Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 2011, 1, 154-162 doi:10.4236/aces.2011.13023 Published Online July 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Enzymatic Formation of Gold Nanoparticles Using Phanerochaete Chrysosporium Rashmi Sanghi1,2, Preeti Verma1, Sadhna Puri3 1Facility for Ecological and Analytical Testing, Southern Laboratories, Institute of Technology, Kanpur, India 2The LNM Institute of Information Technology, Jaipur, India 3Dayanand Girls College, Chhatrapati Shahu Ji Maharaj University, Kanpur, India E-mail: rsanghi@gmail.com Received February 14, 2011; revised March 11, 2011; accepted April 2, 2011 Abstract When fungus Phanerochaete chrysosporium was challenged with gold ions under ambient aqueous con- ditions gold nanoparticles were formed within 90 minutes. Controlling experimental conditions like the age of fungus, incubation temperature and different concentration of gold chloride solution had drastic effect on the morphology of the nanoparticles formed. The enzyme assays indicated the role of enzyme as a reducing and shape directing agent. Laccase was the dominating enzyme in the case of fungal media for the synthesis of extracellular gold nanoparticles. Ligninase was responsible for the intracellular formation of nanoparticles on the fungal mycelium. The stabilization of the nanoparticles (NPs) via protein layer was evident by Atomic Force Microscopy (AFM) which revealed the nanoparticles to be spherical in the range of 10 - 100 nm. This study represents an important advancement in the use of fungal enzymes for the biosynthesis of highly stable gold nanoparticles by a green and mild technique in one pot in aqueous media. Keywords: Gold, Nanoparticles, Fungus, Enzymes, Protein 1. Introduction Synthesis of metal nanoparticles of various size and shape and their colloidal stabilization through biomolecule immobilization is very essential due to their usefulness in many applications such as sensors [1], catalysis [2], che- mical [3], optoelectronics [4], single-electron transistors, light emitters [5], and in vivo imaging [6]. The wide range of applications shown by nanomaterials is mainly due to their large surface area and small size. A wide variety of physical and chemical processes have been employed for the synthesis of metal nanoparticles [7], but these methods have certain disadvantages due to the involvement of toxic chemicals and radiations. So the de- velopment of reliable experimental protocols for the syn- thesis of nanomaterials over a range of chemical com- positions, sizes, and high monodispersity is one of the challenging issues in current nanotechnology. There is a need to develop an environment friendly approach for nanomaterials synthesis and assembly that should be fast and devoid of the use of toxic chemicals in the synthesis protocol. As a result, for the development of clean and environmentally acceptable green procedures, biological systems like bacteria and fungi are fast gaining attention of the researchers. The bacterium Pseudomonas stutzeri AG259 isolated from silver mine, when placed in a concentrated aqueous solution of AgNO3, was able to form silver NPs of well- defined size and distinct morphology within the peri- plasmic space of the bacteria [8]. The bacterium Pseu- domonas stutzeri AG259 and Pseudomonas aeruginosa have also been used for the synthesis of gold nanoparti- cles by the reduction of aqueous Au3+ ions [8,9]. The common Lactobacillus strains found in buttermilk has been used for the formation of gold, silver, and gold- silver alloy crystals [10]. Yeast strains have also been identified for their ability to produce gold nanoparticles, whereby controlled size and shape of the nanoparticles could be achieved by controlling the growth and other cellular activities [11,12]. However, compared to bacteria, the filamentous fungi could be better candidates for such biomimetic processes with significantly higher productivity of nanoparticles as these biomasses are known to secrete much higher amounts of proteins. Besides, fungi are easy to culture on a large scale as it could grow on the surface of an inorganic