Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 2011, 1, 118-124 doi:10.4236/aces.2011.13018 Published Online July 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Effect of Sodium Dodecyl Sulphate on the Composition of Electroless Nickel—Yttria Stabilized Zirconia Coatings Nkem O. Nwosu, Alan M. Davidson, Colin S. Hindle School of Engineering and the Built Environment, Edinburgh Napier University, Edinburgh, UK E-mail: {n.nwosu, a.davidson, c.hindle}@napier.ac.uk Received June 1, 2011; revised June 27, 2011; accept ed July 5, 2011 Abstract The influence of a surfactant on the composition of nickel—yttria stabilised zirconia (YSZ) cermet coatings, applied by electroless nickel plating technique was examined. The amphiphilic characteristics of anionic surfactant sodium dodecyl sulphate (SDS), was relied upon for enhanced dispersion of YSZ particles co-deposited for use as anodes in solid oxide fuel cell technology and potential heat absorbing layers in thermal barrier coatings. Optical microscopy was employed to study the correlation between the plating thickness, level of ceramic loading and SDS concentration while the effect of the surfactant and fineness of YSZ particles on the as-deposited coating’s ceramic to metal ratio, was analysed using energy dispersive X-ray analysis (EDXA) characterisation technique. Keywords: Electroless Nickel Composite Coatings, Solid Oxide Fuel Cell Anodes, Coating Composition, Additives 1. Introduction Electroless nickel plating (ENP) is a highly desirable surface coating technique that has the capability of pro- ducing uniform surface deposits regardless of recesses and bores [1]. Although Brenner & Riddell are credited with developing the process [1,2], Odekerken who while attempting to improve the corrosion resistance of nickel chromium electrodeposits, applied an intermediate layer of alumina (Al2O3) and poly vinyl chloride (PVC) within a metal matrix, is widely acknowledged as the earliest demonstrator of particulate matter incorporation by ENP [3,4]. Since then, numerous studies such as those of Sheela & Pushpavanam [5] who adopted the technique to synthesise high wear resistant diamond-nickel composite coatings, have been carried out to establish potential ap- plications for the technology. Recently, Davidson & Waugh [6] successfully applied ENP to the manufacture of nickel-yttria stabilised zirco- nia cermet coatings for use as solid oxide fuel cell (SOFC) electrodes. This was called electroless co-deposition (ECD) of nickel and ceramic. Simply requiring a source of en- ergy to liberate chelated metal ions maintained in sus- pension by complexing agents, the technique has been shown to have a much faster rate of production and low- er energy consumption than traditional SOFC electrode manufacturing techniques such as silk screening and tape casting [7]. It’s