviation. HNMR data for each capsaicinoid are in good agreement with the assigned chemical Shift typical for each functionalized groups contained in new compound s involved. All th e above-stated figur es tell in flavour that the synthesis of all the members of capsaicinoids we have developed in present study is to be successful. 3.2. Pungency-Structure Dependence A group of systematic and comparative results listed in Table 1 and regarding the pungency—structure depen- dence, permitted us to reveal the following basic laws governing the heading of pungency alternation. Firstly among the many factors affecting the pungency of capcaicinoids, the mutuality of hydroxy and methoxy attached to benzene ring of vanilly group have a decisive influence on the pungency, which is clearly demonstrated by comparing the structure and pungency of capsaicinoid No. 10 as one positive side with that of No. 8 and 9 as opposite side. Keeping methoxy group to be unchanged but eliminating the hydroxy (case No. 8) lead to remark= able dropping of the pungency of No.10, leaving only one third of the originol pungency (1.6 > 0.6 × 106 SHU) alternatively sustaining hydroxy but omitting methoxy enable the pungency continuously dropped to be zero, high lighting the 1-st extreme importance of methoxy and hydroxy mutuality, neither of two groups can be dispensed with, Neith er should be overemphasized at the xpense to the neglect of the other. The two groups e C opyright © 2011 SciRes. PP 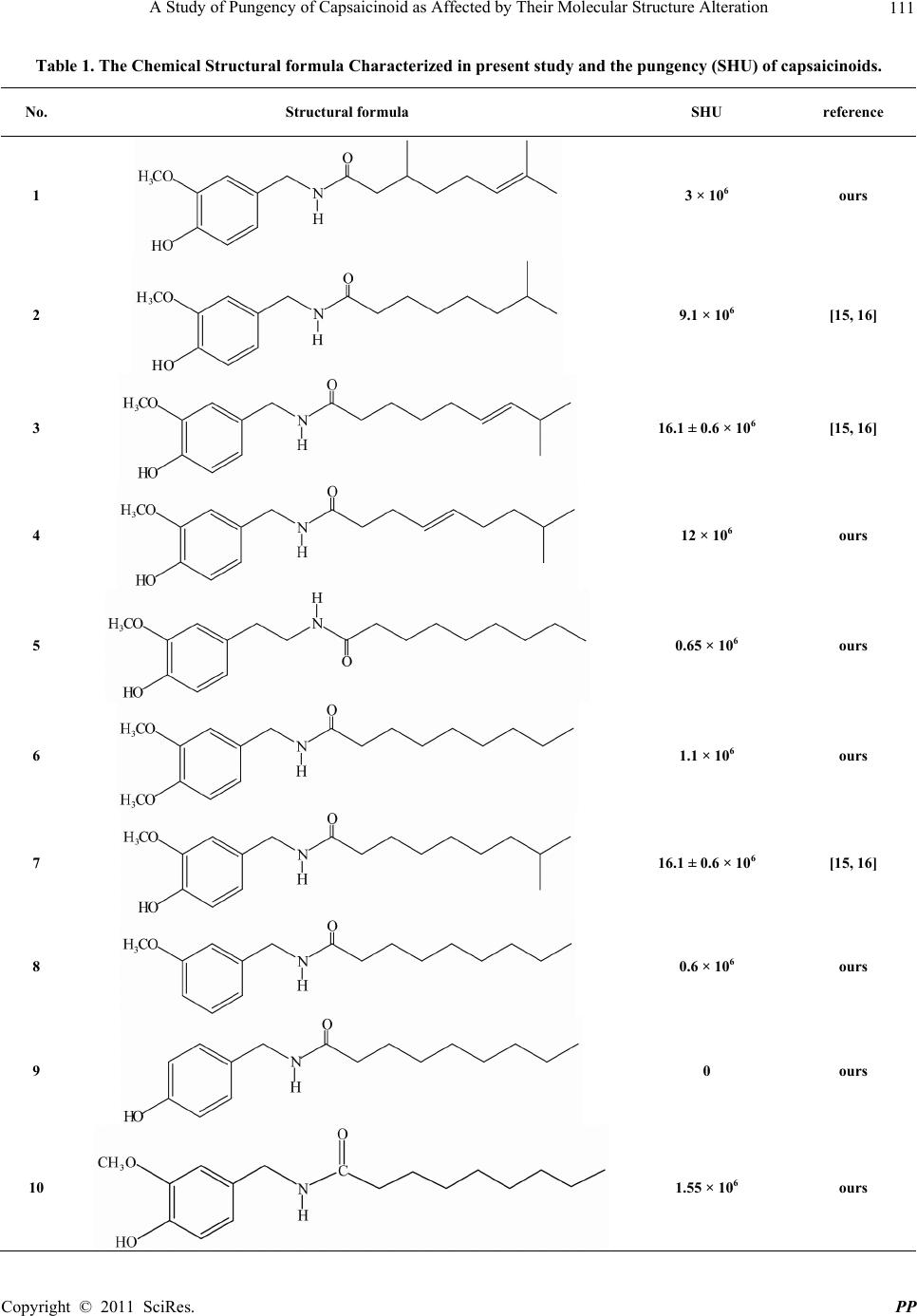 A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration Copyright © 2011 SciRes. PP 111 Table 1. The Chemical Structural formula Charac terized in present study and the pungency (SHU) of capsaicinoids. No. Structural formula SHU reference 1 3 × 106 ours 2 9.1 × 106 [15, 16] 3 16.1 ± 0.6 × 106 [15, 16] 4 12 × 106 ours 5 0.65 × 106 ours 6 1.1 × 106 ours 7 16.1 ± 0.6 × 106 [15, 16] 8 0.6 × 106 ours 9 0 ours 10 1.55 × 106 ours  112 A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration depend on each other for