vector  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 155 during culture. Some researchers have reported the syn- thesis of silver and gold nanoparticles using various fun- gal strains like, Verticillium [13-15] and Aspergillus fla- vus [16]. However, these studies reported an intracellular production of nanoparticles which makes the job of downstream processing difficult and beats the purpose of developing a simple and cheap process. The surface trapped nanoparticles or those formed inside the biomass would require an additional step of processing like ultra- sonication for their release into the surrounding liquid media. Therefore, in recent times the focus of research has been given to development of an extracellular proc- ess which offers a great advantage over intra-cellular process of synthesis from the application point of view. There are some recent reports [17,18] on the extracellular biosynthesis of silver nanoparticles using filamentous fungus Aspergillus fumigatus and Fusarium semitectum. Mukherjee et al. [15] have elucidated the mechanism of nanoparticles formations, as in vitro approach was fol- lowed where species specific NADH dependent reduc- tase, released by the Fusarium oxysporium, were suc- cessfully used to carry out the extracellular reduction of gold ions to gold nanoparticles. Among the lignolytic fungi, Phanerochaete chryso- sporium has been the most intensively studied white rot fungus and is considered as a model strain for the devel- opment and understanding of the lignolytic-enzyme-pro- duction system as it can produce more complete lig- nolytic enzyme complex than most other strains. But surprisingly this particular strain of fungus has not been much explored for the biosynthesis of gold NPs. Al- though, in 2006 Vigneshwaran [19] reported the biosyn- thesis of silver nanoparticles using fungus Phaenero- chaete chrysosporium, to the best of our knowledge the biosynthesis of gold by this fungus has not yet been re- ported. So we explored the utilization of this model strain Phaenerochaete chrysosporium for the biosynthe- sis of gold NPs under varied working conditions. Work- ing towards an ecofriendly, simple yet speedy approach we have developed a one step, easy, cheap and conven- ient method for the biosynthesis of gold nanoparticles using white rot fungus, Phaenerochaete chrysosporium. The objective of the study was the intra as well as ex- tracellular formation of gold NPs in much lesser time than that reported earlier [17,18]. 2. Materials and Methods 2.1. Growth of the Fungus The white rot fungal strain Phanerochaete chrysospo- rium was obtained from Institute of Microbial Technol- ogy (IMTECH), Chandigarh. The strain was maintained at 4˚C on malt agar slants. The liquid growth medium (GM) used for inoculating the fungus, consisted of 20 g/L glucose and 20 g/L malt extract. The medium was autoclaved (‘WidWo’ Cat. AVD 500 ‘horizontal auto- clave’) at 15˚psi for 30˚min and cooled to room tem- perature before use and the pH after autoclaving was 5.6. The fungus was grown in a 150 mL conical flask and harvested for 7 days at 37˚C. The pale white fungal my- celium (FMy) took the shape of a circular mat. The fungal mycelium (FMy) was filtered through Whatman No. 1 filter paper and washed thoroughly with deionized water to remove any adhering growth media (GM) components and the excess water was blotted. For the biosynthesis of gold nanoparticles, two types of ex- periments were conducted in parallel at 37˚C under unal- tered normal pH conditions (3.5) with constant shaking at 200 rpm in an incubator shaker. Experiments were con- ducted with the washed fungal mycelium (FMy) and with the growth media (GM) in which the fungus was grown and harvested for 7 days. The effect of temperature, time, concentration of Auions (1 mM and 2 mM) and age of fungus were studied by varying one parameter at a time, keeping the other experimental conditions the same. 2.2. Synthesis of Gold Nanoparticles Experiments were conducted with the fungal mycelium (FMy) as well as with the growth media (GM) in parallel. Typically around 4 gm of wet fungal mycelium (FMy) or the