non-requirement of a high energy sinter- ing stage, offers an opportunity for cost reduction and complete elimination of high temperature induced de- fects such as cell warpage occasionally associated with existing processes. In SOFC technology, nickel aids fuel catalysation and electronic conductivity while YSZ in addition to preventing nickel from sintering at high SOFC operating temperatures of about 1000˚C, re- lieves the coefficient of thermal expansion (CTE) mismatch between the anode and electrolyte [8,9,10] (nickel –13 × 10–6/˚C & YSZ –10 × 10–6/˚C). High nickel content pro- motes cell cracking while too low a content and corre- spondingly high YSZ presence could compromise cell efficiency and performance [11]. To avoid unwanted occurrences such as cell de-lamination which may arise due to CTE mismatch, an understanding of the structure of ceramic to metal ratios obtained by ECD or the effect of additives that may aid achievement of a desired 60 - 40 composition is essential. In fact for use of ECD de- posits as intermediate layers in thermal barrier coatings (TBC) where material insulation in high temperature environments is sought, the required proportion of ce- ramic to metal is even greater. Surfactants, non-ionic (steric stabilised) or anionic, ca- tionic and zwitter ionic (electrostatic interaction) are  N. O. NWOSU ET AL. Copyright © 2011 SciRes. ACES 119 surface active agents that in addition to lowering the sur- face tension of a liquid also allow for easy spreading and less interfacial tension between two liquids. Its inherent amphiphilic property that arises du e to its molecules dual lyophobic—lyophilic tendency, has been previously em- ployed for dispersion of ceramic powders in polymers [12,13] and polymers in solutions including electroless nickel [14]. While Wu, et al. [15] used sodium dodecyl sulphate (SDS) to increase the wettability a nd dispersion of silicon carbide particles, Elansezhian, et al. [16] per- formed studies on Ni-P coatings influenced by anionic and cationic surfactants SDS and cetyltrimethyl ammo- nium bromide (CTAB). They reported that the concen- tration of surfactant utilised in the process had an effect on the surface morphology of the deposits. Hardness of the coatings was found to progressively increase with SDS content while surface roughness reduced as the lev- el of surfactant reached 0.6 g/l. The resultant smooth surface was attributed to uniform dispersion of fine nick- el particles. While their observation is in line with the findings of Tr ipathy, et al. [17] who studied the effect of sodium lauryl sulphate on zinc electrowinning from acidic sulfate solutions, Karuppusamy & Anantharam [18] also reported that addition of 150ppm SDS resulted in uniform and pit free nickel deposits. In this work, the influen ce of SDS on the compositio n of nickel-YSZ coatings applied by ECD process is inves- tigated. Associated changes in the characteristics of the coatings such as thickn ess is also assessed and reported. 