existence, restrict and interact each other to give full play to the pungency functionality. Secondly, one of the major factors affecting the pun- gency is the length of acyl chain. Neither too long, for example, chain with more than 9 carbon atoms, nor too short for instance, chain with less than 9 carbon atoms can promote to further upgrade the pungency of capsai- cin (case No. 7) and dihydrocapsaicin (case No. 3),whose acyl chain is constituted just from 9 carbon atoms. Thirdly the methyl group attached to the 8- numbered carbon atom is concluded not to be dispensable, hence might be nonessential or neglectful. Let us have a paral- lel comparison on the pungency of two members of cap- saicinoid, one is norddihydrocapsaicin (case No. 7), whose pungency is 12.1 ± 0.6 × 106, the other is (E)-N-vanilly- (4-hydroxy-3-methoxy benzyl-) nonylamide (case No. 10), whose pungency is declined as low as 1.5 × 106 SHU, being one of eighth of the original pungency of capsaicinoid No. 7. Fourthly It has been known that capsaicin and dihy- drocapsaicin differ from each other in the presence(case No. 3) and absence (case No. 7) of do uble bond at the 8 - numbered carbon atom, such a structural distinction failed to create a difference in pungency. However it doesn’t imply that it is of secondary or even no signifi- cance for a factor of double bond. Nothing in chemistry would is invariable. If a double bond’s locatio n is shifted and headed for th e dire ction toward the amide planarity, for example, from 6-nonen-(case No. 3) to 4-nonen am- ide (case No. 4), the pungency value had been lowered from 16.1 × 106 SHU for capsaicinoid No. 3 to 12 × 106 SHU for No. 4. Finally the effect of Carbon atom number by which the benzene ring and amide plane is separated should also considered to be not ignored, which can be clearly seen when the carbon atom number is increased from one (case No. 10) to two (case No. 5). In response to such a increased carbon atom (-CH2-), the pungency is Less- ened from 1.5 × 106 (Case 10) to 0.65 × 106 SHU. 4. Summary 1) A series of capsaicinoids with different structures have been synthesized and characterized. 