 Open Journal of Marine Science, 2011, 1, 50-68 doi:10.4236/ojms.2011.12006 Published Online July 2011 (http://www.SciRP.org/journal/ojms) Copyright © 2011 SciRes. OJMS Scleractinian Corals and Reefs of Vietnam as a Part of th e Pacific Reef Ecos ys tem Yuri Ya Latypov A. V. Zhirmunsky Institute of Marine Biology, Far East Branch of the Rus si an Aca demy of Sci e nces, Vladivostok, Russia E-mail: ltpv@mail.ru Received May 12, 2011; revised June 1, 2011; accepted June 10, 2011 Abstract The paper analyzes both published and unpublished results of the investigations of Vietnamese reef building corals and reefs performed in the last decades of the twentieth century and first decades twenty-first. The state of the art in the study of reef-building scleractinian corals and reefs is presented. The scleractinian fauna of Vietnam is shown to match in species diversity (350 species of 80 genera) the tropical coral fauna of the Indonesian–Malacca fertile center, from which Indo-Pacific reef-building corals originated. The whole Vietnam coast from the Gulf of Tonkin to the Gulf of Siam is a biogeographically single whole and is a part of the Indo-Polynesian Province of the Indo-Pacific Area. Keywords: Vietnam, Reefs, Reef-Building Corals, Pacific Reef Ecosystem 1. Introduction 1.1. Brief the Ecological Characteristic and a History of Studying The coastline of Vietnam is over 3200 km long and cov- ers 15 degrees in latitude, from the Gulf of Siam in the south (8°N) to the Chinese border in the north (23°N). The near shore water area (up to 50-m deep) of Vietnam, including some 3000 islands, is about 206,000 km2. Vietnam and its coastline are divided into 5 parts, the Gulf of Tonkin, Central and Southern Vietnam, Gulf of Siam, and Spratly Islands [1]. Reef-building corals and reef accumulations are confined to hard grounds, typical of the Vietnam coast. Between 16° and 19°N, the coast- line is formed mostly by moving sand with a minor presence of hard substrates. The temperature varies be- tween 18˚C - 32˚C, and the salinity, 28‰ - 40‰. One hundred and fourteen rivers are registered along the coastline. The spread of the reef is limited near the mouths of two large rivers, the Red River in the north and the Mekong in the south, due to adverse conditions. The ecosystems of the coral reefs of Vietnam feature high bioproductivity, with a primary production of up to 30 - 100 mg·C/m3 per day, which is almost 100 times that in open waters [2,3]. Vietnam is situated in the tropics, affected by two sorts of monsoons: the wet southwest, lasting from May till September, and the dry northeast, occurring in Octo- ber-April. Heavy rain showers during the wet monsoon period result in a huge (5 - 400 million m3) freshwater influx and a substantial (up to 200 thous. tons) terri- genous sediment influx into the sea. The daily suspended matter precipitation rate in the reef reaches 70 - 100 g/m2 and increases tenfold during typhoons [4,5]. This results in a remarkable decrease in water transparency, affecting, together with other factors, the development of coral settlement in this region. The reef-building corals and reefs of Vietnam attracted scientific attention as early as the first half of the twenti- eth century [6-8] was the first to analyze the zonation of reef-building corals in reefs of the Khanh Hoa province. He distinguished four scleractinian-dominated facieses. These investigators determined the species composition of scleractinians and demonstrated its similarity to that of Australia and Indonesia. Beginning in 1980, systematic studies of Vietnam corals and reefs have been performed in joint expeditions by the Institute of Marine Biology (Vladivostok), Nha Trang Institute of Oceanography, Haiphong Institute of Oceanology, and WWF (World Wide Fund for Nature). The published results were mainly related to scleractinian composition and distribu- tion, with some papers analyzing common accompanying macrobenthos species and a few publications providing the general characteristics of the reefs. Part of the data obtained was presented only in unpublished reports. 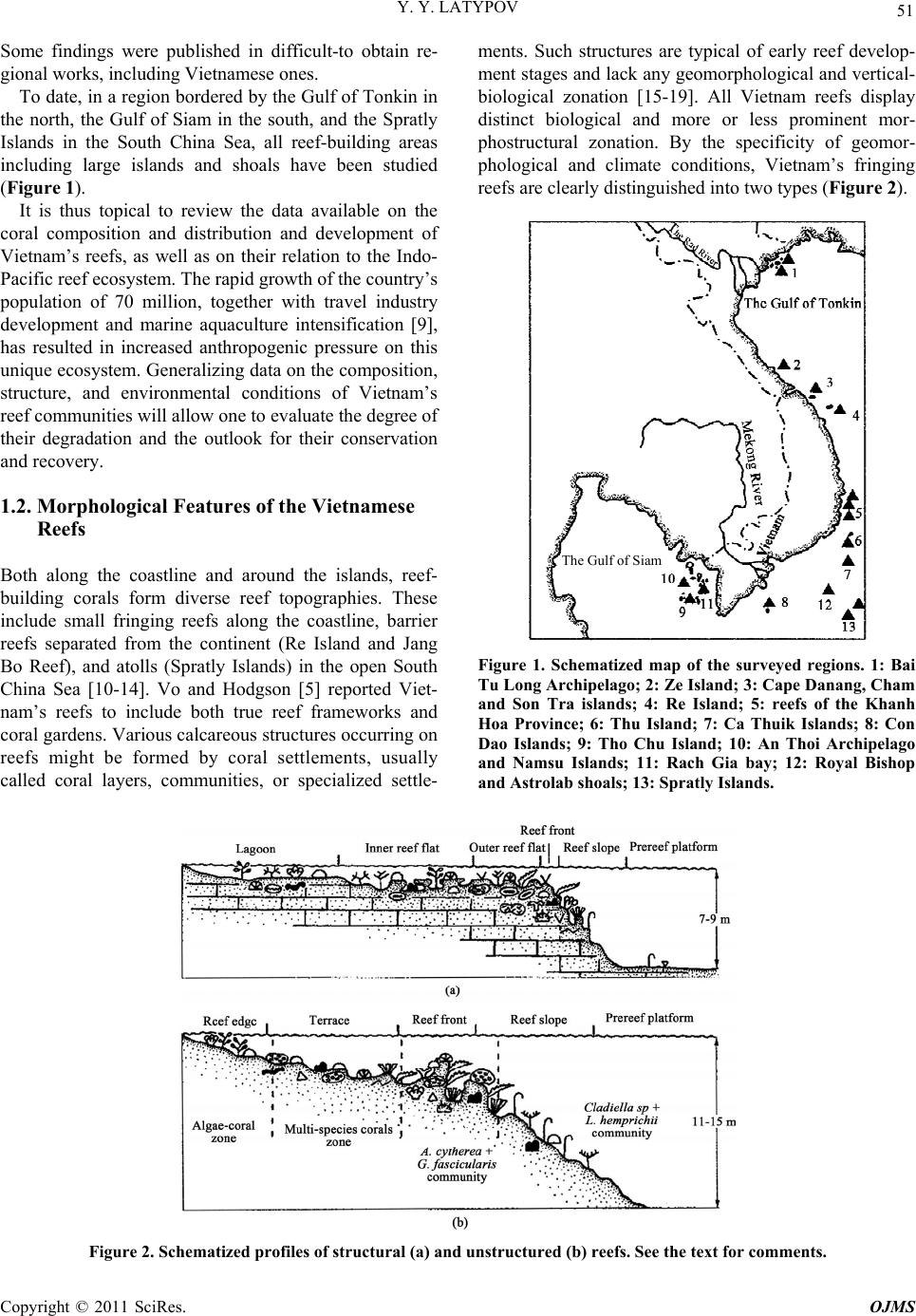 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 51 Some findings were published in difficult-to obtain re- gional works, including Vietnamese ones. To date, in a region bordered by the Gulf of Tonkin in the north, the Gulf of Siam in the south, and the Spratly Islands in the South China Sea, all reef-building areas including large islands and shoals have been studied (Figure 1). It is thus topical to review the data available on the coral composition and distribution and development of Vietnam’s reefs, as well as on their relation to the Indo- Pacific reef ecosystem. The rapid growth of the country’s population of 70 million, together with travel industry development and marine aquaculture intensification [9], has resulted in increased anthropogenic pressure on this unique ecosystem. Generalizing data on the composition, structure, and environmental conditions of Vietnam’s reef communities will allow one to evaluate the degree of their degradation and the outlook for their conservation and recovery. 1.2. Morphological Features of the Vietnamese Reefs Both along the coastline and around the islands, reef- building corals form diverse reef topographies. These include small fringing reefs along the coastline, barrier reefs separated from the continent (Re Island and Jang Bo Reef), and atolls (Spratly Islands) in the open South China Sea [10-14]. Vo and Hodgson [5] reported Viet- nam’s reefs to include both true reef frameworks and coral gardens. Various calcareous structures occurring on reefs might be formed by coral settlements, usually called coral layers, communities, or specialized settle- ments. Such structures are typical of early reef develop- ment stages and lack any geomorphological and vertical- biological zonation [15-19]. All Vietnam reefs display distinct biological and more or less prominent mor- phostructural zonation. By the specificity of geomor- phological and climate conditions, Vietnam’s fringing reefs are clearly distinguished into two types (Figure 2). The Gulf of Siam Figure 1. Schematized map of the surveyed regions. 