Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 20 1 1, 1, 83-89 doi:10.4236/aces.2011.13014 Published Online July 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Biosynthesis of Ethanol and Hydrogen by Glycerol Fermentation Using Escherichia coli Nida Chaudhary1, Michael O. Ngadi1, Benjamin K. Simpson2, Lamin S. Kassama3 1Department of Bioresource Engineering, McGill University, Montreal, Canada 2Department of Foo d Scie nce , McGill University, Montreal, Canada 3Department of Food an d A nimal Sciences , Alabama A&M University, Huntsville, USA E-mail: michael.ngadi@mcgill.ca Received May 13, 201 1; revised June 20, 2011; accepted June 28, 2011 Abstract Production of high value products from glycerol via anaerobic fermentation is of utmost importance for the biodiesel industry. The microorganism Escherichia coli (E. coli) K12 was used for fermentation of glycerol. The effects of glycerol concentration and headspace conditions on the cell growth, ethanol and hydrogen production were investigated. A full factorial experimental design with 3 replicates was conducted in order to test these factors. Under the three headspace conditions tested, the increase of glycerol concentration ac- celerated glycerol fermentation. The yields of hydrogen and ethanol were the lowest when glycerol concen- tration of 10 g/L was used. The maximum production of hydrogen was observed with an initial glycerol concentration of 25 g/L at a final concentration of hydrogen was 32.15 mmol/L. This study demonstrated that hydrogen production negatively affects cell growth. Maximum ethanol yield was obtained with a glyc- erol concentration of 10 g/L and was up to 0.40 g/g glycerol under membrane condition headspace. Statisti- cal optimization showed that optimal conditions for hydrogen production are 20 g/L initial glycerol with ini- tial sparging of the reactor headspace. The optimal conditions for ethanol production are 10 g/L initial glyc- erol with membrane. Keywords: Fermentation, Ethanol, Hydrogen, Glycerol, Cell Growth, Reactor Headspace 1. Introduction The continuous use of fossil fuels is globally accepted as not sustainable largely because of the underlying envi- ronmental factors. The depletion of natural resources and the accumulation of greenhouse gasses posses’ signifi- cant threat to global warming. Schenk et al. [1] reported that the greenhouse gasses in the environment have al- ready exceeded “dangerously higher” threshold of 450 ppm CO2-e. These starling findings are leading govern- ments to establish regulations to reduce CO2 emissions. Furthermore, the increase dependency on foreign oil is of grave concern to national securities and economic stabil- ity. Converting biomass-derived oil to biofuels is a viable substitute for petroleum-based liquid fuel. Glycerol is a by-product that in produced in abundance in biodiesel fuel production. Yazdani [2] reported that 10 lb of crude glycerol is generated for every 100 lb of biodiesel fuel produced by transesterification of vegetable oils or ani- mal facts. The current growth in the biodiesel industry results to surplus glycerol being produced, thus the price of crude