2. Experimental 2.1. Procedure Alumina (Al2O3) tiles, 40 × 15 × 1 mm were used as substrates for the deposition. Overcoming the insulation posed by such ceramic surfaces was achieved via pre- treatment processes. Cuprolite X-96 DP (2-Aminoe- thanols (1% - 4%)) (A lfachimici, Italy), was employed at 60˚C for 15 mins to degrease the substrate’s surface. Next, to sensitise the surface, the subs trate was immersed in 100 ml of stannous chloride solution at room tem- perature for 15 mins. The final step performed for sur- face activation was carried out in a solution containing palladium chloride with temperature ranging between 36˚C to 40˚C. Electroless nickel solution for the deposition process was made up from proprietary slotonip 18 - 51 starter and slotonip 18 - 53 replenisher chemicals supplied by Schloetter company limited. Bath pH and plating tem- perature were 4.9 and 89˚C respectively. SDS (Fisher Scientific, UK) and yttria stabilised zirconia (YSZ) (Un- itec Inc.) were added to the bath prior to the introduction of the pre-treated alumina tile. A magnetic stirrer was used to ensure particle dispersion and deposition time was 1 hr. Governing reactions are as detailed by Gutzeit [19] in Equations 1-4. 22 223 HPOHOHPO2H 2e (1) 2 Ni 2eNi (2) 2 2H 2eH (3) 22 2 HPO2HeP 2HO (4) Electrons from the hypophosphite ion reduce nickel ions to nickel metal (1) & (2). The nickel metal thereafter entraps YSZ particles while adsorbing on the activated alumina substrate. Unfortunately, phosphorus, a resultant element simul- taneously produced from the hypophosphite ion (4), poses a challenge in fuel cell technology. Besides being noted to have a characteristic property of reducing the anode/cell performance [20,21], phosphorus has a ten- dency to form a liquid phase with nickel at temperatures above 850˚C which thereafter precipitation hardens to yield an undesired material, nickel phosphide (Ni3P). Alternatives such as hydrazine (N2H4)—probably with less deleterious effects—do exist, but have considerable cost implications. 2.2. Sample Preparation and Characterisation A Struers Accutom-5 precision cutter was used to cut cross-sections of the coated substrate. The feed rate was set at 0.02 mm/s and the utilised diamond tip cutting disc was maintained at a speed of 3000 rpm. The samples were thereafter mounted in 40 mm diameter epoxy resin and grinding and polishing was carried out with the aid of the TegraForce-5/TegraPol-21. Contact force for both operations was 50 N with an anti-clockwise rotation of 150 rpm . The surface structure of the coatings was observed with a Cambridge Stereoscan 90 Scanning Electron Mi- croscope (SEM) while an in-situ Oxford Instrument Inca Energy Dispersive X-ray Analysis system (EDXA) was relied upon for analysis of its composition. For examina- tion of the thick ness and characteristics of th e coatings, a Leitz Aristomet Hi-power light microscope was em- ployed. All experiments were repeated twice and an average of both results is reported. 