1: Bai Tu Long Archipelago; 2: Ze Island; 3: Cape Danang, Cham and Son Tra islands; 4: Re Island; 5: reefs of the Khanh Hoa Province; 6: Thu Island; 7: Ca Thuik Islands; 8: Con Dao Islands; 9: Tho Chu Island; 10: An Thoi Archipelago and Namsu Islands; 11: Rach Gia bay; 12: Royal Bishop and Astrolab shoals; 13: Spratly Islands. Figure 2. Schematized profiles of structur al (a) and unstr uc tur ed (b) reefs. See the text for commen ts .  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 52 Reefs of the first type have a distinct zonation (reef lagoon, reef-flat, etc.) with a developed carbonate frame- work, so-called structural reefs [20] common for the tropical zone of the World Ocean. Reefs of the second type display a weak morphostructural zonation, with some zones occasionally absent. Carbonate deposits in such reefs comprise only coral settlements of a low thickness, hardly changing the overall substrate profile. These are so-called structureless [20] or encrusting [18] reefs. Vietnam’s structural reefs are mainly formed in closed bights and on the organogenic base of Holocene reefs [21,22], while structureless reefs are formed off promontories and in open bights, mostly on stone and rocky substrates [18,23-25]. Vietnam’s reefs pertain to the epicontinental monsoon type [7,11,21,24,26,27]. They are situated at the South China Sea periphery. The shoal waters of this region are highly eutrophicated, and the grounds are highly silted due to the huge amount of terrigenous influx. Other hy- drological conditions are also not optimal for reef-build- ing coral growth. Thus, in the Gulf of Tonkin, the salinity may drop to 26‰, and winter water temperature to 16˚C. Heavy northeast monsoon winds generate coastal waves up to three meters high with a 6-s period. During south- west monsoons, the Vietnam coast is struck by 5 - 10 typhoons per year with the strongest storm excitements and strong desalination, that sometimes results in almost utter annihilation of coral reef [5,28]. All this renders appreciable influence on features of formation of reefs which occurs, as a rule, on a stony, rock-stony substratum and less often on coralogenous sediments of Holocene reefs. Vietnam’s reefs feature a moderate vertical and horizontal spread and low thickness of modern reef-de- rived deposits. Their offshore spread usually does not exceed 200 - 300 m. They rarely extend to a depth of over 20 m. Sometimes they lack distinct morphological zona- tion. Most of Vietnam’s reefs have an indistinct reef flat and slope. In some reefs, mostly ones on stone and boul- der substrates, the only distinct zone is the reef slope. However, they all have a distinct vertical biological zona- tion, showing up in the dominant species succession and in the change in the composition and structure of coral communities and accompanying macrobenthos. Barrier, platform and fringing reefs on Holocene organogenous deposits always have well expressed morphological zon- ality, characteristic for the majority of reefs Indo-pacific [12,27,28]. 2. Results 2.1. Species Diversity According to the studies performed in the first decades of the twenty first century, Vietnam’s reef-building coral fauna comprises 350 species (see Table 1), pertaining to 80 genera (including 9 ahermatypic corals), of which 137 species, belonging to 26 genera, were not previously known for that region, and 13 species from 6 genera were described for the first time [11,18,29-33]). As in most Indo-Pacific reefs [5,19,24,34-36], the species di- versity of Vietnam’s reefs consists mainly of the mem- bers of 5 families, Acroporidae (117 species), Faviidae (42 species), Fungiidae (32 species), Poritidae (31 spe- cies), and Dendrophylliidae (25 species), making up al- together 64.48% of the total scleractinian species com- position (Figure 3). The five genera most diverse and widespread in all reefs comprise Acropora (90 species), Montipora (28 species), Porites (20 species), Favia (14 species), and Fungia (12 species) are most various and numerous on all reefs, making 47% of all specific riches of scleractinian (Figure 4). In all, some 20 scleractinian species form mono- spe- cific settlements, varying from small “spots” (tens of square meters) to extended zones (hundreds of square meters), with a coverage reaching 60% - 100%. One fifth of all scleractinians occur throughout the Vietnam coast (Figure 5). As a whole, the species diversity of reef-building scleractinians in different areas of the Vietnam coast is quite comparable, ranging from 190 species in the Gulf of Tonkin to 265 in the South Vietnam (Figure 6). Simi- lar (193 - 256) numbers of species were reported for reefs of Indonesia, the Philippines, and Western Austra- lia [36,37]. Central and South Vietnam reefs are most similar in species composition and are quite comparable to Spratly reefs). The degree of similarity of specific composition of Scleractinian various areas of Vietnam is resulted on the clustered diagram (Figure 7). The peculiarity of the coral faunas of the Siam and Tonkin gulfs as revealed by cluster analysis (Figure 8) is consistent with their ecological peculiarities [7,23,38,39]. Their scleractinian diversity is partly caused by their similar hydrological regimes. Both gulfs are shallows with high water eutrophycation and turbidity, with a predominance of clay sediments. These factors cause a similarity of the biological and morphostructural zonation of reefs and species composi- tion of reef communities in the gulfs. At the same time, certain differences in climatic and geomorphological conditions of the gulfs result in some dissimilarities in their scleractinian species composition. The development, zonation, species composition, and structure of the reefs in the gulfs were reported previously [13,23,39,40], so here, only major similarities and differences will be mentioned. 