growth medium(GM) used, was made upto 30mL volume by adding 1 mM concentration of HAuCl4 so- lution in a 150 mL Erlenmeyer flask. It was then agitated at 37˚C at 200 rpm under normal pH. The pH of the so- lution was found to be 3.5. Simultaneously, a positive control of incubating the fungal mycelium (FMy) with deionized water as well as growth media without gold ions (blank experiment) and a negative control contain- ing only gold solution were maintained under similar conditions. The reduction of metal ions was routinely monitored by visual inspection of the solution as well as by mea- suring the UV-Vis spectra of the solution by periodic sampling of aliquots (1 mL) of the aqueous component. The gold nanoparticles thus formed were subjected to dif- ferent instrumental analytical techniques to characterize them and the mechanistic details were further worked out. 2.3. Characterization of Gold Nanoparticles For the measurement of the UV–Vis absorbance, a UV/Vis, Spectrophotometer Lambda 40, (Perkin Elmer, USA) in the wavelength range of 200 - 800 nm was used.  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 156 The deionized water was used as the blank. Infrared (IR) spectra were recorded on a BRUCKER, VERTEX 70, Infrared spectrophotometer making KBr pellets in re- flectance mode. The composition of gold nanoparticles was studied by the method of x-ray diffraction analysis on a recorded in ARL X TRA X-ray Diffractometer and the x-ray diffracted intensities were recorded from 10˚ to 80˚ 2θ angles. For the observations of Scanning electron micrographs SEM, the powered gold nanoparticles were mounted on specimen stubs with double-sided adhesive tape and examined under FEI (QUANTA 200) SEM at 10 - 17.5˚kV with a tilt angle of 45˚. Their corresponding EDX spectrum was recorded by focusing on a cluster of particles. We have also imaged protein capped gold nanoparticles on mica substrate using an atomic force microscope (AFM). Samples for AFM imaging were pre- pared by, a drop of aqueous solution containing the gold nanoparticles were placed on the mica film and then dried under room temperature. Atomic force microscopy (AFM) was obtained using a Picoscan TM Molecular imaging, (U.S.A). The pH of the solution was measured with a Digital pH meter (MK VI Systronics). 2.4. Protein Assay The protein concentration was determined by the BCATM Kit at max 562 nm methods with bovine serum albumin (Sigma) as standard. 2.5. Enzyme Assays The lignin peroxidase activity was determined using the method described by Tien and Kirk, [20]. Laccase activ- ity was monitored using the method described by Leonowicz and Grzywnowicz [21] and the manganese- dependent peroxidase activity was measured using the method described by Kuwahara et al. [22]. 3. Results and Discussion The washed fungal mycelium (FMy) and the growth me- dium (GM) in which the fungus was harvested were separately challenged with 1mM of HAuCl4 solution and incubated in shaker at 200˚rpm. The pH of the solution was found to be 3.5. 3.1. Effect of Temperature For both sets of experiments, FMy and GM at room temperature, no change of color was observed for a long time even under shaking conditions. It is generally be- lieved that the reaction temperature will have a great effect on the rate and shape of particle formation as the rate of formation of the nanoparticles relates to the in- cubation temperature. However on increasing the tem- perature to 37˚C, nanoparticles formation started within 3 min and was found to be maximum at 90 min as evi- dent by the color changes. The UV–visible spectrum of the solutions was recorded to study the change in light absorption profile of the medium. In the first set of ex- periments, the fungal mycelium (FMy) on exposure to aqueous solution of HAuCl4 slowly changed from pale white color to vivid purple after 30 min indicating the intracellular formation of Au nanoparticles (Figure 1). However its corresponding solution remained colorless and showed no discernible absorption in the 500 - 600 nm regions indicating that extracellular reduction of AuCl4 ions has not occurred [23]. The intracellular re- duction of