glycerol has dropped substantially [2]. The col- lapsed glycerol price has led to the shutdown of many glycerol producing plants, hence negatively affected the economic viability of the biodiesel industry [3]. As a re- sult, glycerol becomes a waste stream instead of being a desirable co-product of significant economic value [2] and in compliance to environmental regulation the bur- den of cost for dispo sal of the effluent became a liability for plants [4]. Thus, improving the bioconversion meth- ods of the low-cost glycerol into higher valued product will provide an incentive for the commercialization of glycerol into biofuel. Glycerol (C3H8O3: = 4.7) is a better source of carbon compared to the common fermentable sugars (glucose C6H12O6: = 4.0; xylose C5H10O5: = 4.0) for fermenta- tion [5,6]. The common sugars (glucose, xylose etc) ad- ditionally produce succinate, and propanodiols during fermentation when compared to glycerol [2,7]. The con-  N. CHAUDHARY ET AL. Copyright © 2011 SciRes. ACES 84 version of glycerol to a higher value product requires channelling the processes through chemical and biologi- cal pathways. Glycerol fermentation by organisms are mediated by two-branch-pathway, which results in the synthesis of the glycolytic intermediate dihydroxyacetone (DHA), dihydroxyacetone phosphate (DHAP) and the fermented product 1,3-propanediol (1,3-PDO) [2,8,9]. In effect the biological conversion process improves the function of chemical catalysis to synthesise crude glyc- erol with high levels of contaminants [2]. The microbial fermentation of glycerol can be either aerobic or anaero- bic process; the anaerobic fermentation is preferred be- cause of the low capital and operational costs of anaero- bic fermenters. The microbial conversion of glycerol to various com- pounds has been investigated by many authors [10-14]. Several microorganisms have been used to ferment glyc- erol into 1,3-propanediol (1,3-PDO) which is a basic ingredient for polyester production [15]. Kiebsiella pneumonia has been cited as an excellent producer of 1,3-PDO [16], howeve r, the presences of oxygen inhibits the metabolic pathway of converting glycerol into etha- nol [17]. The Citrobacter species is another microbe that received many interest for fermenting glycerol into 1,3-PDO [18], hence Clostridium species are also good candidates for similar reasons [19]. However, the patho- genicity, the need for strict anaerobic conditions, lack of genetic tools and requirement of rich nutrients can be limiting factors for the use of these microorganisms [20]. Esherishia coli (E. coli) is one of the most commonly used host organisms for metabolic engineering and in- dustrial application s, because it is easy to manipulate ge- netically and can produce wide variety of anaerobic fer- mentation products [10]. Dharmadi [21], Hu and Wood [10] reported that E. coli ferments glycerol in a low pH medium, and the fer- mentation is dependent on CO2 availability. These au- thors reported that oxidation of formate hydrogen lyase (FHL) produces CO2 which severely impedes the fer- mentation process and block the FHL activity. Eighty percent (80%) of