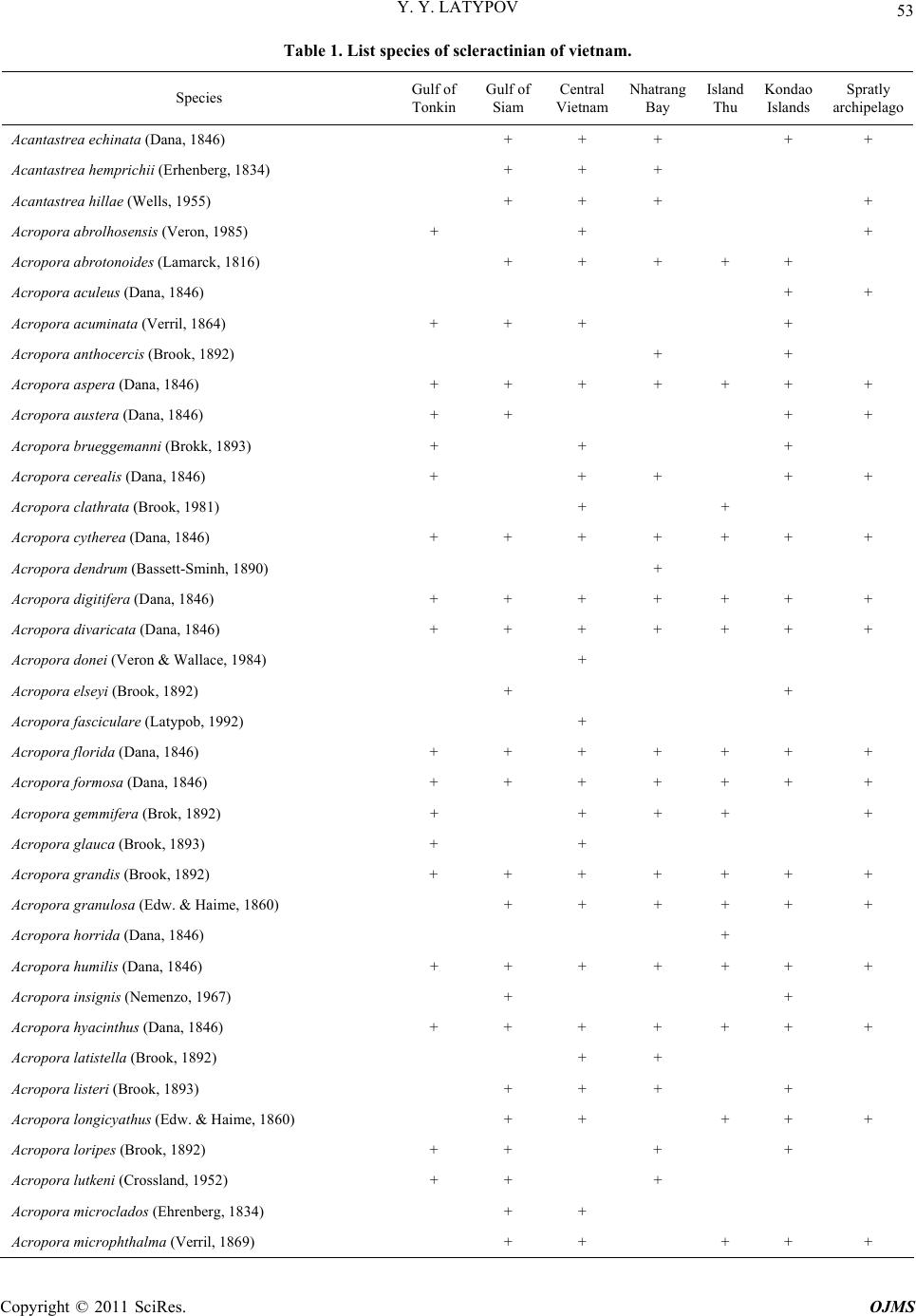 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 53 Table 1. List species of scleractinian of vietnam. Species Gulf of Tonkin Gulf of Siam Central Vietnam Nhatrang Bay Island Thu Kondao Islands Spratly archipelago Acantastrea echinata (Dana, 1846) + + + + + Acantastrea hemprichii (Erhenberg, 1834) + + + Acantastrea hillae (Wells, 1955) + + + + Acropora abrolhosensis (Veron, 1985) + + + Acropora abrotonoides (Lamarck, 1816) + + + + + Acropora aculeus (Dana, 1846) + + Acropora acuminata (Verril, 1864) + + + + Acropora anthocercis (Brook, 1892) + + Acropora aspera (Dana, 1846) + + + + + + + Acropora austera (Dana, 1846) + + + + Acropora brueggemanni (Brokk, 1893) + + + Acropora cerealis (Dana, 1846) + + + + + Acropora clathrata (Brook, 1981) + + Acropora cytherea (Dana, 1846) + + + + + + + Acropora dendrum (Bassett-Sminh, 1890) + Acropora digitifera (Dana, 1846) + + + + + + + Acropora divaricata (Dana, 1846) + + + + + + + Acropora donei (Veron & Wallace, 1984) + Acropora elseyi (Brook, 1892) + + Acropora fasciculare (Latypob, 1992) + Acropora florida (Dana, 1846) + + + + + + + Acropora formosa (Dana, 1846) + + + + + + + Acropora gemmifera (Brok, 1892) + + + + + Acropora glauca (Brook, 1893) + + Acropora grandis (Brook, 1892) + + + + + + + Acropora granulosa (Edw. & Haime, 1860) + + + + + + Acropora horrida (Dana, 1846) + Acropora humilis (Dana, 1846) + + + + + + + Acropora insignis (Nemenzo, 1967) + + Acropora hyacinthus (Dana, 1846) + + + + + + + Acropora latistella (Brook, 1892) + + Acropora listeri (Brook, 1893) + + + + Acropora longicya t h us (Edw. & Haime, 1860) + + + + + Acropora loripes (Brook, 1892) + + + + Acropora lutkeni (Crossland, 1952) + + + Acropora microclados (Ehrenberg, 1834) + + Acropora microphthalma (Verril, 1869) + + + + + 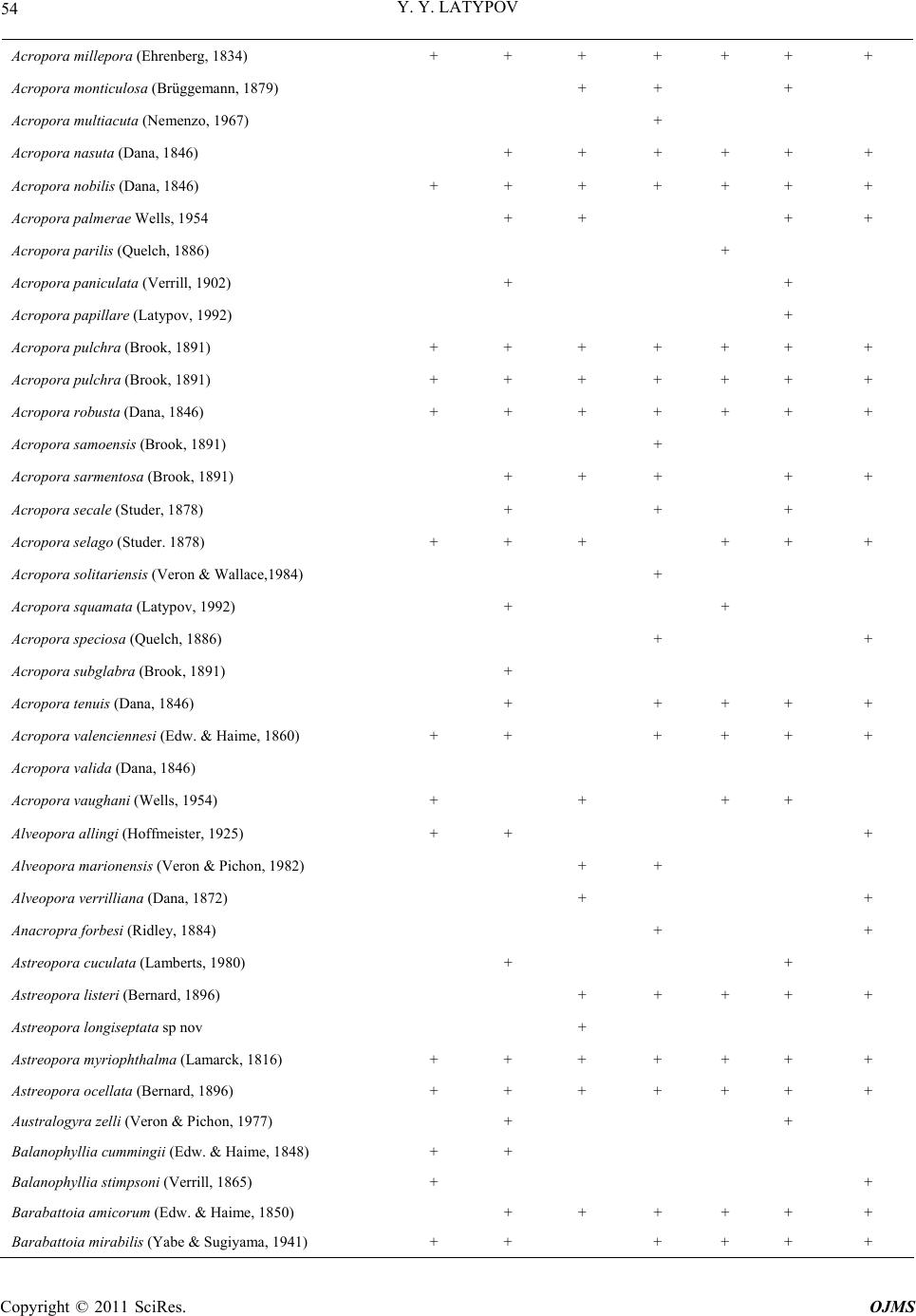 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 54 Acropora millepora (Ehrenberg, 1834) + + + + + + + Acropora monticulosa (Brüggemann, 1879) + + + Acropora multiacuta (Nemenzo, 1967) + Acropora nasuta (Dana, 1846) + + + + + + Acropora nobilis (Dana, 1846) + + + + + + + Acropora palmerae Wells, 1954 + + + + Acropora parilis (Quelch, 1886) + Acropora paniculata (Verrill, 1902) + + Acropora papillare (Latypov, 1992) + Acropora pulchra (Brook, 1891) + + + + + + + Acropora pulchra (Brook, 1891) + + + + + + + Acropora robusta (Dana, 1846) + + + + + + + Acropora samoensis (Brook, 1891) + Acropora sarmentosa (Brook, 1891) + + + + + Acropora secale (Studer, 1878) + + + Acropora selago (Studer. 1878) + + + + + + Acropora solitariensi s (Veron & Wallace,1984) + Acropora squamata (Latypov, 1992) + + Acropora speciosa (Quelch, 1886) + + Acropora subglabr a (Brook, 1891) + Acropora tenuis (Dana, 1846) + + + + + Acropora valenciennesi (Edw. & Haime, 1860) + + + + + + Acropora valida (Dana, 1846) Acropora vaughani (Wells, 1954) + + + + Alveopora allingi (Hoffmeister, 1925) + + + Alveopora marionensis (Veron & Pichon, 1982) + + Alveopora verrilliana (Dana, 1872) + + Anacropra forbesi (Ridley, 1884) + + Astreopora cuculata (Lamberts, 1980) + + Astreopora listeri (Bernard, 1896) + + + + + Astreopora longiseptata sp nov + Astreopora myriophthalma (Lamarck, 1816) + + + + + + + Astreopora ocellata (Bernard, 1896) + + + + + + + Australogyra zelli (Veron & Pichon, 1977) + + Balanophyllia cummingii (Edw. & Haime, 1848) + + Balanophyllia stimpsoni (Verrill, 1865) + + Barabattoia amicorum (Edw. & Haime, 1850) + + + + + + Barabattoia mirabilis (Yabe & Sugiyama, 1941) + + + + + +  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 55 Caulastrea furcata (Dana, 1846) + + + Caulastrea tumida (Matthai, 1928) + + + + Coeloseris mayeri (Vaughan, 1918) + + + Coscinaraea columna (Dana, 1846) + + + + + + + Coscinaraea exesa (Dana, 1846) + + + Coscinaraea mcneil li (Wells, 1962) + Ctenactis echinata (Pallas, 1766) + + + + + + Cycloseris cf. Sinensis (Edw. & Haime, 1851) + + + Cycloseris costulata (Ortman, 1889) + + + + Cycloseris cyclolites (Lamarck, 1801) + + + + + Cycloseris densicolummelus sp. nov. + Cycloseris patelliformis (Boschma, 1923) + + + Cycloseris somervillei (Gardiner, 1909) + Cycloseris tenuis (Dana, 1846) + + + Cycloseris vaughani (Boschma, 1923) + + + Cynarina lacrymalis (Edw. & Haime, 1848) + + + + Cyphastrea chalcidicum (Forskål, 1775) + + + + + Cyphastrea japonica (Yabe & Sugiyama, 1932) + + Cyphastrea microphthalm a (Lamarck, 1816) + + + + + + Cyphastrea serailia (Forskål, 1775) + + + + + + + Dendrophyllia aculeata sp. nov. + + Dendrophyllia arbuscula (Van der Horst, 1922) + + + Dendrophyllia cornige r a (Lamarck, 1816) + Dendrophyllia horsti (Gard. & Waugh, 1939) + + + Dendrophyllia japonica (Regberg, 1892) + + Dendrophyllia laborelli (Zibr. & Brilo, 1984) + Dendrophyllia robusta (Bourne, 1905) + + Dendrophyllia sphaerica (Nemenzo, 1981) + + Diaseris fragilis (Alcock, 1893) + + + + Diploastrea heliopora (Lamarck, 1816) + + + + + + + Echinophyllia aspera (Ellis & Solander, 1786) + + + + + Echinophyllia echinata (Saville-Kent, 1975) + + + + E. echinoporoides (Veron & Pichon, 1980) + + + + + + Echinophyllia nichihirai (Veron & Pichon, 1990) + + + 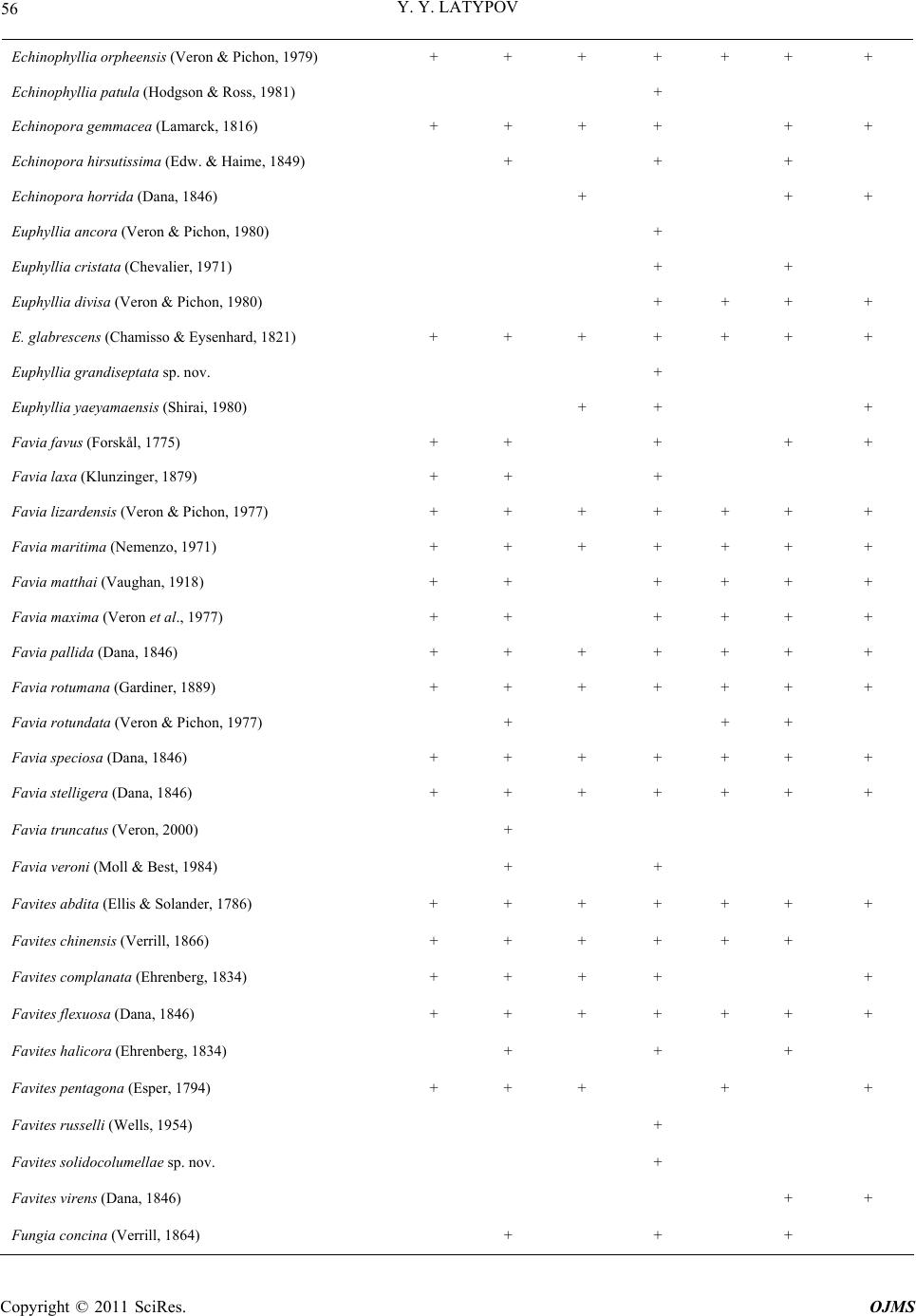 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 56 Echinophyllia orpheensis (Veron & Pichon, 1979) + + + + + + + Echinophyllia patu la (Hodgson & Ross, 1981) + Echinopora gemmacea (Lamarck, 1816) + + + + + + Echinopora hirsutissima (Edw. & Haime, 1849) + + + Echinopora horrida (Dana, 1846) + + + Euphyllia ancora (Veron & Pichon, 1980) + Euphyllia cristata (Chevalier, 1971) + + Euphyllia divisa (Veron & Pichon, 1980) + + + + E. glabrescens (Chamisso & Eysenhard, 1821) + + + + + + + Euphyllia grandiseptata sp. nov. + Euphyllia yaeyamaensis (Shirai, 1980) + + + Favia favus (Forskål, 1775) + + + + + Favia laxa (Klunzinger, 1879) + + + Favia lizardensis (Veron & Pichon, 1977) + + + + + + + Favia maritima (Nemenzo, 1971) + + + + + + + Favia matthai (Vaughan, 1918) + + + + + + Favia maxima (Veron et al., 1977) + + + + + + Favia pallida (Dana, 1846) + + + + + + + Favia rotumana (Gardiner, 1889) + + + + + + + Favia rotundata (Veron & Pichon, 1977) + + + Favia speciosa (Dana, 1846) + + + + + + + Favia stelligera (Dana, 1846) + + + + + + + Favia truncatus (Veron, 2000) + Favia veroni (Moll & Best, 1984) + + Favites abdita (Ellis & Solander, 1786) + + + + + + + Favites chinensis (Verrill, 1866) + + + + + + Favites complanata (Ehrenberg, 1834) + + + + + Favites flexuosa (Dana, 1846) + + + + + + + Favites halicora (Ehrenberg, 1834) + + + Favites pentagona (Esper, 1794) + + + + + Favites russelli (Wells, 1954) + Favites solidocolumellae sp. nov. + Favites virens (Dana, 1846) + + Fungia concina (Verrill, 1864) + + + 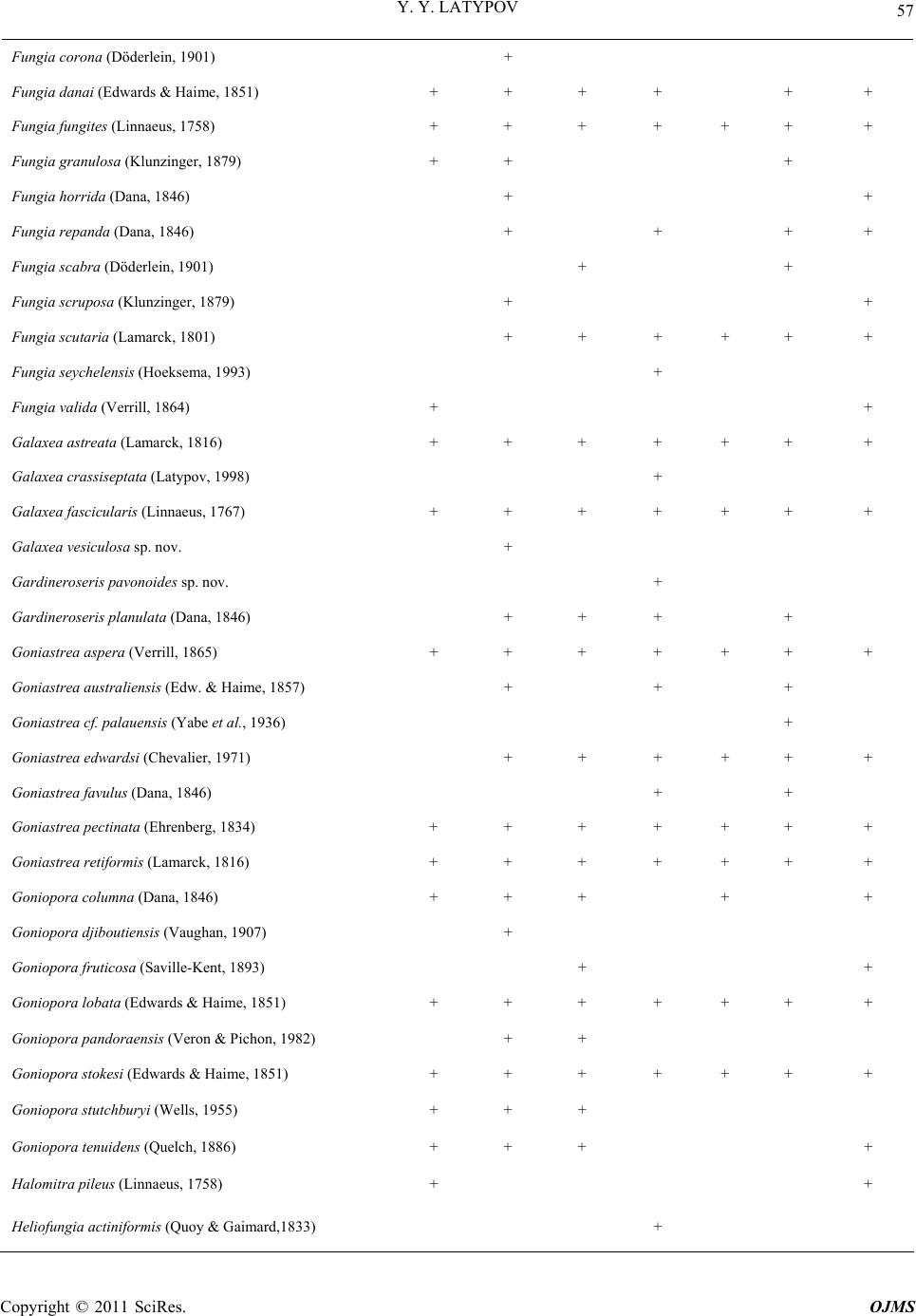 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 57 Fungia corona (Döderlein, 1901) + Fungia danai (Edwards & Haime, 1851) + + + + + + Fungia fungites (Linnaeus, 1758) + + + + + + + Fungia granulosa (Klunzinger, 1879) + + + Fungia horrida (Dana, 1846) + + Fungia repanda (Dana, 1846) + + + + Fungia scabra (Döderlein, 1901) + + Fungia scruposa (Klunzinger, 1879) + + Fungia scutaria (Lamarck, 1801) + + + + + + Fungia seychelensis (Hoeksema, 1993) + Fungia valida (Verrill, 1864) + + Galaxea astreata (Lamarck, 1816) + + + + + + + Galaxea crassiseptata (Latypov, 1998) + Galaxea fascicularis (Linnaeus, 1767) + + + + + + + Galaxea vesiculosa sp. nov. + Gardineroseris pavonoides sp. nov. + Gardineroseris planulata (Dana, 1846) + + + + Goniastrea aspera (Verrill, 1865) + + + + + + + Goniastrea australiensis (Edw. & Haime, 1857) + + + Goniastrea cf. palauensis (Yabe et al., 1936) + Goniastrea edwardsi (Chevalier, 1971) + + + + + + Goniastrea favulus (Dana, 1846) + + Goniastrea pectinata (Ehrenberg, 1834) + + + + + + + Goniastrea retiformis (Lamarck, 1816) + + + + + + + Goniopora columna (Dana, 1846) + + + + + Goniopora djiboutiensis (Vaughan, 1907) + Goniopora fruticosa (Saville-Kent, 1893) + + Goniopora lobata (Edwards & Haime, 1851) + + + + + + + Goniopora pandoraensis (Veron & Pichon, 1982) + + Goniopora stokesi (Edwards & Haime, 1851) + + + + + + + Goniopora stutchburyi (Wells, 1955) + + + Goniopora tenuidens (Quelch, 1886) + + + + Halomitra pileus (Linnaeus, 1758) + + Heliofungia actiniformis (Quoy & Gaimard,1833) + 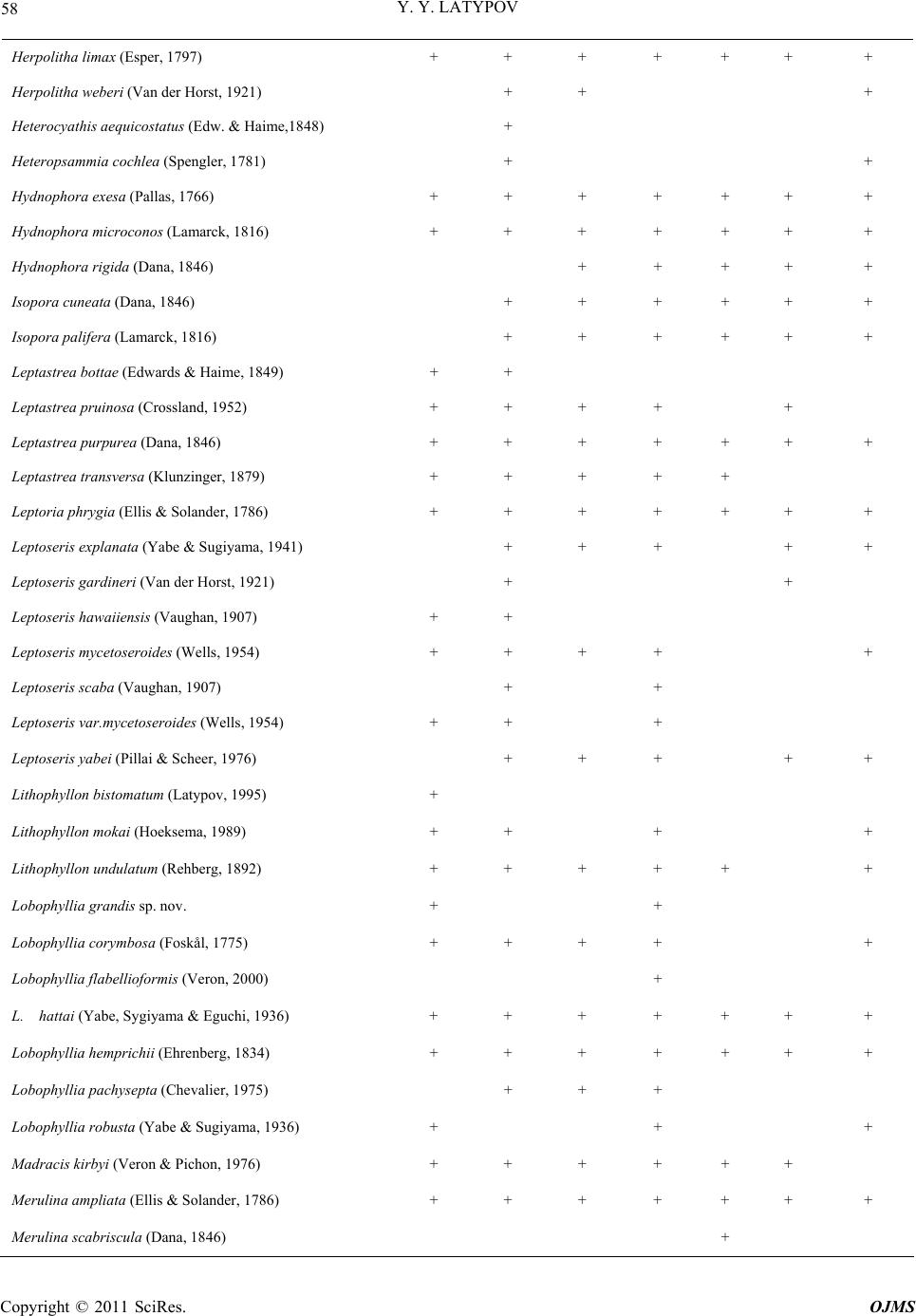 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 58 Herpolitha limax (Esper, 1797) + + + + + + + Herpolitha weberi (Van der Horst, 1921) + + + Heterocyathis aequicostatus (Edw. & Haime,1848) + Heteropsammia cochlea (Spengler, 1781) + + Hydnophora exesa (Pallas, 1766) + + + + + + + Hydnophora microconos (Lamarck, 1816) + + + + + + + Hydnophora rigida (Dana, 1846) + + + + + Isopora cuneata (Dana, 1846) + + + + + + Isopora palifera (Lamarck, 1816) + + + + + + Leptastrea bottae (Edwards & Haime, 1849) + + Leptastrea pruinosa (Crossland, 1952) + + + + + Leptastrea purpurea (Dana, 1846) + + + + + + + Leptastrea transversa (Klunzinger, 1879) + + + + + Leptoria phrygia (Ellis & Solander, 1786) + + + + + + + Leptoseris explanata (Yabe & Sugiyama, 1941) + + + + + Leptoseris gardineri (Van der Horst, 1921) + + Leptoseris hawaiiensis (Vaughan, 1907) + + Leptoseris mycetoseroides (Wells, 1954) + + + + + Leptoseris scaba (Vaughan, 1907) + + Leptoseris var.mycetoseroides (Wells, 1954) + + + Leptoseris yabei (Pillai & Scheer, 1976) + + + + + Lithophyllon bistomatum (Latypov, 1995) + Lithophyllon mokai (Hoeksema, 1989) + + + + Lithophyllon undulatum (Rehberg, 1892) + + + + + + Lobophyllia grandis sp. nov. + + Lobophyllia corymbosa (Foskål, 1775) + + + + + Lobophyllia flabellioformis (Veron, 2000) + L. hattai (Yabe, Sygiyama & Eguchi, 1936) + + + + + + + Lobophyllia hemprichii (Ehrenberg, 1834) + + + + + + + Lobophyllia pachyse pt a (Chevalier, 1975) + + + Lobophyllia robusta (Yabe & Sugiyama, 1936) + + + Madracis kirbyi (Veron & Pichon, 1976) + + + + + + Merulina ampliata (Ellis & Solander, 1786) + + + + + + + Merulina scabriscula (Dana, 1846) + 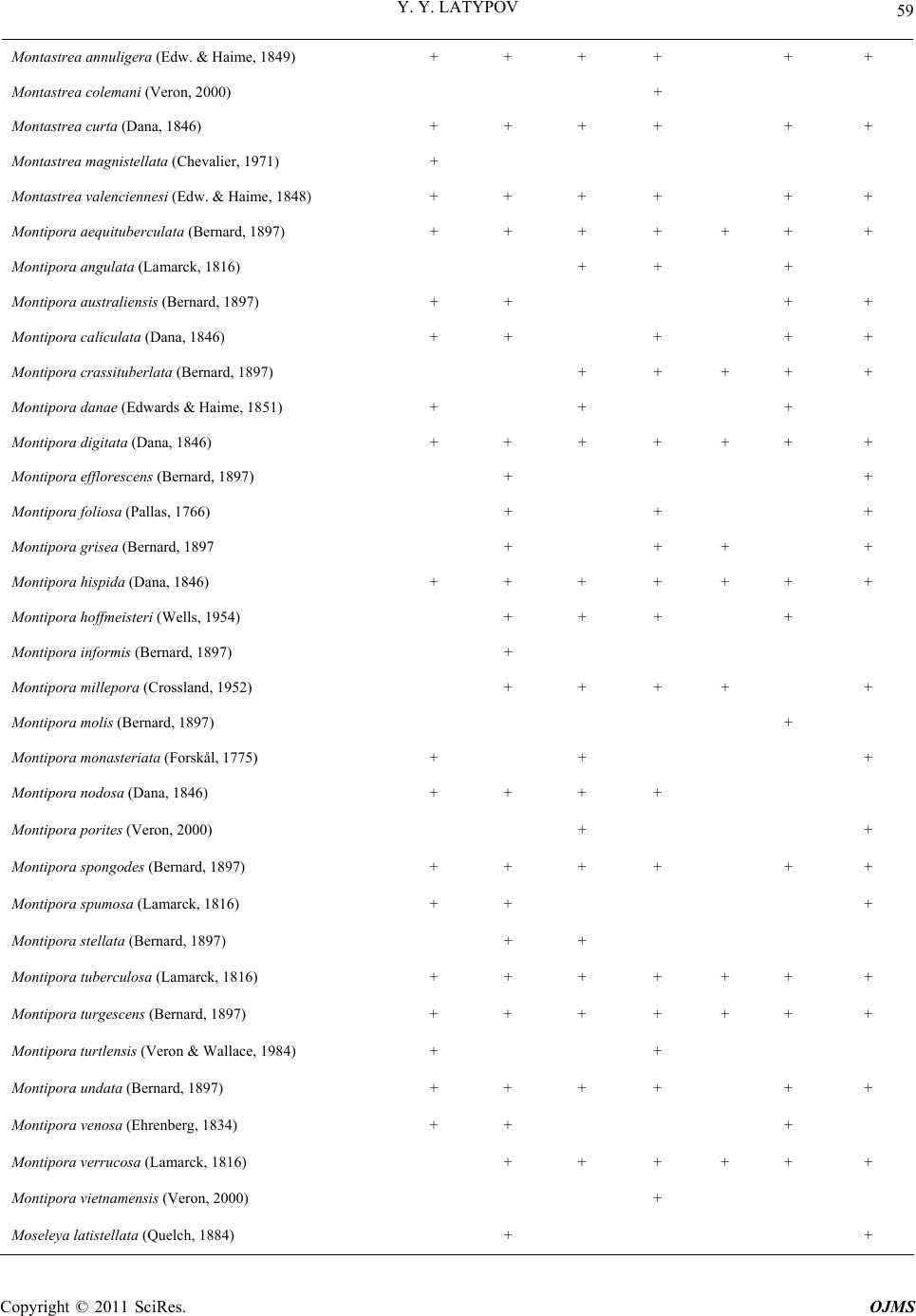 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 59 Montastrea annuligera (Edw. & Haime, 1849) + + + + + + Montastrea colemani (Veron, 2000) + Montastrea curta (Dana, 1846) + + + + + + Montastrea magnistellata (Chevalier, 1971) + Montastrea valenci ennesi (Edw. & Haime, 1848) + + + + + + Montipora aequituberculat a (Bernard, 1897) + + + + + + + Montipora angulata (Lamarck, 1816) + + + Montipora australie ns i s (Bernard, 1897) + + + + Montipora caliculata (Dana, 1846) + + + + + Montipora crassituberl at a (Bernard, 1897) + + + + + Montipora danae (Edwards & Haime, 1851) + + + Montipora digitata (Dana, 1846) + + + + + + + Montipora efflorescen s (Bernard, 1897) + + Montipora foliosa (Pallas, 1766) + + + Montipora grisea (Bernard, 1897 + + + + Montipora hispida (Dana, 1846) + + + + + + + Montipora hoffmeisteri (Wells, 1954) + + + + Montipora informis (Bernard, 1897) + Montipora millepora (Crossland, 1952) + + + + + Montipora molis (Bernard, 1897) + Montipora monasteriata (Forskål, 1775) + + + Montipora nodosa (Dana, 1846) + + + + Montipora porites (Veron, 2000) + + Montipora spongodes (Bernard, 1897) + + + + + + Montipora spumosa (Lamarck, 1816) + + + Montipora stellata (Bernard, 1897) + + Montipora tuberculosa (Lamarck, 1816) + + + + + + + Montipora turgescens (Bernard, 1897) + + + + + + + Montipora turtlensis (Veron & Wallace, 1984) + + Montipora undata (Bernard, 1897) + + + + + + Montipora venosa (Ehrenberg, 1834) + + + Montipora