gold ions completed within 90 min as ob- served visually. In the second set of experiments, the growth media (GM) when exposed to HAuCl4 solution, changed color with time from colorless to light orange, light purple and finally dark or vivid purple [24] in 35 min, suggesting the formation of gold nanoparticles. The reaction mixture exhibited an absorbance band at about 525 nm character- istic of gold nanoparticles due to its SP absorbance, which is responsible for the vivid purple color. With further increase in temperature to 45˚C, the time taken for the initiation of gold nanoparticles formation was much increased to 1 h with the maximum intensity at 2 h. Also, the maximum absorption wavelengths of gold colloids take on a red shift (from 525 to 528˚nm), showing an increasing tendency for the size of gold nanoparticles at 45˚C. Since 37˚C showed the best results in terms of reduced time as well as smaller particle size, further studies were all carried out at this temperature. The absorption spectrum for the blank experiment showed a peak at 280 nm indicating the presence of Figure 1. Test tube containing fungal mycelium after the reaction with gold ions. 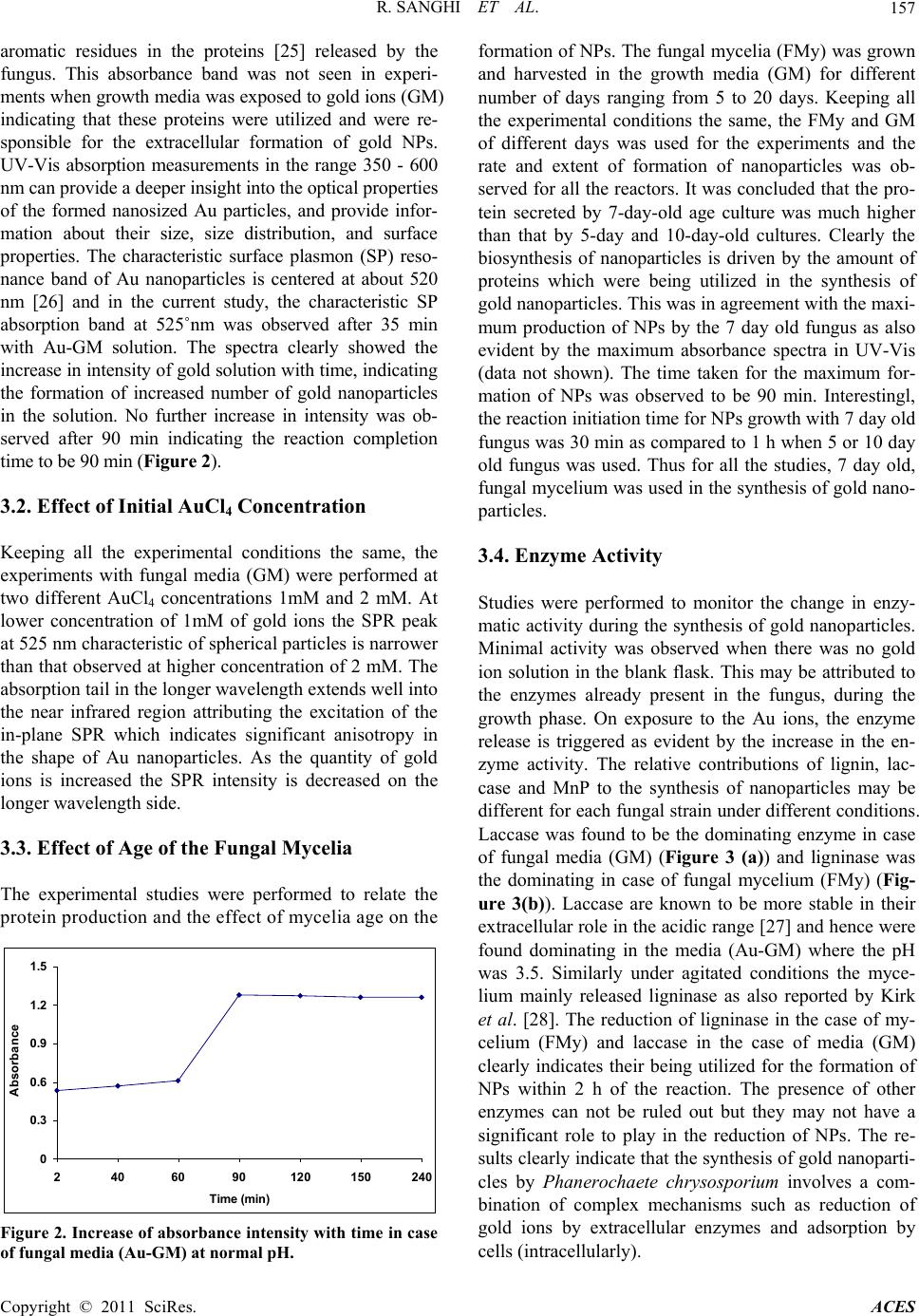 R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 157 aromatic residues in the proteins [25] released by the fungus. This absorbance band was not seen in experi- ments when growth media was exposed to gold ions (GM) indicating that these proteins were utilized and were re- sponsible for the extracellular formation of gold NPs. UV-Vis absorption measurements in the range 350 - 600 nm can provide a deeper insight into the optical properties of the formed nanosized Au particles, and provide infor- mation about their size, size distribution, and surface properties. The characteristic surface plasmon (SP) reso- nance band of Au nanoparticles is centered at about 520 nm [26] and in the current study, the characteristic SP absorption band at 525˚nm was observed after 35 min with Au-GM solution. The spectra clearly showed the increase in intensity of gold solution with time, indicating the formation of increased number of gold nanoparticles in the solution. No further increase in intensity was ob- served after 90 min indicating the reaction completion time to be 90 min (Figure 2). 3.2. Effect of Initial AuCl4 Concentration Keeping all the experimental conditions the same, the experiments with fungal media (GM) were performed at two different AuCl4 concentrations 1mM and 2 mM. At lower concentration of 1mM of gold ions the SPR peak at 525 nm characteristic of spherical particles is narrower than that observed at higher concentration of 2 mM. The absorption tail in the longer wavelength extends well into the near infrared region attributing the excitation of the in-plane SPR which indicates significant anisotropy in the shape of Au nanoparticles. As the quantity of gold ions is increased the SPR intensity is decreased on the longer wavelength side. 3.3. Effect of Age of the Fungal Mycelia The experimental studies were performed to relate the protein production and the effect of mycelia age on the 0 0.3 0.6 0.9 1.2 1.5 2406090120 150240 Time (min) Absorbance Figure 2. Increase of absorbance intensity with time in case of fungal media (Au-GM) at normal pH. formation of NPs. The fungal mycelia (FMy) was grown and harvested in the growth media (GM) for different number of days ranging from 5 to 20 days. Keeping all the experimental conditions the same, the FMy and GM of different days was used for the experiments and the rate and extent of formation of nanoparticles was ob- served for all the reactors. It was concluded that the pro- tein secreted by 7-day-old age culture was much higher than that by 5-day and 10-day-old cultures. Clearly the biosynthesis of nanoparticles is driven by the amount of proteins which were being utilized in the synthesis of gold nanoparticles. This was in agreement with the maxi- mum production of NPs by the 7 day old fungus as also evident by the maximum absorbance spectra in UV-Vis (data not shown). The time taken for the maximum for- mation of NPs was observed to be 90 min. Interestingl, the reaction initiation time for NPs growth with 7 day old fungus was 30 min as compared to 1 h when 5 or 10 day old fungus was used. Thus for all the studies, 7 day old, fungal mycelium was used in the synthesis of gold nano- particles. 