2) The pungency of different capsaicinoids has been determined by sensory method expressed as SHU. 3) The pungency is found to be very sensitive an d de- pendent upon the molecular structure alteration occurred within the capsaicinoids. 4) The structure pungency dependence discovered in present study has provided a preliminary basis to further study the structure-curative effect dependence of capsai- cinoids with respect to various cancer cells and dis- eases. 5. References [1] A. Athanasiou, T. E. Bates, et al., “Vanilloid Receptor Agonists and Antagonists Are Mitochon-Drial Inhibitors: How Vanilloids Causenon-Vanilloid Receptor Mediated Cell Death,” Biochemcal and Biophysical Research Communications, Vol. 354, No. 1, 2007, pp. 50-55. doi:10.1016/j.bbrc.2006.12.179 [2] A. Mori, S. Lehmann, J. O’kelly et al., “Capsaicin, a Component of Red Peppers, Inhibits the Growth of An- dro-Gen-Independent, p53 Mutant Prostate Cancer Cells,” Cancer Research, Vol. 66, No. 6, March 2006, pp. 3222-3229. doi:10.1158/0008-5472.CAN-05-0087 [3] S. Y. Choi, H. Ha and K. T. Kim, “Capsaicin Inhibits Platelet—Activating Factor—Induced Cytosolic Ca2+ Rise and Superoxide Production,” The Journal of Immu- nology, Vol. 165, No. 7, October 2000, pp. 3992-3998. [4] D. J. Morre, A. Caldwell, A. Mgyorga, et al., “NADA Oxidase Activity from Sera Altered by Capsaicin Is Widely Distributed among Cancer Patients,” Archives of Biochemistry and Biophysics, Vol. 342, No. 2, 1997, pp. 224-230. doi:10.1006/abbi.1997.0110 [5] G. Galati and P. L. O’Brien, “Cytoprotective and Anti- cancer Properties of Coenzyme Quersus Capsacic,” Bio- factors, Vol. 18, No. 1-4, 2003, pp. 195-205. doi:10.1002/biof.5520180222 [6] A. M. Sanchez, J. Martin ez-Botas, I. Di az-Laviada , et al., “Induction of the Endoplasmic Reticulum Stress Protein GADD153/CHOP by Capsaicin in Prostate PC-3 Cell: A Microarray Study,” Biochemical and Bio-Physical Re- search Communications, Vol. 372, No. 4, 