verrucosa (Lamarck, 1816) + + + + + + Montipora vietnamensis (Veron, 2000) + Moseleya latistellata (Quelch, 1884) + + 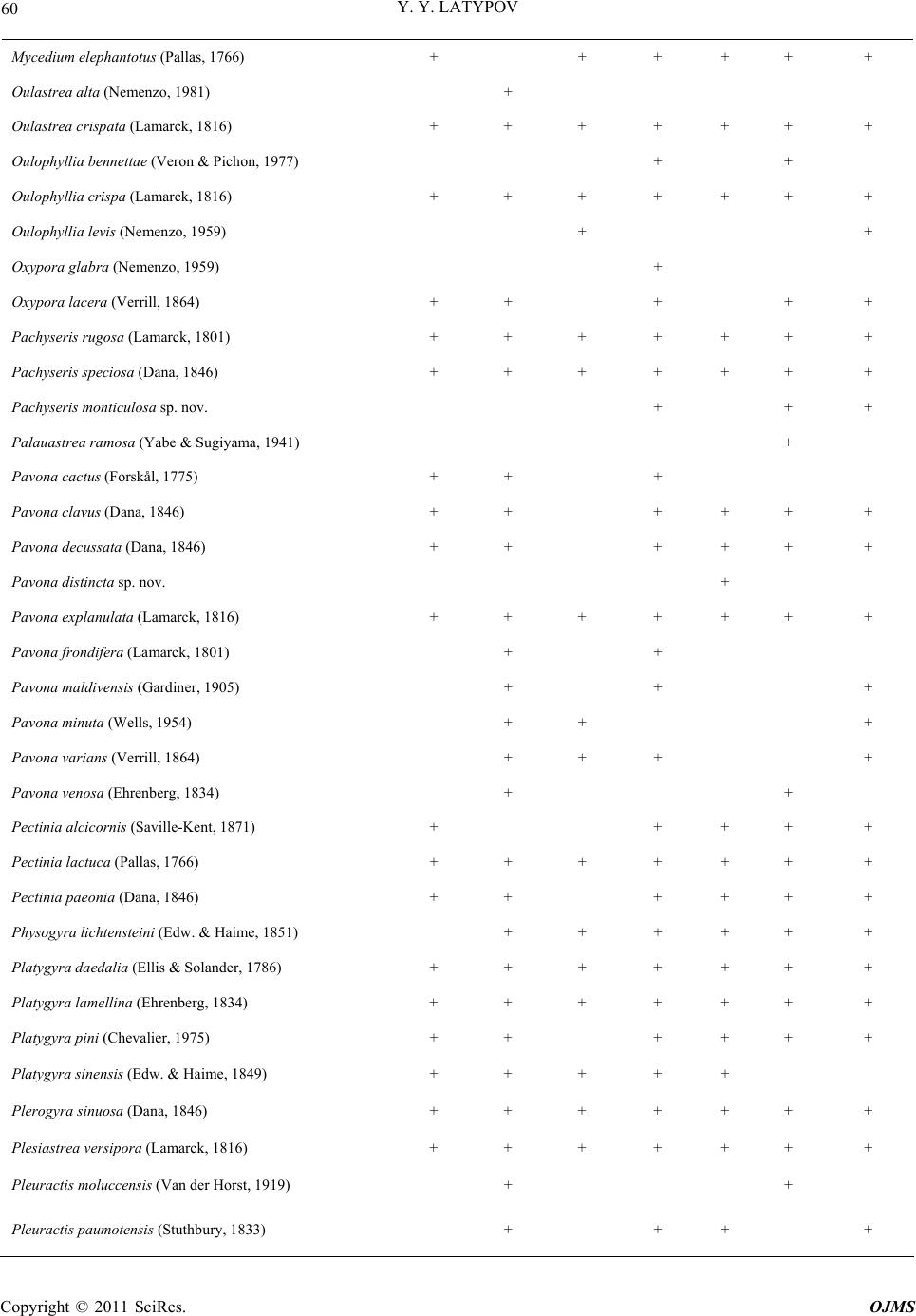 Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 60 Mycedium elephantotus (Pallas, 1766) + + + + + + Oulastrea alta (Nemenzo, 1981) + Oulastrea crispata (Lamarck, 1816) + + + + + + + Oulophyllia bennettae (Veron & Pichon, 1977) + + Oulophyllia crispa (Lamarck, 1816) + + + + + + + Oulophyllia levis (Nemenzo, 1959) + + Oxypora glabra (Nemenzo, 1959) + Oxypora lacera (Verrill, 1864) + + + + + Pachyseris rugosa (Lamarck, 1801) + + + + + + + Pachyseris speciosa (Dana, 1846) + + + + + + + Pachyseris monticulosa sp. nov. + + + Palauastrea ramosa (Yabe & Sugiyama, 1941) + Pavona cactus (Forskål, 1775) + + + Pavona clavus (Dana, 1846) + + + + + + Pavona decussata (Dana, 1846) + + + + + + Pavona distincta sp. nov. + Pavona explanulata (Lamarck, 1816) + + + + + + + Pavona frondifera (Lamarck, 1801) + + Pavona maldivensis (Gardiner, 1905) + + + Pavona minuta (Wells, 1954) + + + Pavona varians (Verrill, 1864) + + + + Pavona venosa (Ehrenberg, 1834) + + Pectinia alcicornis (Saville-Kent, 1871) + + + + + Pectinia lactuca (Pallas, 1766) + + + + + + + Pectinia paeonia (Dana, 1846) + + + + + + Physogyra lichtensteini (Edw. & Haime, 1851) + + + + + + Platygyra daedalia (Ellis & Solander, 1786) + + + + + + + Platygyra lamellina (Ehrenberg, 1834) + + + + + + + Platygyra pini (Chevalier, 1975) + + + + + + Platygyra sinensis (Edw. & Haime, 1849) + + + + + Plerogyra sinuosa (Dana, 1846) + + + + + + + Plesiastrea versipora (Lamarck, 1816) + + + + + + + Pleuractis moluccensis (Van der Horst, 1919) + + Pleuractis paumotensis (Stuthbury, 1833) + + + +  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 61 Pocillopora capitata (Verrill, 1864) + Pocillopora damicornis (Linnaeus, 1758) + + + + + + + Pocillopora eydouxi (Edwards & Haime, 1860) + + + + + + Pocillopora kelleheri (Veron, 2000) + Pocillopora meandrina (Dana, 1846) + + + + Pocillopora verruc os a (Ellis & Solander) + + + + + + + Pocillopora woodjones i (Vaughan, 1918) + + + + + + Podabacia crustacea (Pallas, 1766) + + + + + + + Polyphyllia novaehiberniae (Lesson, 1831) + Polyphyllia talpina (Lamarck, 1831) + + + + + + + Porites annae (Crossland, 1952) + + + + Porites attenuata (Nemenzo, 1955) + + + Porites australiensis (Vaughan, 1918) + + + + + + + Porites cylindrica (Dana, 1846) + + + + + + Porites deformis (Nemenzo, 1955) + + Porites densa (Vaughan, 1918) + + Porites lichen (Dana, 1846) + + + + + + + Porites lobata (Dana, 1846) + + + + + + + Porites lutea (Edwards & Haime, 1860) + + + + + + + Porites mayeri (Vaughan, 1918) + + + + Porites monticulosa (Dana, 1846) + + Porites mordax (Dana, 1846) + + + Porites murrayensis (Vaughan, 1918) + + + + + Porites nigrescens (Dana, 1848) + + + + + + Porites rus (Forskål, 1775) + + + + + + + Porites solida (Forskål, 1775) + + + + + Porites sp. 1 + Porites sp. 2 + + + + + Porites stephensoni (Crossland, 1952) + + + + + Porites vaughani (Crossland, 1952) + + + Psammocora contigua (Esper, 1979) + + + + + + + Psammocora digitata (Edwards & Haime, 1851) + + + + + Psammocora explanulata (Van der Horst, 1922) + + Psammocora nierstraszi (Van der Horst, 1921) + + + + + +  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 62 Psammocora profundacell a (Gardiner, 1898) + + + + + + Psammocora superficialis (Gardiner, 1898) + + + + + Pseudosiderastrea tayamai (Yabe & Sug., 1935) + + + + + + + Sandalolitha dentata (Quelch, 1884) + + + + + + + Sandalolitha robusta (Quelch, 1886) + + + + + + + Scolymia aff. Vitiensis (Brüggemann, 1877) + + + + Scolymia australis (Edw. & Haime, 1849) + + + + + Seriatopora calien dr u m (Ehrenberg, 1834) + Seriatopora hystrix (Dana, 1846) + + + + + + Stylocoeniella gue ntheri (Basset-Smith, 1890) + + + + + Stylophora pistillata (Esper, 1797) + + + + + + + Stylophora subseriata (Ehrenberg, 1834) + + Symphyllia agaricia (Edwards & Haime, 1849) + + + + + Symphyllia erythraea (Klunzinger, 1879) + Symphyllia hassi (Pillai & Scheer, 1976) + + + Symphyllia radians (Edwards & Haime, 1849) + + + + + + + Symphyllia recta (Dana, 1846) + + + + + + + Symphyllia valenciennesii (Edw. & Haime, 1849) + + + + + + + Trachyphyllia geoffroyi + + + + Tubastrea aurea (Quoy & Gaimard, 1833) + + + + + + + Tubastrea coccinea (Ehrenberg, 1834) + + + + + + Tubastrea diaphana (Dana, 1846) + + + Tubastrea micrantha (Ehrenberg, 1834) + + + + + + + Turbinaria bifrons (Brüggemann, 1877) + + + + + + Turbinaria conspicua (Bernard, 1896) + + + Turbinaria contort a (Bernard, 1896) + + Turbinaria crater (Pallas, 1766) + + + Turbinaria frondens (Dana, 1846) + + + + + + + Turbinaria mesenterina (Dana, 1846) + + + + + + + Turbinaria patula (Dana, 1846) + + + Turbinaria peltata (Esper, 1794) + + + + + + + Turbinaria radical is (Bernard, 1896) + + + + Turbinaria reniformis (Bernard, 1896) + + + + + + + Turbinaria stellulata (Lamarck, 1816) +  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 63 Species composi t i on 29% 34% 12% 9% 9% 7% 6 1 2 3 4 5 Figure 3. Relative species diversity of major coral genera in Vietnam. 1: Acroporidae; 2: Faviidae; 3: Fungiidae; 4: Poritidae; 5: Dendrophillidae; 6: The rest genera. Diversity 53% 26% 8% 6%4%3% 6 1 2 3 4 5 Figure 4. Reative species diversity of major coral species in Vietnam. 1: Acropora; 2: Montipora; 3: Porites; 4: Faia; 5: Fungia; 6: The res species. Figure 5. Monospecific settlement on reef Myeu Island at Nhatrang Bay, depth 2 m. Species r i chness 0 100 200 300 400 12345678 Numb er s pec i es Figure 6. Scleractinian species diversity in different regions of Vietnam. 1: total number of species (347); 2: Gulf of Tonkin (177); 3: Central Vietnam (204); 4: South Vietnam (240); 5: Thu Island; 6: Con Dao Islands (211); 7: Spratly Island (211), 8: Gulf of Siam (251). Figure 7. Cluster analysis of similarity species composition on different regions of Vietnam. Figure 8. Similarity dendrogram of sclera ctinians faunas in different regions of Vietnam. 