3.4. Enzyme Activity Studies were performed to monitor the change in enzy- matic activity during the synthesis of gold nanoparticles. Minimal activity was observed when there was no gold ion solution in the blank flask. This may be attributed to the enzymes already present in the fungus, during the growth phase. On exposure to the Au ions, the enzyme release is triggered as evident by the increase in the en- zyme activity. The relative contributions of lignin, lac- case and MnP to the synthesis of nanoparticles may be different for each fungal strain under different conditions. Laccase was found to be the dominating enzyme in case of fungal media (GM) (Figure 3 (a)) and ligninase was the dominating in case of fungal mycelium (FMy) (Fig- ure 3(b)). Laccase are known to be more stable in their extracellular role in the acidic range [27] and hence were found dominating in the media (Au-GM) where the pH was 3.5. Similarly under agitated conditions the myce- lium mainly released ligninase as also reported by Kirk et al. [28]. The reduction of ligninase in the case of my- celium (FMy) and laccase in the case of media (GM) clearly indicates their being utilized for the formation of NPs within 2 h of the reaction. The presence of other enzymes can not be ruled out but they may not have a significant role to play in the reduction of NPs. The re- sults clearly indicate that the synthesis of gold nanoparti- cles by Phanerochaete chrysosporium involves a com- bination of complex mechanisms such as reduction of gold ions by extracellular enzymes and adsorption by cells (intracellularly).  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 158 0 0.5 1 1.5 2min 2h4h Time Absorbance MnPGMBMnPGM LiGM LaGMB LaGM LiGMB (a) 0 0.4 0.8 1.2 1.6 2min 2h4h Time Absorbance LiFMyB LiFMyLaFMy LaFMyB MnPFMyB MnPFMy (b) Figure 3. (a) Change in max as an indicator of enzyme ac- tivity in case of fungal media (Au-GM) incubated with gold ions at normal pH (La-Laccase max 525 nm, Li-Lignin Peroxidase max 310 nm, MnP-Manganese Peroxidase max 431 nm, B-blank); (b) Change in max as an indicator of enzyme activity in case of fungal mycelium (Au-FMy) in- cubated with gold ion solution at normal pH (La-Laccase max 525 nm, Li-Lignin Peroxidase max 310 nm, MnP- Manganese Peroxidase max 431 nm, B-blank). 4. Characterization of Nanoparticles 4.1. X-Ray Diffraction Studies Further evidence for the formation of gold nanoparticles is provided by X-ray diffraction (XRD) analysis of the Au nanoparticles formed during the course of the studies. As shown by the XRD pattern in (Figure 4) which cor- responds to the gold nanoparticles, which could be in- dexed on the basis of the face-centered cubic (fcc) gold structure. A strong diffraction peak at 38.05˚ was ascribed to the fcc gold structures, while diffraction peaks of other four facets were much weaker. The four strong Bragg diffraction peaks at 38.05, 44.6, 64.4, and 77.45 closely matched that of gold [29]. As expected, the XRD peaks of the nanoparticles were considerably broadened be- cause of the finite size of the nanoparticles. Figure 4. X-ray diffraction pattern of Au/fungal mycelium, filled square indicate the peaks corresponding to that gold nanoparticles. 4.2. Fourier Transform Infrared Spectroscopy FTIR measurements (Figure 5) were carried out both for the native fungal mycelium before the reaction with gold ions as well as for the gold nanoparticles formed after the reaction, to identify the possible interactions between gold ions with fungal proteins. The broad band contour which appears in the range of 3000 - 3400 cm−1 is the summation of association inter- molecular hydrogen bonds arising from NH2 and OH groups in protein molecules which becomes much broader after the reaction with gold ions. The significant change of transmittance related to the bonds with N atoms reveal that nitrogen atoms are the binding sites for gold