glycero l initially pr esented in the media is consumed within 84 h of the active growth period, producing 86% ethanol and 7% succinic acid with minor fraction of acetate and no detectable formate or lactate [22]. Yazdani et al. [22] created two strains of E. coli for the co-production of ethanol-hydrogen and ethanol-for- mate. They also reported high yield of ethanol with both strains and minimal synthesis of the by-products such as succinate and acetate. The yield observed in their study was higher than those obtained with other organisms. The accumulation of hydrogen was observed, and is problematic to the metabolic recycling and generates an unfavourable internal redox state [3]. Thus, controlling and preventing hydrogen accumulation during glycerol fermentation is significant in glycerol production [20]. Esherishia coli can be used as a microorganism for a high-yield production of ethanol from low cost glycerol. However, there is lack of adequate information in litera- ture on the ideal culture and effective fermentation con- ditions. Therefore, the aim of this research is to study ef- fect different gro wth condition s and how it a ffects ethanol and hydrogen production during glycerol fermentation with E. coli in lab-scale bioreactors. 2. Materials and Methods 2.1. Microorganism and Maintenance Esherishia coli MG1655 (ATCC 700926) was obtained from Cedar Lane Labs. The M9 minimal medium con- tained 990 ml distilled water, 2 ml of MgSO4, 10 ml of 20% glucose, 6.0 g Na2HPO4, 3.0 g KH2PO4, 0.5 g NaCl and 1.0 g NH4Cl were mixed. Agar (3.6 g) was added to 240 ml of this medium and autoclaved and subsequently poured into Petri dishes. Sixty millilitres of the medium was used to rehydrate the bacteria pellet. The bacteria was then transferred to the plates and grown overnight at 37˚C. Single colonies were then replaced onto Luria Bertani agar plates and incubated overnight at 37˚C. Sin- gle colonies selected from these agar plates were used to generate 80% glycerol stock solutions which were then stored at –80˚C. At the beginning of each reactor run, a sterile pipette tip was used to transfer cells from glycerol stock into 5 ml of Luria Bertani media broth and incubated overnight at 37˚C. During the exponential growth phase, a sample was drawn and plated onto Luria Burtani agar plates and incubated at 37˚C in anaerobic state inside an anaerobic jar (Oxoid Anaerojar, AG0025A) for 24 hours. 2.2. Inocul ums an d Cul ture Medi um A modified rich buffer (10X MOPS) minimal media kit (Teknova, M2106) was used to make MOPS minimal media and supplemented with 10 ml of 0.132 N2HPO4 and 1 ml 1 mol sodium selenite adjusted to pH 6.3 to serve as experimental media. The media was also sup- plemented with 5 g/L yeast extract and 10 g/L tryptone. Hungate tubes (Bellco Glass, 2047-16125) filled with media with 10 g/L glycerol as carbon source and inocu- lated with a single colony taken from the anaerobically grown agar plate. The hungate tubes were incubated at 37˚C until an initial optical density of 0.1 O.D was reached. Each run required 2.25 L of media supplemented with 10, 25 or 50 g/L of glycerol in a bioreactor and in- oculated to maintain an initial O.D of 0.05.  