1: Gulf of Tonkin; 2: Central Vietnam; 3: South Vietnam; 4: Gulf of Siam; 5: Spratly Island. The reef communities in both gulfs lack members of Palauastrea and Caulastrea and Acropora cuneata, the latter occurring in most Vietnam reefs. Plerogyra and Physogyra are absent in the closed part of the Gulf of Tonkin, and Pachyseris, Mycedium, and Pectinia, in the innermost and coastal areas of the Gulf of Siam. How- ever, some members of the latter three genera and rare Physogyra and Plerogyra species are found in the open parts of both gulfs, off Hainan and Tho Chu islands. Corals having large polyp forms and capable of self cleaning—Galaxea, Echinopora, Lobophyllia, Echino- phyllia, Turbinaria, Podobacia, Lithophyllon, Fungia, and Goniopora—are widespread in both gulfs. The reefs in both gulfs are dominated by many species of these genera (Galaxea fascicularis, Goniopora stokesi, Echi- nopora lamellosa, and Lobophyllia hemprichii), as well as by Acropora cytherea, A. nobilis, Montipora hispida, Porites lobata, and P. cylindrica, widespread in Indo-  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 64 Pacific reefs. Altogether, these species cover 60% - 80% of the substrate. Massive Porites colonies (at least 10 species) forming vast monospecific settlements are typi- cal of both gulfs. At the same time, members of Pocil- lopora, abundant in most Indo-Pacific reefs (5-7 species), only rarely occur in the innermost parts of the gulfs (2 species maximum) but are common for island reefs in the open parts of the gulfs (Tho Chu, Hainan). By and large, the two gulfs are quite similar in coral diversity and share 74.3% of species. 2.2. Communities The distribution and peculiarities of benthic communities in the coastal part of Vietnam reefs is rather constant (See Figure 2). As a rule, these are algal-coral commu- nities, composed of several biocenoses (zones, facieses), dominated by individual algal or coral species or by groups of species (Figure 9). The predominance of Laurencia, Turbinaria, and Sargassum algae in the coastal zones of the reefs has been reported for many reef development areas. This may be indicative of an increase in water eutrophication or later stages of reef development [38,41-43]. Both along the Vietnam coast and in the whole Indo- Pacific, in reef zones characterized by relatively stable conditions (lagoons, deep stony and coral terraces, and reef slopes), branched, plate, and trumpet colonies of A. cytherea, A. hyacinthus, Montipora danae, M. foliosa, Porites cylindrica, P. nigrescens, and others successfully compete with differently shaped scleractinian colonies [43-45]. A wider distribution of encrusting and plate colonies of Euphyllia, Echinophyllia, Mycedium, Pachyseris, and Turbinaria compared to that of branched forms is di- rectly caused by lowered illumination. This is also the case in many reefs of the Indo-Pacific and Caribbean basins [46-48]. In Vietnam’s reefs, such corals are com- mon for communities of the slope base, byoherms zone, and fore reef platform. Caused by abiotic factors, the vertical distribution of reef-building corals has a strong effect on the develop- ment of biotic zonation across a whole reef community, beginning with settlement-site choice and ending with interspecific trophic relationships. The relationships be- tween the species composition of benthic communities of some reefs as revealed by cluster analysis correlated with the ecological and physiographical zonation of the reefs (Figure 10). Algal-coral lagoon and reef flat communities domi- nated by red and brown algae and similar in coral and common macrobenthos species composition formed a single cluster group, that of communities developing under similar conditions. The high similarity between coral faunas from different sites reflected similar, some- times extreme, conditions of reef flat and shallow-water stone terrace. At the same time, communities of these reef zones were set apart from those of neighboring reef zones. Both in structural and unstructured reefs, poly- specific reef slope communities sharing a relatively greater number of corals also formed a distinct cluster. Polyspecific coral communities of a reef slope of struc- tural and unstructured reefs distinctly stands apart on the greatest level of similarity of their specific variety. Figure 9. Algal-coral community on reef Bathlongvi, depth 2 m. Figure 10. Cluster dendrogram of the species composition of benthic communities. Algal-coral community, unstruc- tured (1) and structural (2) reefs; Acropora community, unstructured (3) and structural (4) reefs; Acropora + Dip- loastrea community, unstructured (5) and structural (6) reefs; (7) bioherm community (reef slope); (8) Junceella + Diaseris community; (9) Maleus + Junceella community. A: lagoon, B: reef flat and terrace zone, C: reef slope, D: for-reef platform.  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 65 3. Discussion and Conclusions To summarize the above, both structural and unstruc- tured reefs feature vertical biological and geomor- phological zonations. The latter is mainly determined by peculiarities of the underwater reef slope substrate. Similar biological zonation reflecting interzonal differ- ences in environmental conditions (substrate, wave re- gime, sedimentation rate, illumination) has been reported for many of Vietnam’s reefs and various Pacific and Caribbean reefs [21,23,40,42,43]. Shallow-water Vietnam reefs growing in highly eutro- phic conditions lack thick reef deposits [18,23] and fea- ture high coral diversity and distinct biological zonation, that is, the presence of inner heterotrophic (lagoon, reef flat) and outer autotrophic (reef slope) zones [26,49,50], which is characteristic of typical Indo-Pacific reefs. In reefs of