3. Results and Discussion 3.1. Pre-surfactant Ceramic Loading Prior to the introduction of SDS, the optimum pre-sur- factant YSZ concentration for maximum incorporation of 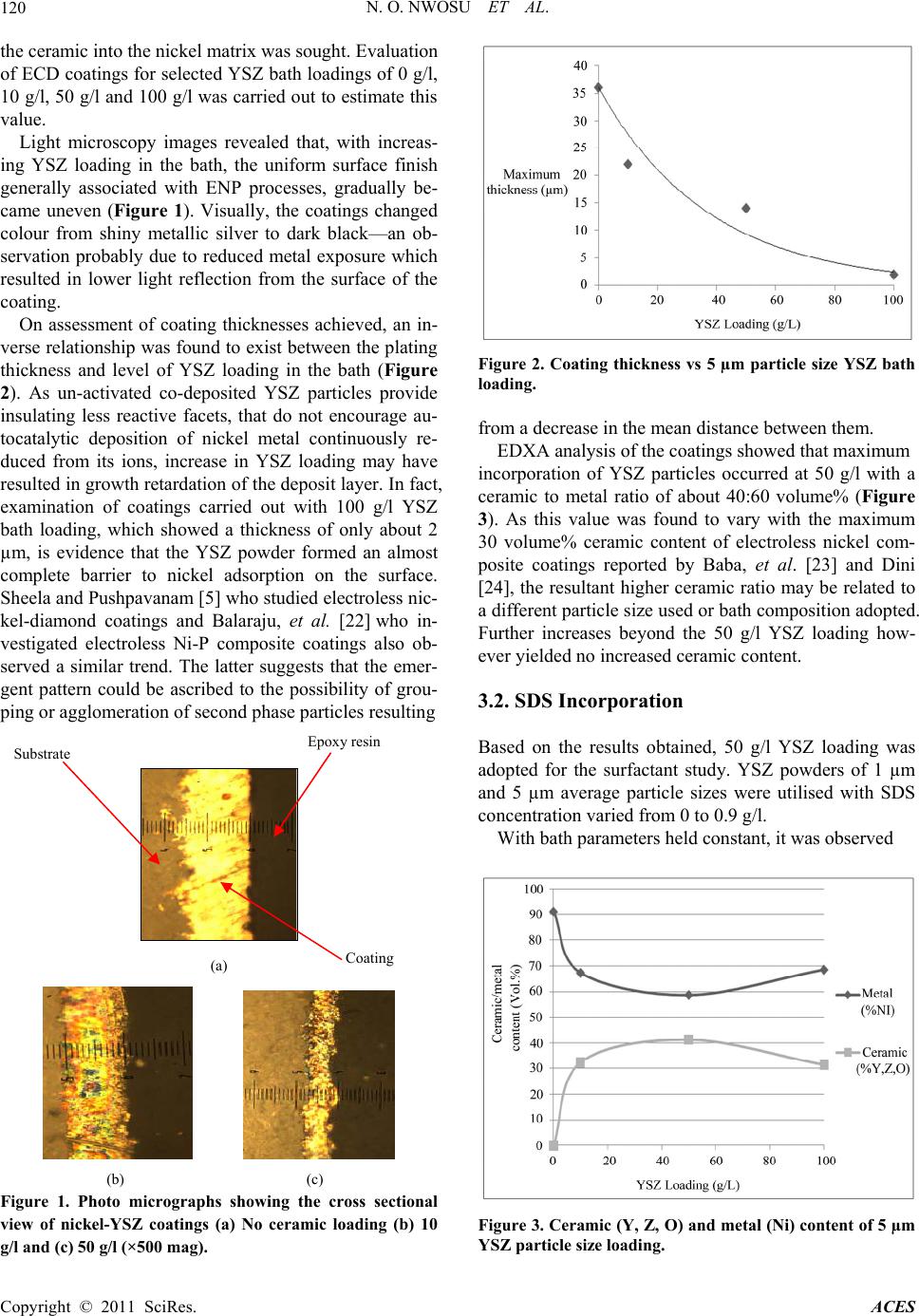 N. O. NWOSU ET AL. Copyright © 2011 SciRes. ACES 120 the ceramic into the nickel matrix was sought. Evaluation of ECD coatings for selected YSZ bath loadings of 0 g/l, 10 g/l, 50 g/l and 100 g/l was carried out to estimate this value. Light microscopy images revealed that, with increas- ing YSZ loading in the bath, the uniform surface finish generally associated with ENP processes, gradually be- came uneven (Figure 1). Visually, the coatings changed colour from shiny metallic silver to dark black—an ob- servation probably due to reduced metal exposure which resulted in lower light reflection from the surface of the coating. On assessment of coating thicknesses achieved, an in- verse relationship was foun d to exist between the plating thickness and level of YSZ loading in the bath (Figure 2). As un-activated co-deposited YSZ particles provide insulating less reactive facets, that do not encourage au- tocatalytic deposition of nickel metal continuously re- duced from its ions, increase in YSZ loading may have resulted in growth retardation of the depo sit layer. In fact, examination of coatings carried out with 100 g/l YSZ bath loading, which showed a thickness of only about 2 µm, is evidence that the YSZ powder formed an almost complete barrier to nickel adsorption on the surface. Sheela and Pushpavanam [5] who studied electro less nic- kel-diamond coatings and Balaraju, et al. [22] who in- vestigated electroless Ni-P composite coatings also ob- served a similar trend. The latter suggests that the emer- gent pattern could be ascribed to the possibility of grou- ping or agglomeration of second phase particles resulting (a) (b) (c) Figure 1. Photo micrographs showing the cross sectional view of nickel-YSZ coatings (a) No ceramic loading (b) 10 g/l and (c) 50 g/l (×500 mag). Figure 2. Coating thickness vs 5 µm particle size YSZ bath loading. from a decrease in the mean distance between them. EDXA analysis of the coatings showed that maximum incorporation of YSZ particles occurred at 50 g/l with a ceramic to metal ratio of about 40:60 volume% (Figure 3). As this value was found to vary with the maximum 30 volume% ceramic content of electroless nickel com- posite coatings reported by Baba, et al. [23] and Dini [24], the resultant higher ceramic ratio may be related to a different particle size used or bath composition adopted. Further increases beyond the 50 g/l YSZ loading how- ever yielded no increased ceramic content. 