N. CHAUDHARY ET AL. Copyright © 2011 SciRes. ACES 85 2.3. Analytical Methods Biomass was measured by measuring dry weight which was in turn correlated with optical density. A 15 ml sam- ple was collected and the optical d en sity measu red at 550 nm in spectrophotometer zeroed with sterile broth. The sample was then centrifuged for 10 minutes at 10 000 rpm. The pellet was collected and washed twice with 35 ml distilled water. It was then dried at 105˚C for 24 hours and weighed. The supernatant was collected and stored for analysis at –20˚C. Glycerol concentration was determined using the glycerol assay kit (Megazyme, K-GCROL). Ethanol con- centration was measured using the ethanol assay kit (Megazyme, K-ETOH). Hydrogen (H2) concentration of the headspace was measured by taking samples through a septa with a syringe and direct injection into an HP Packard Series II 5890 GC with TCD detector and Pora- pak Q Molecular Sieve 5A column with argon carrier at 75 psig. A one point calibration was carried out to de- termine the relationship between peak size and H2 con- centration. 2.4. Experimental Design A full (2 × 3) factorial design with 3 replicates was used. The factors were glycerol concentration and headspace and three levels were 10, 25, 50 g/L and initial sparging, continuous sparging membrane, respectively, were used as shown in Table 1. Statistical analysis was conducted with SAS® version 9.1 (SAS, 2003) statistical software package was used for data analysis. 3. Results and Discussion 3.1. Bioprocessing of Ethanol Table 1. Experimental design and set-up of the bior e act or s. Levels Factor Low Medium High Glycerol Concentration 10 g/L 25 g/L 50 g/L Headspace Initial Sparge Continuous Sparge Membrane A typical profile for hydrogen, ethanol and dry weight production during fermentation of glycerol by E. coli strain MG1655 (ATCC 700926) is shown of Figure 1. Exponential production of hydrogen was observed during the first 12 h of fermentation and maximum concentration was achieved after 84 h of fermentation. Glycerol was almost completely consumed within the 84 hrs of fer- mentation when only 0.55 g/L of the initial 9.6 g/L of glycerol remained. The remaining time after the 84 h ap- parently falls within the stationary period. Dharmadi et al. [21] reported that within 84 h, 80% of the glycerol ini- tially present in the medium was consumed, a trend dif- ferent from this study large due to different microbial strains used as well as varied experimental conditions. Cell concentration in the first 36 h was observed to be 0.5 g/L and sluggishly reached 0.73 g/L at the end of 120 h fermentation. This could be attributed to the accumulation of hydrogen, t h us i nhi bi t development of bi omass [20]. Glycerol concentration has an important impact from the economic point of view. It is desirable to avoid in- cluding more water than necessary in the system in or- der to minimize the energy consumption for heating and pumping in the reactor [23]. Figure 2 shows the com- parison between cell growth and H2 production in three different glycerol concentrations. The