on fun- gus which further broadens and becomes symmetrical, indicates that the N-H vibration was affected due to the gold attachment. The band at 1456 cm–1 is assigned to methylene scissoring vibrations from the proteins in the solution. It is reported earlier that proteins can bind to nanopar- ticles either through free amine groups or cysteine residues in the proteins and via the electrostatic attraction of negatively charged carboxylate groups in enzymes present in the cell wall of mycelia and therefore, stabili- zation of the gold nanoparticles by protein is a possibility [3,30]. The two bands observed at 1367 cm–1 and 1029 cm–1 can be assigned to the C–N stretching vibrations of aro- matic and aliphatic amines, respectively. The aromatic amine band becomes less intensified in gold nanoparti- cles formed after the reaction. Infrared active modes at- tributed to side chain vibrations include C-H stretching antisymetric and symmetric modes at 2917 cm–1 and 2850 cm–1 corresponding to aliphatic and aromatic modes re- spectively broaden after the reaction with gold solution at normal pH.  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 159 0.4 0.7 1 3998 3419 2841 226216841105527 Wavelength (cm-1) Int e ns ity Nat iv e FMyAu- FMy Figure 5. FTIR Spectra of plain fungal mycelium as well as gold nanoparticles. The significant changes observed for peaks at 1367 cm–1, and 2850 cm–1 is indicative of the role of aromatic groups in reduction of Au ions in Au/mycelium (FMy) which possibly arise from aromatic amino acids trypto- phan or tyrosine as discussed above. It is notable that a new band at about 1735 cm−1 (Fig- ure 5) corresponding to carbonyl stretch vibrations in ketones, aldehydes and carboxylic acids indicating that the reduction of the gold ions is coupled to the oxidation of the hydroxyl groups and/or its hydrolyzates, which may be attributed to the formation of a quinone structure due to the oxidation of the phenolic group of aromatic amino acids. The amide linkages between amino acid residues in polypeptides and proteins give rise to well known signa- tures in the infrared region of the electromagnetic spec- trum. The strong and narrow peak at 1648 cm−1 is due to the presence of amide I band which is primarily a C=O stretching mode. After the reaction the amide band I bi- furcate in two peaks at 1668 cm−1 and at 1631 cm−1. The amide II band due to the N-H stretching modes of vibra- tion in the amide linkage was not visible after the reac- tion with gold ions but showed up at 1540 cm−1 in the native fungal mycelium. A shift of v cm−1 39 is also seen in the more complex Amide III band located near 1228 cm−1 to 1267 cm−1 in gold nanoparticles. The position of these bands is close to that reported for native proteins in earlier papers 2. The peaks at 1648 cm−1 and 1145 cm−1 arise from a carbonyl stretching vibration and phenolic groups of tyrosine and tryptophan, respectively, which shows the carbonyl stretching vibration from the car- boxylate ions and the hydroxyl stretching vibration from the phenolic ions in Tyr of the native fungus [30,31]. This indicated that the secondary structure of the proteins is affected as a consequence of reaction with the Au ions or binding with the gold nanoparticles. Some very interesting observations were made after the reaction. Peaks which could be ascribed to the pres- ence of proteins disappear after the reaction with gold ions. An aromatic C-C stretch at 1145 cm−1 and a C-H bend at 844 cm–1 could be well assigned to the aromatic residue tyrosine and the C-H bending mode of the aro- matic residue tryptophan detected at 786 cm–1. Apart from this the spectrum also shows peaks at 621 cm–1, 653 cm–1 and 703 cm–1 due to C-S stretching; these C-S stretching modes of the sulfur-bearing residues confirm the presence of cysteine and methionine. The peaks at around 1145 cm−1, 1068 cm−1, 1029 cm–1 in the n–C–O stretching vibration region, are indicative of gold ions interaction with the fungal protein of mycelium (FMy). On comparison of the spectra of native fungal myce- lium and gold nanoparticles, the most significant point to be noted is that the