3.2. SDS Incorporation Based on the results obtained, 50 g/l YSZ loading was adopted for the surfactant study. YSZ powders of 1 µm and 5 µm average particle sizes were utilised with SDS concentration varied from 0 to 0.9 g/l. With bath parameters held constant, it was observed Figure 3. Ceramic (Y, Z, O) and metal (Ni) content of 5 µm YSZ particle size loading. Su b strate E p ox y resin Coatin g 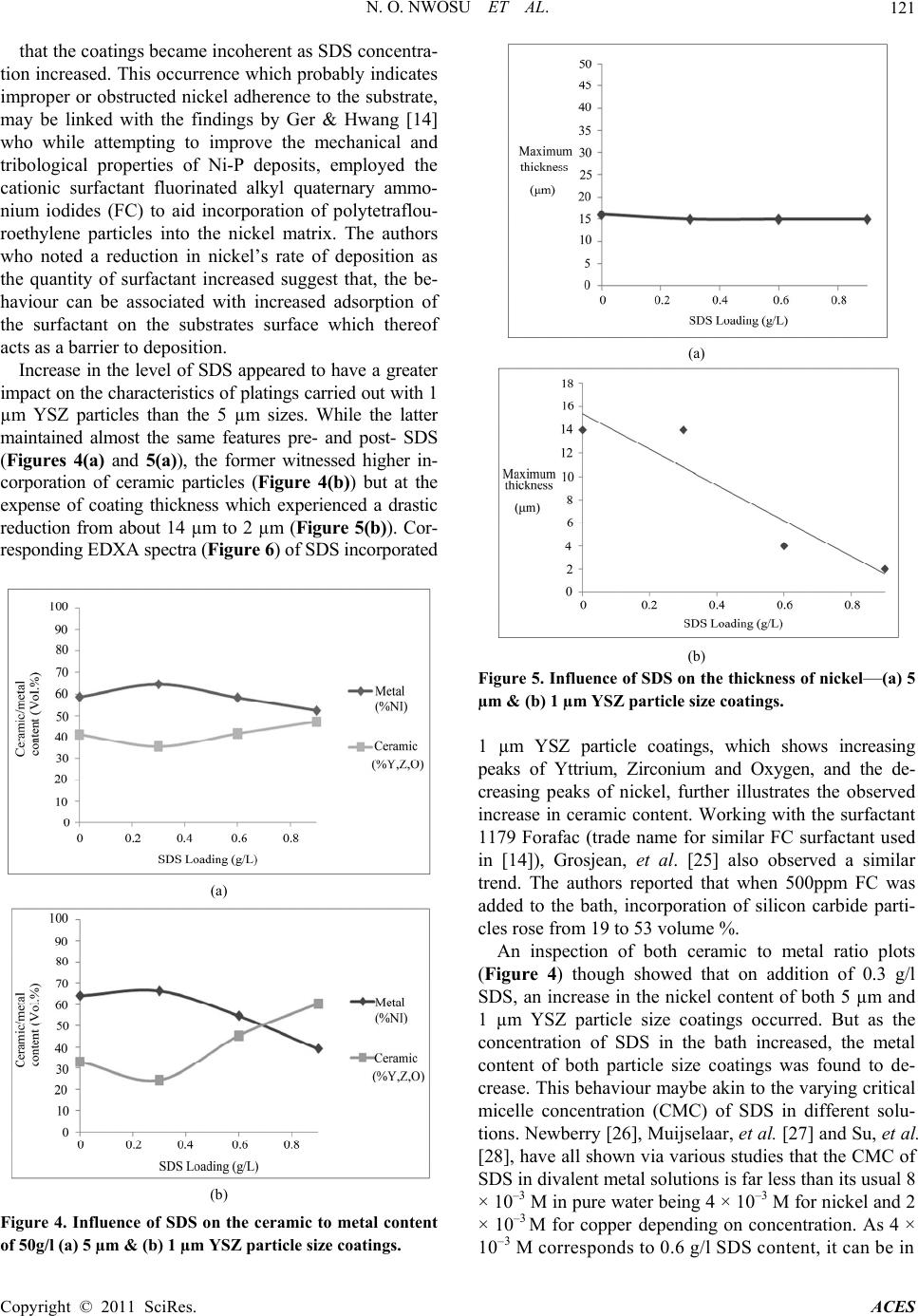 N. O. NWOSU ET AL. Copyright © 2011 SciRes. ACES 121 that the coatings became incoherent as SDS concentra- tion increased. This occurrence which probably indicates improper or obstructed nick el adherence to the substrate, may be linked with the findings by Ger & Hwang [14] who while attempting to improve the mechanical and tribological properties of Ni-P deposits, employed the cationic surfactant fluorinated alkyl quaternary ammo- nium iodides (FC) to aid incorporation of polytetraflou- roethylene particles into the nickel matrix. The authors who noted a reduction in nickel’s rate of deposition as the quantity of surfactant increased suggest that, the be- haviour can be associated with increased adsorption of the surfactant on the substrates surface which thereof acts as a barrier to deposition. Increase in the level of SDS appeared to have a greater impact on the characteristics o f platings carried out with 1 µm YSZ particles than the 5 µm sizes. While the latter maintained almost the same features pre- and post- SDS (Figures 4(a) and 5(a)), the former witnessed higher in- corporation of ceramic particles (Figure 4(b)) but at the expense of coating thickness which experienced a drastic reduction from about 14 µm to 2 µm (Figure 5(b)). Cor- responding EDXA spectra (Figure 6) of SDS incorporated (a) (b) Figure 4. Influence of SDS on the ceramic to metal content of 50g/l (a) 5 µm & (b) 1 µm YSZ particle size coatings. (a) (b) Figure 5. Influence of SDS on the thickness of nickel—(a) 5 µm & (b) 1 µm YSZ particle size coatings. 1 µm YSZ particle coatings, which shows increasing peaks of Yttrium, Zirconium and Oxygen, and the de- creasing peaks of nickel, further illustrates the observed increase in ceramic content. Working with the surfactant 1179 Forafac (trade name for similar FC surfactant used in [14]), Grosjean, et al. [25] also observed a similar trend. The authors reported that when 500ppm FC was added to the bath, incorporation of silicon carbide parti- cles rose from 19 to 53 volume %. An inspection of both ceramic to metal ratio plots (Figure 4) though showed that on addition of 0.3 g/l SDS, an increase in the nickel content of both 5 µm and 1 µm YSZ particle size coatings occurred. But as the concentration of SDS in the bath