highest final dry weight concentration was observed at glycerol concen- tration of 25 g/L followed by 50 g/L and 10 g/L. This result is different from the results obtained by Zhu et al. [24] who reported that high levels of glycerol signifi- cantly inhibit cell growth. This difference may have oc- curred due to the different strains of E. coli used. The effect of headspace may have also contributed to some of the differences. The lowest final cell dry weight and highest hydrogen concentration wer e observed under ini- tial sparging headspace condition. Continuous sparging and the membrane conditions promoted the stripping of fermentative gases from the process, hence the hydrogen production was limited [20]. Statistical analysis revealed that glycerol concentration and headspace condition sig- nificantly (at the 5% level) affected cell dry weight, ethanol and hydrogen production. Although hydrogen is a growth-associated product (as shown from Figure 1), it inhibits the growth of Escherichia coli during glycerol fermentation. In general, as higher concentrations of hy- drogen are produced, there was a decrease in the final cell dry weight concentrations. Figure 3 shows the comparison between cell growth and ethanol production in three different glycerol con- centrations. As expected, final ethanol concentrations are somewhat related to cell growth since ethanol production is growth-dependent. The highest amount of ethanol pro- duced and the highest cell dry weight were in the mem- 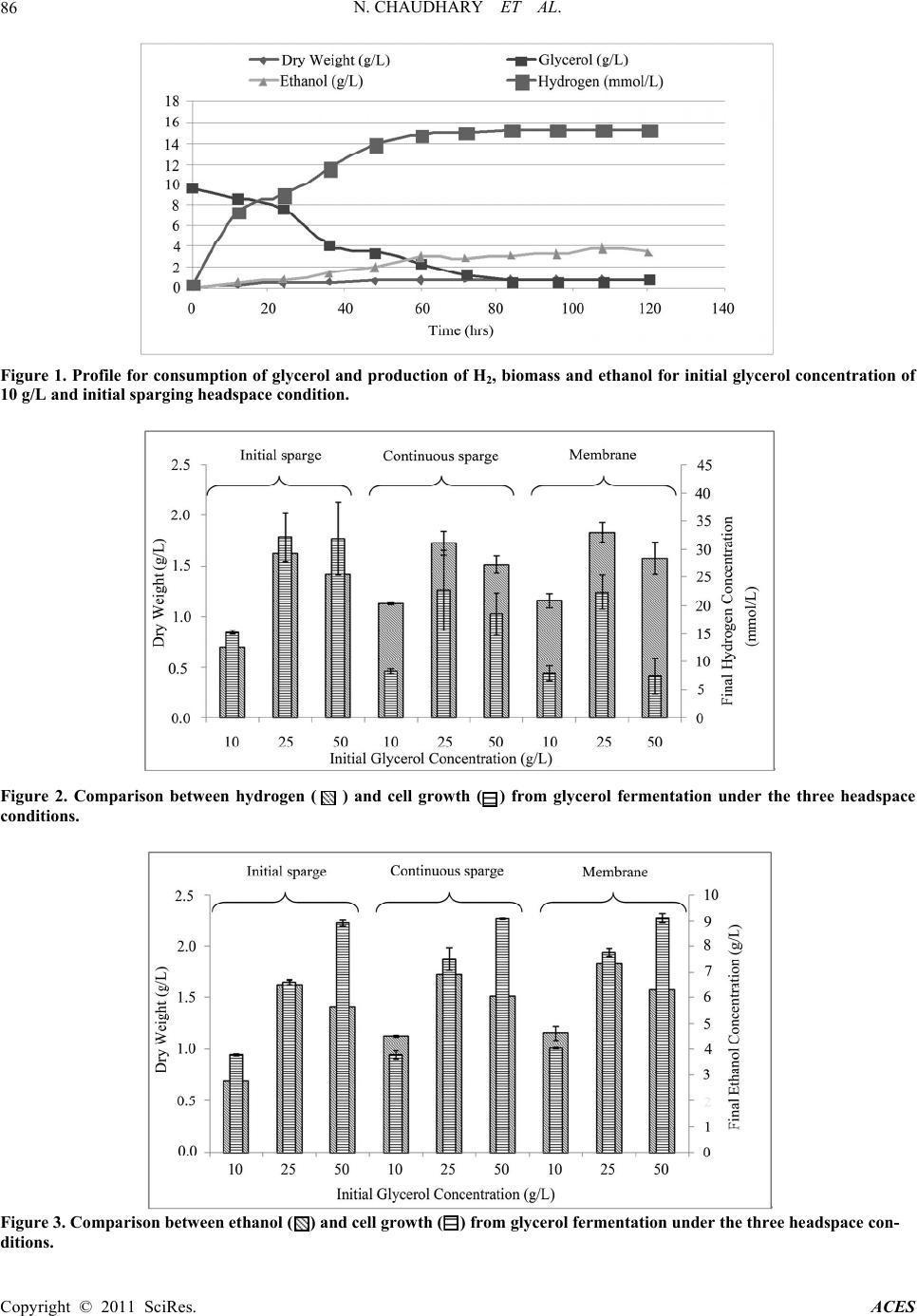 N. CHAUDHARY ET AL. Copyright © 2011 SciRes. ACES 86 Figure 1. Profile for consumption of glycerol and production of H2, biomass and ethanol for initial glycerol concentration of 10 g/L and initial sparging headspace condition. Figure 2. Comparison between hydrogen ( ) and cell growth ( ) from glycerol fermentation under the three headspace conditions. Figure 3. Comparison between ethanol ( ) and cell growth ( ) from glycerol fermentation under the three headspace con- ditions. 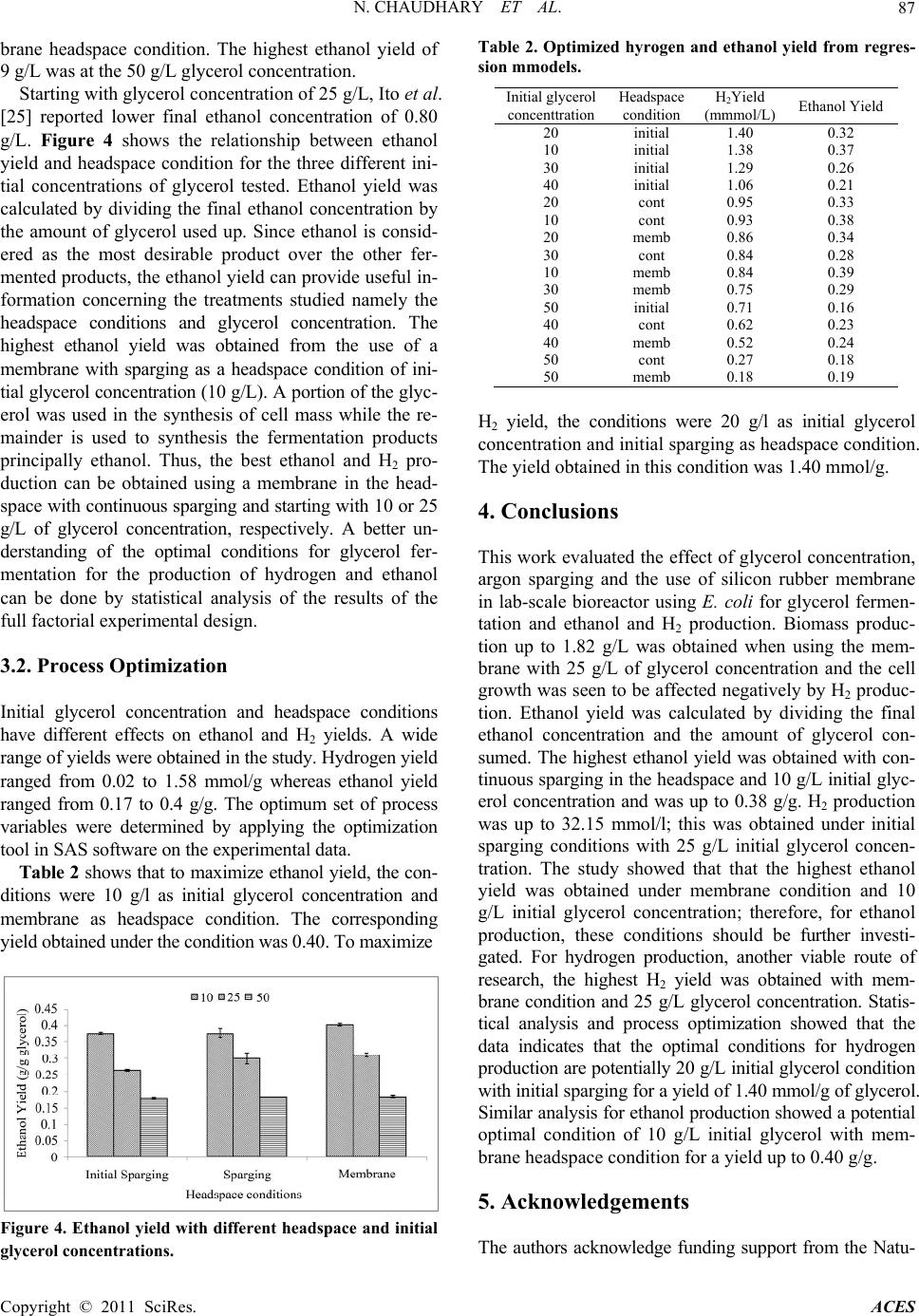 N. CHAUDHARY ET AL. Copyright © 2011 SciRes. ACES 87 brane headspace condition. The highest ethanol yield of 9 g/L was at the 50 g/L glycer o l concentration. Starting with glycerol concentration of 25 g/L, Ito et al. [25] reported lower final ethanol concentration of 0.80 g/L. Figure 4 shows the relationship between ethanol yield and headspace condition for the three different ini- tial concentrations of glycerol tested. Ethanol yield was calculated by dividing the final ethanol concentration by the amount of glycerol used up. Since ethanol is consid- ered as the most desirable product over the other fer- mented products, the ethanol yield can provide useful in- formation concerning the treatments studied namely the headspace conditions and glycerol concentration. The highest ethanol yield was obtained from the use of a membrane with sparging as a headspace condition of ini- tial glycerol concentration (10 g/L). A portion of the glyc - erol was used in the synthesis of cell mass while the re- mainder is used to synthesis the fermentation products principally ethanol. Thus, the best ethanol and H2 pro- duction can be obtained using a membrane in the head- space with continuous sparging and starting with 10 or 25 g/L of glycerol concentration, respectively. A better un- derstanding of the optimal conditions for glycerol fer- mentation for the production of hydrogen and ethanol can be done by statistical analysis of the results of the full factorial experimental design. 