2008, pp. 785- 791. doi:10.1016/j.bbrc.2008.05.138 [7] S. Malagarie-Cazenave, N. Olea-Herrero, I. Diaz-Laviada, et al., “Capsaicin, a Component of Red Peppers, Induces Expression of Androgen Receptor via PI3K and MAPK Pathways in Prostate LNCaP Cells,” FEBS Letters, Vol. 583, No. 1, 2009, pp. 141-147. doi:10.1016/j.febslet.2008.11.038 [8] I. Diaz-Laviada, “Effect of Capsaicin on Prostate Cancer Cells,” Future Oncology, Vol. 6, No. 10, 2010, pp. 1545- 1550. doi:10.2217/fon.10.117 [9] A. M. Sanchez, S. Malagarie-Cazenave, I. Diaz-Laviada et al., “Apoptosis Induced by Capsaicin in Prostate PC-3 Cell Involves Ceramide Accumulation, Neutral Sphin- gomyelinase, and JNK Activation,” Apoptosis, Vol. 12, No. 11, 2007, pp. 2013-2024. doi:10.1007/s10495-007-0119-z [10] K. C. Brown, T. R. Witte, P. Dasgupta, et al., “Capsaicin Displays Anti-Proliferative Activity against Human Small Cell Lung Cancer in Cell Culture and Nude Mice Models via the E2F Pathway,” PloS ONE, Vol. 5, No. 4, 2010, pp. 1-15. doi:10.1371/journal.pone.0010243 [11] K. Ito, T. Nakazato, M. Kizaki, et al., “Induction of Apoptosis in Leukemic Cell by Homovanillic Acid De- rivative, Capsaicin, through Oxidative Stress: Implication of Phosphorylation of p52 at Ser-15 Residue by Reactive Oxygen Species,” Cancer Research, Vol. 64, 2004, pp. 1071-1078. doi:10.1158/0008-5472.CAN-03-1670 C opyright © 2011 SciRes. PP  A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration 113 [12] P. M. Gannett , D. L. Na gel, P. J. Re illy, et al., “The Cap- saicinoids: Their Separation, Synthesis, and Mutagenic- ity,” Journal of Organic Chemistry, Vol. 53, No. 5, 1988, pp. 1064-1071. doi:10.1021/jo00261a047 [13] H. Kaga, M. Masakatsu and K. Orito, “A Facile Proce- dure of Capsaicin,” Journal of Organic Chemistry, Vol. 54, No. 14, 1989, pp. 3477-3478. doi:10.1021/jo00275a040 [14] S.-Z. Zhou, X.-F. Zhou and B.