increased, the metal content of both particle size coatings was found to de- crease. This behaviour maybe akin to the varying critical micelle concentration (CMC) of SDS in different solu- tions. Newberry [26], Muijselaar, et al. [27] and Su, et al. [28], have all shown via various studies that the CMC of SDS in divalent metal solutions is far less than its usu al 8 × 10–3 M in pure water being 4 × 10–3 M for nickel and 2 × 10–3 M for copperdepending on concentration. As 4 × 10–3 M corresponds to 0.6 g/l SDS content, it can be in  N. O. NWOSU ET AL. Copyright © 2011 SciRes. ACES 122 (a) (b) (c) Figure 6. Energy Dispersive X-ray spectra of (a) 0.3 g/l (b) 0.6 g/l (c) 0.9 g/l SDS incorporated Ni-YSZ coatings. ferred that the attained CMC enhanced YSZ particle dis- persion which yielded an increased level of ceramic in- corporation in the coating. Examination of SEM micrographs (Figure 7) appear to suggest that surface roughness of the coatings in- creased with SDS content—an observation consistent with the report by Alsari, et al. [29]. While investigating the effect of SDS solutions as gelation media on the for- mation of polyethersulfone (PES) membranes, the au- thors noted that upon SDS attaining its CMC, the conse- quential increase in pore size resulted in increased rough- ness of the membranes. 4. Conclusions The effect of the surfactant SDS on the composition of keV  N. O. NWOSU ET AL. Copyright © 2011 SciRes. ACES 123 (a) (b) (c) Figure 7. SEM images of (a) 0.3 g/l (b) 0.6 g/l (c) 0.9 g/l SDS incorporated 50 g/l YSZ loading/1 µm particle size coatings × 1,000 mag. nickel-YSZ ECD composite coatings, has been investi- gated and reported. Results obtained show that, by adding SDS to an elec-troless nickel bath containing a pre-determined op- timum YSZ loading of 50 g/l, up to 60 volume% YSZ can be incorporated into cermet coatings applied by ECD. A trade-off though w as observed w ith increasing SDS con- tent as plating thickness decrea sed f r om 14 µm to about 2 µm and the coatings became incoherent. Inspection of the surfaces via micrographs obtained by SEM appeared to support the fact that surface roughness also increased with SDS concentration. Overall, the achievement of 60:40 ceramic to metal ra- tio may suggest, existence of materials that may be in- corporated to enhance the ceramic content of SOFC electrodes synthesised by ECD. However, with a rela- tively high metal content still present, further optimisa- tion may be needed to adapt the process to technologies such as thermal barrier coatings. 