band at 2552 cm–1 and 910 cm–1 cor- responding to –SH stretching 32 and bending mode are absent in the spectrum of gold nanoparticles while these spectra are present in native fungal mycelium (FMy). The disappearance of the –SH stretching band indicates the formation of a bond between the S atoms and gold clusters. It indicates chemisorption on gold surface as thiolate by forming an Au-S bond. 4.3. Scanning Electron Microscopy Figure 6 shows an SEM picture of the fungal mycelium (FMy) after exposure to 1 mM aqueous gold solution for 90 minutes. The presence of uniformly distributed gold nanoparticles on the surface of the fungal mycelium (FMy) is observed, indicating that the nanoparticles formed by the reduction of gold ions are bound to the surface of cells. Aspot-profile energy-dispersive analysis of X-rays (EDX) of one of the gold nanoparticles shows the presence of strong signals from the gold atoms to- gether with weaker signals from C, O, S and Cl atoms. The C and O signals arise from X-ray emission from Figure 6. SEM of Au/mycelium (Au-FMy) at normal pH. Wavelength (cm–1)  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 160 proteins/enzymes either directly bound to the gold nanoparticles or in the vicinity of the particle, while the presence of a weak Cl signal indicates the presence of a small fraction of Au(Cl)4− ions in the region being in- vestigated. 4.4. Atomic Force Microscopy In order to confirm the size and shape of the synthesized gold nanoparticles, the samples were analyzed under the atomic force microscopy. The image revealed the syn- thesized nanoparticles are in the form of spheres (data not shown). Gold nanoparticles were formed in several different sizes, ranging from polydisperse small nanopar- ticles to large nanoparticles. The particles were in the range of 10 - 100 nm in size, depicted by AFM. 5. Mechanism The studies with fungal mycelium (FMy) revealed that the gold ions were first trapped and reduced by the pro- teins/enzymes on the cell surface at normal pH (2 - 3.5), forming nuclei, followed by extensive crystal growth into the final shapes. The positive amino and sulfhydryl (SH) groups of fungal protein at low pH makes Au (III) avail- able for the binding and allow the reduction of Au (III) to Au (0) 33. Carboxylic groups, which are abundant in biomass, are known to be protonated at low pH and con- tribute to the binding of Au (III) ions 33. In the case of experiments with growth media (GM), extracellular synthesis of gold nanoparticles takes place with in 90 min. This is possible because the extracellular enzyme laccase are more stable in their extracellular role as they are often produced as highly glycosylated deriva- tives where the carbohydrate moieties increase their hy- drophilicity [27]. In general, laccase levels are substan- tially higher in media (GM) containing sufficient nitro- gen and the optimum pH for laccase production and ac- tivity ranges from 3.0 to 4.5 and the optimum tempera- ture ranges from 20˚C to 37˚C [27]. So the current ex- perimental conditions were very favorable for the ex- tracellular formation of nanoparticles in the media (GM) and intracellular formation of nanoparticles in the fungal mycelium (FMy). The fact that the resulting gold nanoparticles prepared by this method are stable for very long periods of time in spite of the absence of any additives indicates that the particles are electrostatically stabilized. 6. Conclusions Gold nanoparticles were formed within 90 minutes by fungal protein of Phanerochaete chrysosporium when challenged with gold ions, under normal pH in aqueous solution when temperature was incresaed to 37˚C. The rate of particle formation and therefore the size of the nanoparticles could be manipulated by controlling para- meters such as temperature, gold concentration and ex- posure time to gold ions. The entrapment of the gold nanoparticles occurs by electrostatic interaction between the gold nanoparticles and within the surface-bound fungal protein for the protein-capped gold nanoparticles. The extracellularly nanoparticles synthesis was due to the presence of extracellular laccase enzyme and the in- tracellular nanoparticles synthesis on the mycelium sur- face was due to the presence of ligninase as evident by enzyme assays. 