3.2. Process Optimization Initial glycerol concentration and headspace conditions have different effects on ethanol and H2 yields. A wide range of yields were obtained in the study. Hydrogen yield ranged from 0.02 to 1.58 mmol/g whereas ethanol yield ranged from 0.17 to 0.4 g/g. The optimum set of process variables were determined by applying the optimization tool in SAS soft ware on the experiment al dat a. Table 2 shows that to maximize ethanol yield, the con- ditions were 10 g/l as initial glycerol concentration and membrane as headspace condition. The corresponding yield obtained under the condition was 0.40. To maximize Figure 4. Ethanol yield with different headspace and initial glycerol concent ra t io ns . Table 2. Optimized hyrogen and ethanol yield from regres- sion mmodels. Initial glycerol concenttration Headspace condition H2Yield (mmmol/L) Ethanol Yield 20 initial 1.40 0.32 10 initial 1.38 0.37 30 initial 1.29 0.26 40 initial 1.06 0.21 20 cont 0.95 0.33 10 cont 0.93 0.38 20 memb 0.86 0.34 30 cont 0.84 0.28 10 memb 0.84 0.39 30 memb 0.75 0.29 50 initial 0.71 0.16 40 cont 0.62 0.23 40 memb 0.52 0.24 50 cont 0.27 0.18 50 memb 0.18 0.19 H2 yield, the conditions were 20 g/l as initial glycerol concentration and initial sparging as headspace condition. The yield obtained in this condition was 1.40 mmol/g. 4. Conclusions This work evaluated the effect of glycerol concentration, argon sparging and the use of silicon rubber membrane in lab-scale bioreactor using E. coli for glycerol fermen- tation and ethanol and H2 production. Biomass produc- tion up to 1.82 g/L was obtained when using the mem- brane with 25 g/L of glycerol concentration and the cell growth was seen to be affected negatively by H2 produc- tion. Ethanol yield was calculated by dividing the final ethanol concentration and the amount of glycerol con- sumed. The highest ethanol yield was obtained with con- tinuous sparging in the headspace and 10 g/L initial glyc- erol concentration and was up to 0.38 g/g. H2 production was up to 32.15 mmol/l; this was obtained under initial sparging conditions with 25 g/L initial glycerol concen- tration. The study showed that that the highest ethanol yield was obtained under membrane condition and 10 g/L initial glycerol concentration; therefore, for ethanol production, these conditions should be further investi- gated. For hydrogen production, another viable route of research, the highest H2 yield was obtained with mem- brane condition and 25 g/L glycerol concentration. Statis- tical analysis and process optimization showed that the data indicates that the optimal conditions for hydrogen production are potentially 20 g/L initial gl ycerol condition with initial sparging for a yield of 1.40 mmol/g of glycerol. Similar analysis for ethanol production showed a potential optimal condition of 10 g/L initial glycerol with mem- brane headspace condition for a yield up to 0.40 g/g. 5. Acknowledgements The authors acknowledge funding support from the Natu-  N. CHAUDHARY ET AL. Copyright © 2011 SciRes. ACES 88 ral Sciences and Engineering Research C ouncil of C anada. 