5. References [1] G. O. Mallory and J. B. Hajdu, “Electroless Plating: Fundamentals and Applications,” William Andrew Publishing, Burlington, 1990. [2] A. Brenner and G. Riddel, “Nickel Plating on Steel by Chemical Reduction,” US Patent 2532282, 1950. [3] R. C. Agarwala and V. Agarwala, “Electroless Alloy/Com- posite Coatings: A Review,” Sadhana, Vol. 28, No. 3-4, 2003, pp. 475-493. doi:10.1007/BF02706445 [4] J. M. Odekerken, “Use of Co-deposited Non-conducting Materials to Improve the Corrosion Resistance of Nickel- Chromium Electrodeposits,” British Patent 1041753, U.S. Patent 3644183 and DDR Patent 414061964. [5] G. Sheela and M. Pushpavanam, “Diamond-Dispersed Electroless Nickel Coatings,” Journal of Metal finishing, Vol. 100, No. 1, 2002, pp. 45-47. [6] A. Davidson and W. Waugh, “Method of Manufacture of an Electrode for a Fuel Cell,” World intellectual Property Organisation Patent No. O/2009/044144, 2009. [7] N. Nwosu, A. Davidson and W. Waugh, “Characterisa- tion of Solid Oxide Fuel Cell Cathodes Manufactured by Traditional and Novel (Low Cost) Techniques,” Proceed- ings of the First International Conference on Materials for Energy, Karlsruhe, 4-8 July 2010, pp. 81-85. [8] J. Mizusaki, S. Tsuchiya, K. Waragai, H. Tagawa, A. Yoshihidi and Y. Kuwayama, “Simple Mathematical Model for the Electrical Conductivity of Highly Porous Ceramics,” Journa l of American Ceramic Society, Vol. 79, No. 1, 1996, pp. 109-113. doi:10.1111/j.1151-2916.1996.tb07887.x [9] W. Dees, T. D. Claar, T. E. Easler, D. C. Fee and F. C. Mrazek, “Conductivity of Porous Ni/ZrO2-Y2O3 Cermets,” Journal of Electrochemical Society, Vol. 134, No. 9, 1987, pp. 2141-2146. doi:10.1149/1.2100839 [10] T. Iwata, “Characterization of Ni-YSZ Anode Deg radation for Substrate-Type Solid Oxide Fuel Cells,” Journal of Electrochemical So c i e ty, Vol. 143, No. 5, 1996, pp. 1521- 1525. doi:10.1149/1.1836673 [11] R. Bauri, “Development of Ni-YSZ Cermet Anode for Solid Oxide Fuel Cells by Electroless Ni Coating,” Jour- nal of Coating Technology and Research, 2009. doi:10.1007/s11998-009-9223-z [12] Y. Rao, A. Takahashi and C. P. Wong, “Di-block Co- polymer Surfactant Study to Optimize F il ler D ispersion in High Dielectric Constant Polymer-Ceramic Composite,” Journal of Composites Part A: A pplied Sci ence and Manu -  N. O. NWOSU ET AL. Copyright © 2011 SciRes. ACES 124 facturing, Vol. 34, No. 11, 2003, pp. 1113-1116. doi:10.1016/S1359-835X(03)00202-1 [13] L. Gabrieson and M. J. Edirisinghe, “On the Dispersion of Fine Ceramic Powders in Polymers,” Journal of Materials Science Letters, Vol. 15, No. 13, 1996, pp. 1105-1107. doi:10.1007/BF00539950 [14] M.-D. Ger and B. J. Hwang, “Effect of Surfactants on Co-deposition of PTFE Particles with Electroless Ni-P Coating,” Materials Chemistry and Physics, Vol. 76, No. 1, 2002, pp. 38-45. doi:10.1016/S0254-0584(01)00513-2 [15] Y. C. Wu, G. H., Li L. Zhang and B. Yan, “Study on Constitution and Wear Resistance of Nickel Phosphorus Alloy-Silicon Carbide Composite Coatings,” Materials Research and Advanced Techniques, Vol. 91, 2000, pp. 788-793. [16] R. Elansezhian, B. Ramamoorthy and P. K. Nair, “The In- fluence of