Indonesia and Philippines and in the Great Barrier Reef, a total of 360 - 410 reef-building scler- actinians pertaining to 70 - 80 genera have been recorded [36]. This region of the Western Pacific is considered the center of origin of tropical coral faunas. The maximum coral diversity is observed in the so-called Coral Triangle [36,51,52] with apices in the Philippines, the Malacca Peninsula, and New Guinea (Figure 11). To same fertile the center should expense and coast of Vietnam, scler- actinian fauna which totals 350 species belonging to 80 genera. Vietnam’s reefs, too, obviously belong to this center, which is evidenced by their high similarity in coral spe- cies composition to reefs of Thailand, Indonesia, and the Philippines (76.4%, 72.3%, and 81.6%, respectively). In the greater Western Pacific Coral Triangle (with apices in Vietnam, South Japan, and the Great Barrier Reef), coral faunas are also highly similar and homogenous. The similarities between the Vietnam coral fauna and those of Japan and Australia are 77.5% and 86%, respec- tively, suggesting homogeneity of the coral fauna of the Western and Southwest Pacific. As a whole, the species complex of Vietnam scleractinians, as well as those of alcyonarians and gorgonarians, belongs to the tropical fauna as the majority of Vietnam corals are also common for the equatorial Indo-Pacific reef zone. The scleractin- ian species composition of this area exceeds 80% of that of the Pacific, and the alcyonarian diversity of Vietnam’s reefs is one of the greatest in the Indo-Pacific [18,31,53]. The species composition and high diversity of Viet- nam’s coral fauna, as well as its close similarity to the Southwest Pacific coral fauna, allow one to refer it to the Indonesia-Polynesian center of origin of the coral faunas of the tropical Indo-Pacific (Figure 12) The whole Viet- nam coast, from the Gulf of Tonkin to the Gulf of Siam, is a biogeographically single whole and is part of the Indo-Polynesian Province of the Indo-Pacific Area. Figure 11. Schematized map of the generic diversity of reef-building corals in different regions of the Indo-Pacific (partly after Veron, 1995). The dotted line and arrow indicate new and old 70 genera diversity isolines, respectiv ely.  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 66 Figure 12. Optimal Vietnamese coral reef. 4. Acknowledgements The author is sincerely grateful to the domestic and for- eign colleagues V. Brykov, I. Budin, N. Selin, Yu. Yakovlev, Lang Van Ken, Нгуен Van Tien, Vo Si Tuan, assisting to me in researches and sent prints of their pub- lications, special gratitude to Dr. Douglas Fenner for editing of the English text. 5. References [1] T. D. Thanh, “Change in Environment and Ecosystems Relative to the Land-Sea Ineraction in the Vietnam Coastal Zone,” EALOICZ Worshop, Qingdao, 1999, pp. 1-10. [2] Y. I. Sorokin, “Some Issues of Productivity, Trophology, and Energy Balance of a Coral Reef Ecosystem,” Biolo- gia Morya, No. 6, 1986, pp. 3-14. [3] N. T. An, “Biological Productivity of Vietnam Marine, Waters, Monography on Vietnam Seas,” Science and Technology Publishing Housing, Ha Noi, 1994, pp. 502-518. [4] T. N. Dautova, Y. Y. Latypov and S. S. Dautov, “Com- position and Structure of the Coral Communities of the Bai Ty Long Archipelago under Different Ecological Conditions,” Biologia Morya, Vol. 25, No. 2, 1999, pp. 106-107. [5] S. T. Vo and G. Hodgson, “Coral Reefs of Vietnam: Re- cruitment Limitation and Physical Forcing,” Proceedings of 8th International Coral Reef Symposium, Vol. 1, 1997, pp. 477-482. [6] R. Sérene, “Inventaires des Invertebres Marine de l’Indochine,” Institute of Oceanography Indochine, Vol. 30, 1937, pp. 3-83. [7] C. Dawydoff, “Contribution et l’Etude des Invertebres de la Faune Marine Benthique de l’Indochina,” Buletin Biologique de la France et de la Belgique, Vol. 37, 1952, pp. 1-158. [8] T. N. Loi, “Peuplements Animaux et Vegetaux du Substrat des Intertidal de la Baie de Nha Trang (Vietnam),” Memories Institute of Oceanography, Vol. 11, 1967, pp. 1-236. [9] C. Cheung, “Vietnam Marine Conservation,” Coastal Management in Tropical Asia, Vol. 4, 1994, pp. 21-23. [10] Y. Y. Latypov, “Scleractinian Corals of Vietnam. Tham- nasteriidae, Astroceniidae, Pocilloporidae, Dendrophyl- liidae),” Nauka, Moscow, 1990. [11] Y. Y. Latypov, “Coral Reefs of Vietnam,” Nauka, Mos- cow, 2007. [12] Y. Y. Latypov, “Species Composition and Structure of Coral Community of a Platform Reef at Bach Long Vi Island in the South China Sea,” Russian Journal of Ma- rine Biology, Vol. 34, No. 4, 2008, pp. 249-253. 0doi:10.1134/S1063074008040068  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 67 [13] L. V. Ken, “The Stony Corals in the Sea of Vietnam, Marine Environment and Resources, (1986-1990),” Sci- entific and Technic Publishing House, Hanoi, 1991, pp. 127-135. [14] N. H. Yet, “Characteristic Species and Coral Reef Struc- tures Fishermen Dao (Spratly Islands),” Resources and Marine Environment, Vol. 4, 1997, pp. 299-313. [15] J. A. Hubbard, “Scleractinian Corals Behavior in Cali- brated Carrent Experiment: An Index to Their Distribu- tion Patterns,” Proceeding of 2nd International Coral Reef Symposium, Vol. 2, 1974, pp. 107-126. [16] M. Pichon, “Dynamic of Benthic Communities in the Coral Reefs of Tule’ar (Madagaskar): Succession and Transformation of the Biotops Trough Reef Tract Evolu- tion,” Proceeding of 2nd International Coral Reef Sym- posium, Vol. 2, 1974, pp. 55-68. [17] Y. Loya, “The Red Sea Coral Stylophora Pistillata is an Rstrategist,” Nature, Vol. 259, 1976, pp. 478-480. 