-X. Peng, “A Novel Syn- thesis Method of Capsaicinoids,” CN-2007100877050. [15] W. Scoville, “Note on Capsicums,” The Journal of the American Pharmacists Association, Vol. 1, No. 5, 1912, pp. 453-454. [16] D. R. Trainer and A. T. Grenis, “Spices and Seasonings, a Food Technology Handbook,” 2nd Edition, Wiley IEEE, Hoboken, 2001. Copyright © 2011 SciRes. PP 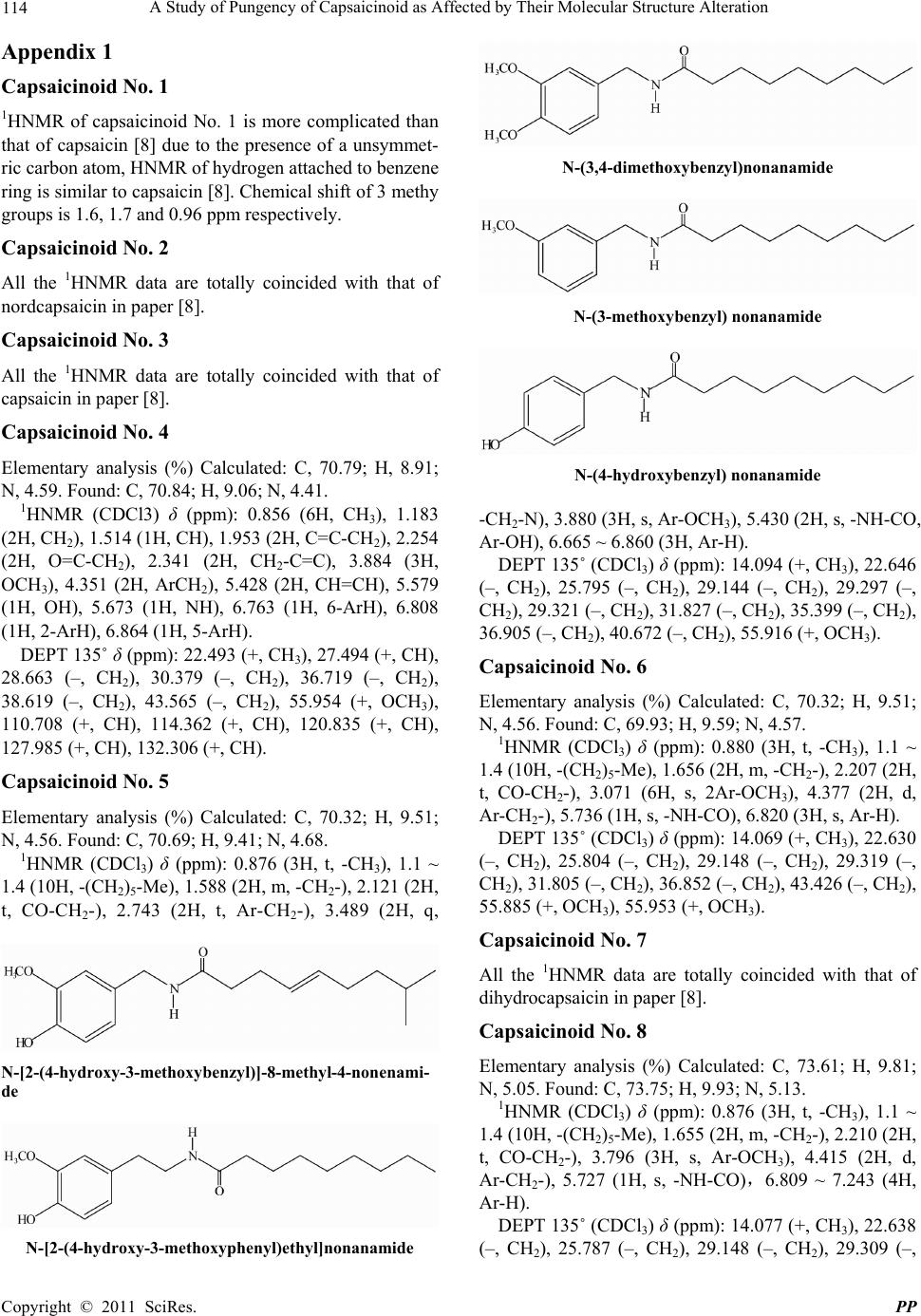 A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration Copyright © 2011 SciRes. PP 114 Appendix 1 Capsaicinoid No. 1 1HNMR of capsaicinoid No. 1 is more complicated than that of capsaicin [8] due to the presence of a unsymmet- ric carbon atom, HNMR of hydrogen attached to benzene ring is similar to capsaicin [8]. Chemical shift of 3 methy groups is 1.6, 1.7 and 0.96 ppm respectively. Capsaicinoid No. 2 All the 1HNMR data are totally coincided with that of nordcapsaici n i n p aper [8]. Capsaicinoid No. 3 All the 1HNMR data are totally coincided with that of capsaicin in paper [8]. Capsaicinoid No. 4 Elementary analysis (%) Calculated: C, 70.79; H, 8.91; N, 4.59. Found: C, 70.84; H, 9.06; N, 4.41. 1HNMR (CDCl3) δ (ppm): 0.856 (6H, CH3), 1.183 (2H, CH2), 1.514 (1H, CH), 1.953 (2H, C=C-CH 2), 2 .254 (2H, O=C-CH2), 2.341 (2H, CH2-C=C), 3.884 (3H, OCH3), 4.351 (2H, ArCH2), 5.428 (2H, CH=CH), 5.579 (1H, OH), 5.673 (1H, NH), 6.763 (1H, 6-ArH), 6.808 (1H, 2-ArH) , 6.864 (1H, 5-ArH) . DEPT 135˚ δ (ppm): 22.493 (+, CH3), 27.494 (+, CH), 28.663 (–, CH2), 30.379 (–, CH2), 36.719 (–, CH2), 38.619 (–, CH2), 43.565 (–, CH2), 55.954 (+, OCH3), 110.708 (+, CH), 114.362 (+, CH), 120.835 (+, CH), 127.985 (+, CH), 132.306 (+, CH). Capsaicinoid No. 5 Elementary analysis (%) Calculated: C, 70.32; H, 9.51; N, 4.56. Found: C, 70.69; H, 9.41; N, 4.68. 1HNMR (CDCl3) δ (ppm): 0.876 (3H, t, -CH3), 1.1 ~ 1.4 (10H, -(CH2)5-Me), 1.588 (2H, m, -CH2-), 2.121 (2H, t, CO-CH2-), 2.743 (2H, t, Ar-CH2-), 3.489 (2H, q, N-[2-(4-hydroxy-3-methoxybenzyl)]-8-methyl-4-nonenami- de N-[2-(4-hydroxy-3-methoxyphenyl)ethyl]nonanamide N-(3,4-dimethoxybenzyl)nonanamide N-(3-methoxybenzyl) nonanamide N-(4-hydroxybenzyl) nonanamide -CH2-N), 3.880 (3H, s, Ar-OCH3), 5.430 (2H, s, -NH-CO, Ar-OH), 6.665 ~ 6.860 (3H, Ar-H). DEPT 135˚ (CDCl3) δ (ppm): 