SDS and CTAB Surfactants on the Surface Morphology and Surface Topography of Electroless Ni-P Deposits,” Journal of Materials Processing Technology, Vol. 209, No. 1, 2009, pp. 33-240. doi:10.1016/j.jmatprotec.2008.01.057 [17] B. C. Tripathy, S. C. Das., G. T. Hefter and P. Singh, “Electro Winning from Acidic Sulphate Solution. Part 1. Effects of SLS,” Journal of Applied Electrochemistry, Vol. 27, No. 6, 1997, pp. 673-674. doi:10.1023/A:1018431619595 [18] K. Karuppusamy and R. Anantharam, “Pit-Free Nickel Electroplating,” Metal finishing , Vol. 90, No. 13, 1992, pp. 15-19. [19] G. Gutzeit, “Catalytic Nickel Deposition from Aqueous Solution. I-IV,” Plating surface finishing, Vol. 46, 1959, pp. 1158-1164, 1275-1278, 1377-1378. [20] K. Haga, Y. Shiraton, Y. Nojiri, K. Ito and K. Sasaki, “Phosphorus Poisoning of Ni-Cermet Anodes in Solid Oxide Fuel Cells,” Journal of Electrochemical Society, Vol. 157, No. 11, 2010, pp. 1693-1700. doi:10.1149/1.3489265 [21] A. Tsoga, A. Naoumidis and P. Nikolopoulos, “Wettabil- ity and Interfacial Reactions in the Systems Ni/YSZ and Ni/Ti-TiO2/YSZ,” Acta Materialia, Vol. 44, No. 9, 1996, pp. 3679-3692. doi:10.1016/1359-6454(96)00019-5 [22] J. N. Balaraj u, T. S. N. S. Naraya nan an d S. K. Sesha dri, “Electroless Ni-P Composite Coatings,” Journal of Ap- plied Electr ochemistry, Vol. 33 , No. 9, 2003, pp. 807 -816. doi:10.1016/1359-6454(96)00019-5 [23] N. B. Baba, W. Waugh and A. Davidson, “Manufacture of Electroless Nickel/YSZ Composite Coatings,” Proceed- ings of World Academy of Science, Engineering and Technology, Vol. 49, 2009, pp. 715-720. [24] J. W. Dini, “Electrodeposition: The Materials Science of Coatings and Substrates,” William Andrew Publishing, Noyes, 1993, p. 336. [25] A. Grosjean, M. Rezrazi and M. Tachez, “Study of the Surface Charge of Silicon Carbide (SIC) Particles for Electroless Composite Deposits: Nickel-SIC,” Surface and Coating technology, Vol. 96, No. 2-3, 1997, pp. 300- 304. [26] J. E. Newberry, “Surface Interactions of Micelles and Divalent Metal Ions,” Journal of Colloid and Interface Science, Vol. 74, No. 2, 1979, pp. 483-488. doi:10.1016/0021-9797(80)90217-9 [27] P. G. Muijselaar, K. Otsuka and S. Terabe, “Micelles as Pseudo-Stationary Phases in Micellar Electrokinetic Chromatography,” Journal of Chromatography, Vol. 780, No. 1, 1997, pp. 41-61. doi:10.1016/S0021-9673(97)00632-8 [28] S. Su, Y. L. Chen and C. Y. Mou, “Micelle-Counterion Interaction, I. Critical Micelle Concentrations of SDS under the Influence of Copper Counterion,” Journal of Chinese Chemical Society, Vol. 32, No. 1, 1985, pp. 5-10. [29] A. M. Alsari, K. C. Khulbe and T. Matsuura, “The Effect of Sodium Dodecyl Sulfate Solutions as Gelation Media on the Formation of PES Membranes,” Journal of Mem- brane Science, Vol. 188, No. 2, 2001, pp. 279-293. doi:10.1016/S0376-7388(01)00395-7 |