1doi:10.1038/259478a0 [18] Y. Y. Latypov, “Community Structure of Scleractinian Reefs in the Baitylong Archipelago (South China Sea),” Asian Marine Biology, Vol. 12, 1995, pp. 27-37. [19] Y. Y. Latypov, “Coral Reefs of the Gulf of Tonkin,” Vestnik DVO RAN, No. 2, 1997, pp. 92-98. [20] S. A. Wainwright, “Reef Communities Visited by the Israel South Red Expedition, 1962,” Bulletin of Sea Fishery Research. Station, Vol. 8, 1965, pp. 40-53. [21] Y. Y. Latypov, “Species Composition and Distribution of Scleractinians in Reefs of the Khanh Hoa Province (South Vietnam),” Biologia Morya, No. 6, 1982, pp. 5-12. [22] B. V. Preobrazhenskii, “Sovremennye Rify,” Nauka, Mos- cow, 1986. [23] Y. Y. Latypov, “Coral Communities of the Namsu Is- lands (Gulf of Siam, South China Sea),” Marine Ecology Progress Series, Vol. 29, 1986, pp. 261-270. 2doi:10.3354/meps029261 [24] Y. Y. Latypov, “Scleractinian Corals of South Vietnam,” The Soviet Journal of Marine Biology, Vol. 13, No. 5, 1987, pp. 246-252. [25] N. H. Yet and L. V. Ken, “Some Data on Species Compo- sition and Distribution of Scleractinian Corals in Ha Long Bay,” Journal of Biology, Vol. 18, 1996, pp. 7-13. [26] Y. I. Sorokin, “Ecosystems of Coral Reefs,” Nauka. Moscow, 1990 [27] Y. Y. Latypov, “Reef-Building Corals of Vietnam as a Part of the Indo-Pacific Reef Ecosystem,” Russian Jour- nal of Marine Biology, Vol. 31, Supplement 1, 2005, pp. S34-S40. 3doi:10.1007/s11179-006-0013-5 [28] Y. Y. Latypov and N. I. Selin, “Coral Communities of Barrier Reefs of Vietnam,” Russian Journal of Marine Biology, Vol. 34, No. 3, 2008, pp. 143-150. 4doi:10.1134/S1063074008030012 [29] Y. Y. Latypov, “Reef-Building Corals and Reefs of Vietnam: 1. The Gulf of Thailand,” Russian Journal of Marine Biology, Vol. 29, Supplement 1, 2003, pp. S22-S33. 5doi:10.1023/B:RUMB.0000011714.71499.75 [30] Y. Y. Latypov, “Reef-Building Corals and Reefs of Vietnam: 2. The Gulf of Tonkin,” Russian Journal of Marine Biology, Vol. 29, Supplement 1, 2003, pp. S34-S45. 6doi:10.1023/B:RUMB.0000011715.09137.b0 [31] Y. Y. Latypov and P. Q. Long, “The Common Hard Cor- als of Vietnam,” Ministry of Agriculture and Rural De- velopment, Hanoi, 2010. [32] S. T. Vo, N. H. Yet and P. M. Alino, “Coral and Coral Reefs in the North of Spratly Archipelago the Results of RP-VN JOMSRE-SCS 1996,” Proceeding of Scientific Conference on Philippines-Vietnam JOMSRE 1996, Hamoi, 22-23 April 1997, pp. 87-101. [33] World Wildlife Fund, “Vietnam Marine Conservation Southern Survey Team,” Survey Report on the Biodiver- sity Resourse Utilization and Conservation Potential of Coto Islands, Switzerland, 1994. [34] M. B. Best, B. W. Hoeksema, W. Moka, et al., “Recent Scleractinian Corals Species Collected during the Snel- lius-II Expedition in Eastern Indonesia,” Netherland Journal of Sea Research, Vol. 23, No. 2, 1989, pp. 107-115. [35] J. E. N. Veron and G. Hodgson, “Annotated Checklist of the Hermatypic Corals of the Philippines,” Pacific Sci- ence, Vol. 43, No. 3, 1989, pp. 234-287. [36] J. E. N. Veron, “Corals in Space and Time: The Biogeog- raphy and Evolution of the Scleractinia,” Australian Insti- tute of Marine Sciences, Townsville, 1995, p. 321. [37] J. E. N. Veron and L. M. Marsh, “Records and Annotated Check List of the Hermatypic Corals of Western Australia,” Records of the Western Australia Museum, 1988, p. 29. [38] Y. Y. Latypov, “Benthic Communities of Coral Reefs of Tho Chu Island (Gulf of Siam, South China Sea),” Rus- sian Journal of Marine Biology, Vol. 25, No. 3, 1999, pp. 233-241. [39] Y. Y. Latypov, “Macrobenthos Communities on Reefs of the an Thoi Archipelago of the South China Sea,” Rus- sian Journal of Marine Biology, Vol. 26, No. 1, 2000, pp. 18-26. 7doi:10.1007/BF02759489 [40] K. Sakai, T. Yeemin, A. Svidvong, et al., “Distribution and Community Structure of Germatypic Corals in the Sichang Islands, Inner Part of the Gulf of Thailand,” Galaxea, Vol. 5, 1986, pp. 27-74. [41] H. Mergner, “Quantitative Okologische Analyse Eines Rifflagunenareals bei Agaba (Golf von Agaba, Rotes Meer),” Helgoland Wissenschsft Meeresuntersuch, Vol. 32, No. 4, 1979, pp. 476-507. 8doi:10.1007/BF02277991 [42] S. Dollar, “Wave Stress and Coral Community Structure in Hawaii,” Coral Reefs, Vol. 1, No. 2, 1982, pp. 71-81. 9doi:10.1007/BF00301688 [43] C. F. Dai, “Patterns of Coral Distribution and Benthic Space Partitioning on the Fringing Reefs of Southern Taiwan,” Marine Ecology, Vol. 14, No. 3, 1993, pp. 185-204. 1doi:10.1111/j.1439-0485.1993.tb00479.x [44] W. D. Liddel and S. L. Ohlhorst, “Patterns of Reef Coral Community Structure. North Jamaica,” Bulletin of Biol-  Y. Y. LATYPOV Copyright © 2011 SciRes. OJMS 68 ogy, Vol. 40, 1987, pp. 311-329. [45] Y. Y. Latypov, “Reefs and Communities of Sclerac- tini- ans of the Island (South Vietnam),” In: Biology of Coastal Waters of Vietnam: Hydrobiological Investiga- tions of Intertidal and Subtidal Zones of South Vietnam, DVO AN SSSR, Vladivostok, 1988, pp. 11-19. [46] Y. Loya, “Effects of Water Turbidity and Sedimentation on the Community Structure of Puerto Rican Corals,” Bulletin Marine Sciences, Vol. 26, No. 4, 1976, pp. 450-466. [47] J. W. Porter, “Autotrophy, Heterotrophy and Recourses Partitioning in Caribbean Reef-Building Corals,” Ameri- can Naturalist, Vol. 110, No. 975, 1976, pp. 731-742. 1doi:10.1086/283100 [48] T. Tomascik and F. Sander, “Effect of Eutrophication on Reef-Building Corals. 3. Reproduction of the Reef- Building Coral Porites Porites,” Marine Biology, Vol. 94, No. 1, 1987, pp. 77-94. 1doi:10.1007/BF00392901 [49] B. V. Preobrazhenskii, “Morphology and Paleoecology of Tabulate Corals,” Nauka, Moscow, 1982 [50] Y. Y. Latypov, “Benthic Communities of the Corals Reef of the Kondao Islands in the South China Sea,” Russian Journal of Marine Biology, Vol. 20, No. 3, 1994, pp. 136-143. [51] S. Ekman, “Zoogeography of the Sea,” Sidgwik and Jackson, London, 1953. [52] E. G. Stehli and I. W. Wells, “Diversity and Age Patterns in Hermatypic Corals,” Systematic Zoology, Vol. 20, No. 20, 1971, pp. 115-126. 1doi:10.2307/2412052 [53] A. N. Malyutin and Yu. Ya. Latypov, “Distribution of Corals and Biogeographic Zonation of the Shelf of Viet- nam,” Biologia Morya, No. 4, 1991, pp. 26-35.
|