6. References [1] P. M. Schenk, S. R. Tho ma s-Ha ll, E. Stephe ns, U. C. Mar x, J. H. Mussgnug, C. Posten, O. Kruse and B. Hankamer, “Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Production,” Bioengineering Research, Vol. 1, No. 1, 2008, pp. 20-43. [2] S. S. Yazdani and R. Gonzalez, “Anaerobic Fermentation of Glycerol: A Path to Economic Viability for the Biofu- els Industry,” Current Opinion in Biotechnology, Vol. 18, No. 3, 2007, pp. 213-219. doi:10.1016/j.copbio.2007.05.002 [3] M. McCoy, “Gly cerin Surplus,” Chemical & Engineering News, Vol. 84, No. 6, 2006, p. 7. doi:10.1021/cen-v084n006.p007a [4] T. Willke and K. D. Vorlop, “Industrial Bioconversion of Renewable Resources as an Alternative to Conventional Chemistry,” Applied Microbiology and Biotechnology, Vol. 66, No. 2, 2004, pp. 131-142. doi:10.1007/s00253-004-1733-0 [5] G. Durnin, J. Clomburg, Z. Yeates, P. J. J. Alvarez, K. Zygourakis, P. Campbell and R. Gonzalez, “Understand- ing and Harnessing the Microaerobic Metabolism of Glycerol in Escherichia coli,” Biotechnology and Bioen- gineering, Vol. 103, No. 1, 2008, pp. 148-161. doi:10.1002/bit.22246 [6] J. Nielsen, J. Villadsen and G. Liden, “Bioreaction Engi- neering Principles,” Kluwer Academic/Plenum Publishers, New York, 2003, pp. 60-73. [7] K. S. Tyson, J. Bozell, R. Wallace, E. Peterson and L. Moens, “Biomass Oil Analysis: Research Needs and Reco- mmendations,” Technical Report, National Renewable En- ergy Laboratory, US Department of Energy. NREL/TP- 510-34796, 2004. [8] I. Booth, “Glycerol and Methylglyoxal Metabolism,” In: F. C. Neidhardt, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger, Eds., Escherichia coli and Salmo- nella: Cellular and Molecular Biology, ASM Press, Washington D.C., 2005.www.ecosal.org [9] O. M. Bouvet, P. Lenormand, E. Ageron, and P. A. Gri- mont, “Taxonomic Diversity of Anaerobic Glycerol Dis- similation in the Enterobacteriaceae,” Research Microbi- ology, Vol. 146, No. 4, 1995, pp. 279-290. doi:10.1016/0923-2508(96)81051-5 [10] H. Hu and T. K. Wood, “An Evolved Escherichia Coli Strain for Prooducing Hydrogen and Ethanol from Glyc- erol,” Biochemical and Biophysical Research Communi- cations, Vol. 391, No. 1, 2010, pp. 1033-1038. doi:10.1016/j.bbrc.2009.12.013 [11] G. Sabourin-Provost and P. C. Hallenbeck, “High Yield Conversion of a Crude Glycerol Fraction from Biodiesel Production to Hydrogen by Photofermentation,” Biore- source Technology, Vol. 100, No. 14, 2009, pp. 3513- 3517. doi:10.1016/j.biortech.2009.03.027 [12] W. Parawira, “Biotechnological Production of Biodiesel Fuel Using Biocatalysed Transesterification: A Review,” Critical Review Biotechnology, Vol. 29, No. 2, 2009, pp. 82-93. doi:10.1080/07388550902823674 [13] B. Katryniok, S. Paul, M. Capron and F. Dumeignil, “To- wards the Sustainable Production of Acrolein by Glycerol Dehydration, ChemSusChem, Vol. 2, No. 8, 2009, pp. 719-730. doi:10.1002/cssc.200900134 [14] T. Maeda, V. Sanchez-Torres and T. K. Wood, “Enhanced Hydrogen Production from Glucose by Metabolically Engineered Escherichia coli,” Applied Microbiology and Biotechnology, Vol. 77, No. 4, 2007, pp. 879-890. doi:10.1007/s00253-007-1217-0 [15] Z.