14.094 (+, CH3), 22.646 (–, CH2), 25.795 (–, CH2), 29.144 (–, CH2), 29.297 (–, CH2), 29.321 (– , CH2), 31 .827 ( –, CH2) , 35.399 (– , CH2), 36.905 (–, CH2), 40.672 (–, CH2), 55.916 (+, OCH3). Capsaicinoid No. 6 Elementary analysis (%) Calculated: C, 70.32; H, 9.51; N, 4.56. Found: C, 69.93; H, 9.59; N, 4.57. 1HNMR (CDCl3) δ (ppm): 0.880 (3H, t, -CH3), 1.1 ~ 1.4 (10H, -(CH2)5-Me), 1.656 (2H, m, -CH2-), 2.207 (2H, t, CO-CH2-), 3.071 (6H, s, 2Ar-OCH3), 4.377 (2H, d, Ar-CH2-), 5.7 3 6 (1 H, s, -N H-CO), 6.820 (3H, s, Ar-H). DEPT 135˚ (CDCl3) δ (ppm): 14.069 (+, CH3), 22.630 (–, CH2), 25.804 (–, CH2), 29.148 (–, CH2), 29.319 (–, CH2), 31.805 (– , CH2), 36 .852 ( –, CH2) , 43.426 (– , CH2), 55.885 (+, OCH3), 55.953 (+, OCH3). Capsaicinoid No. 7 All the 1HNMR data are totally coincided with that of dihydrocapsaicin in paper [8]. Capsaicinoid No. 8 Elementary analysis (%) Calculated: C, 73.61; H, 9.81; N, 5.05. Found: C, 73.75; H, 9.93; N, 5.13. 1HNMR (CDCl3) δ (ppm): 0.876 (3H, t, -CH3), 1.1 ~ 1.4 (10H, -(CH2)5-Me), 1.655 (2H, m, -CH2-), 2.210 (2H, t, CO-CH2-), 3.796 (3H, s, Ar-OCH3), 4.415 (2H, d, Ar-CH2-), 5.727 (1H, s, -NH-CO),6.809 ~ 7.243 (4H, Ar-H). DEPT 135˚ (CDCl3) δ (ppm): 14.077 (+, CH3), 22.638 (–, CH2), 25.787 (–, CH2), 29.148 (–, CH2), 29.309 (–,  A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration 115 CH2), 29.328 (– , CH2), 31 .807 ( –, CH2) , 36.832 (– , CH2), 43.557 (–, CH2), 55.232 (+, OCH3). Capsaicinoid No. 9 Elementary analysis (%) Calculated: C, 72.97; H, 9.57; N, 5.32. Found: C; H; N. 1HNMR (CDCl3) δ (ppm): 0.876 (3H, t, -CH3), 1.1 ~ 1.4 (10H, -(CH2)5-Me), 1.646 (2H, m, -CH2-), 2.202 (2H, t, CO-CH2-), 4. 359 (2H, d, Ar-CH2-). DEPT 135˚ (CDCl3) δ (pp m): 14.081 (+, CH3), 22.629 (–, CH2), 25.774 (–, CH2), 29.127 (–, CH2), 29.258 (–, CH2), 29.273 (– , CH2), 31 .794 ( –, CH2) , 36.856 (– , CH2), 43.268 (–, CH2). Capsaicinoid No. 10 Except methyl group at 8-numbered carbon atom for No.7 all the 1HNMR data are totally coincided with that of dihydrocapsaicin in paper [8]. Copyright © 2011 SciRes. PP
|