-X. Wang, J. Zhuge, H. Fang and B. A. Prior, “Glycerol Production by Microbial fermentation: A Review,” Bio- technology Advances, Vol. 19, No. 3, 2001, pp. 201-223. doi:10.1016/S0734-9750(01)00060-X [16] G. L. Zhang, B. B. Ma, X. L. Xu, C. Li and L. W. Wang, “Fast Conversion of Glycerol to 1,3-Propanediol by a New Strain of Klebsiella Pneumonia,” Biochemical En- gineering Journal, Vol. 37, No. 3, 2007, pp. 256-260. doi:10.1016/j.bej.2007.05.003 [17] B. B. Ma, X. L. Xu, G. L. Zhang, L. W. Wang, M. Wu and C. Li, “Microbial Production of 1,3-Propanediol by Klebsiella Pneumoniae XJPD-Li Under Different Aeration Strategies,” Applied Biochemistry and Biotechnology, Vol. 152, No. 1, 2009, pp. 127-134. doi:10.1007/s12010-008-8220-5 [18] T, Homann, C. Tag, H. Biebl, W. D. Deckwer and B. Schink, “Fermentation of Glycerol to 1,3-Propanediol by Klebsiella and Citrobacter Strains,” Applied Microbiology and Biotechnology, Vol. 33, No. 2, 1990, pp. 121-126. doi:10.1007/BF00176511 [19] C. W. Forsberg, “Production of 1,3-Propanediol from Glycerol by Clostridium-Acetobutylicum and Other Clos- tridium Species,” Applied and Environmental Microbiol- ogy, Vol. 53, No. 4, 1987, pp. 639-643. [20] A. Murarka, Y. Dharmadi, S. S. Yazdani and R. Gonzalez, “Fermentative Utilization of Glycerol by Escherichia coli and Its Implications for the Production of Fuels and Chemicals,” Applied and Environmental Microbiology, Vol. 74, No. 4, 2008, pp. 1124-1135. doi:10.1128/AEM.02192-07 [21] Y. Dharmadi, A. Murarka and R. Gonzalez, “Anaerobic Fermentation of Glycerol by Escherichia coli: A New P lat- form for Metabolic Engineering,” Bio tec hnology and Bio- engineering, Vol. 94, No. 5, 2006, pp. 821-829. doi:10.1002/bit.21025 [22] S. S. Yazdani and R. Gonzalez, “Engineering Escherichia coli for the Efficient Conversion of Glycerol to Ethanol and Co-products,” Metabolic Engineering, Vol. 10, No. 6, 2008, pp. 340-351. doi:10.1016/j.ymben.2008.08.005 [23] A. J. Byrd, K. K. Pant and R. B. Gupta, “Hydrogen Pro- duction from Glycerol by Reforming in Supercritical Wa- ter Over Ru/Al2O3 Catalyst,” Fuel, Vol. 87, No. 13-14, 2008, pp. 2956-2960. doi:10.1016/j.fuel.2008.04.024 [24] M. M. Zhu, P. D. Lawman and D. C. Cameron, “Improving 1,3-Propanediol Production from Glycerol in a Metaboli- cally Engineered Escherichia coli by Reducing Accumula-  N. CHAUDHARY ET AL. Copyright © 2011 SciRes. ACES 89 tion of sn-Glycerol-3-Phosphate,” Biotechnology Progress, Vol. 18, No. 4, 2002, pp. 694-699. doi:10.1021/bp020281+ [25] T. Ito, Y. Nakashimada, K. Senba, T. Matsui and N. Nishio, “Hydrogen and Ethanol Production from Glyc- erol-Containing Wastes Discharged after Biodiesel Manu- facturing Process,” Journal of Bioscience and Bioengi- neering, Vol. 100, No. 3, 2005, pp. 260-265. doi:10.1263/jbb.100.260 |