7. Acknowledgements The authors are thankful to International Foundation for Science, Sweden for the financial assistance to carry out this work. 8. References [1] C. K. Kim, R. R. Kalluru, J. P. Singh, A. Fortner, J. Grif- fin, G. K. Darbha and P. C. Ray, “Gold-Nanoparticle- Based Miniaturized Laser-Induced Fluorescence Probe for Specific DNA Hybridization Detection: Studies on Size-Dependent Optical Properties,” Nanotechnology, Vol. 17, 2006, pp. 3085-3093. [2] A. Gole, C. V. Dash, V. Ramachandran, A. B. Mandale, S. R. Sainkar, M. Rao and M. Sastry, “Pepsin-Gold Colloid Conjugates: Preparation, Characterization and Enzymatic Activity,” Langmuir, Vol. 17, No. 5, 2001, pp. 1674-167. doi:10.1021/la001164w [3] A. Kumar, S. Mandal, P. R. Selvakannan, R. Parischa, A. B. Mandale and M. Sastry, “Investigation into the Interaction between Surface-Bound Alkylamines and Gold Nanoparti- cles,” Langmuir, Vol. 19, No. 15, 2003, pp. 6277-6282. doi:10.1021/la034209c [4] Y. Wang, “Non-linear Optical Properties of Nanometer- Sized Semiconductor Clusters,” Accounts of Chemical Research, Vol. 24, No. 5, 1991, pp. 133-139. doi:10.1021/ar00005a002 [5] D. L. Klein, R. Roth, A. K. L. Lim, A. P. Alivisatos and P. L. McEven, “A Single-Electron Transistor Made from a Cadmium Selenide Nanocrystal,” Nature, Vol. 389, 1997, pp. 699-701. [6] B. Dubertret, P. Skourides, D. J. Norris, V. Noireaux, A. H. Brivanlou and A. Libchaber, “In vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles,” Science, Vol. 298, No. 5599, 2002, pp. 1759-1762. doi:10.1126/science.1077194 [7] S. S. Shankar, A. Ahmad, R. Pasrichaa and M. Sastry, “Bioreduction of Chloroaurate Ions by Geranium Leaves and Its Endophytic Fungus Yields Gold Nanoparticles of Different Shapes,” Journal of Material Chemistry, Vol.  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 161 13, No. 7, 2003a, pp. 1822-1826. doi:10.1039/b303808b [8] T. Klaus, R. Joerger, E. Olssona and C. G. Granqvist, “Silver-Based Crystalline Nanoparticles, Microbially Fa- bricated,” Proceedings of the National Academy of Sci- ence USA, Vol. 96, No. 24, 1999, pp. 13611-13614. doi:10.1073/pnas.96.24.13611 [9] M. I. Husseiny, M. A. El-Aziz, Y. Badr and M. A. Mah- moud, “Biosynthesis of Gold Nanoparticles Using Pseu- domonas Aeruginosa,” Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, Vol. 67, No. 3-4, 2007, pp. 1003-1006. [10] B. Nair and T. Pradeep, “Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactoba- cillus Strains,” Crystal Growth Design, Vol. 2, No. 4, 2002, pp.293-298. doi:10.1021/cg0255164 [11] M. Gericke and A. Pinches, “Biological Synthesis of Metal Nanoparticles,” Hydrometallurgy, Vol. 83, No. 1-4, 2006a, pp. 132-140. doi:10.1016/j.hydromet.2006.03.019 [12] M. Gericke and A. Pinches, “Microbial Production of Gold Nanoparticles,” Gold Bulletin, Vol. 39, No. 1, 2006b, pp. 22-28. doi:10.1007/BF03215529 [13] P. Mukherjee, A. Ahmad, D. Mandal, S. Senapati, S. R. Sainkar, M. I. Khan, R. Ramani, R. Parischa, P. V. Ajaya- kumar, M. Alam, R. Kumar and M. Sastry, “Fungus Medi- ated Synthesis of Silver Nanoparticles and Their Im- mo- bilization in the Mycelial Matrix: A Novel Biological Ap- proach to Nanoparticle Synthesis,” Nano Letters, Vol. 1, No. 10, 2001, pp. 515-519. doi:10.1021/nl0155274 [14] P. Mukherjee, A. Ahmad, D. Mandal, S. Senapati, S. R. Sainkar, M. I. Khan, R. Ramani, R. Parischa, P. V. Ajayakumar, M. Alam, M. Sastry and R. Kumar, “Biore- duction of AuCl4–Ions by the Fungus, Verticillium sp. and Surface Trapping of the Gold Nanoparticles Formed,” Angewandte Chemie International Edition, Vol. 40, No. 19, 2001, pp. 3585-3588. [15] P. Mukherjee, S. Senapati, D. Mandal, A. Ahmad, M. I. Khan, R. Kumar and M. Sastry, “Extracellular Synthesis of Gold Nanoparticles by the Fungus Fusarium Oxy- sporum,” ChemBioChem, Vol. 3, No. 5, 2002, pp. 461- 463. doi:10.1002/1439-7633(20020503)3:5<461::AID-CBIC4 61>3.0.CO;2-X [16] N. Vigneshwaran, N. M. Ashtaputre, P. V. Varadarajan, R. P. Nachane, K. M. Paralikar and R. H. Balasubramanya, “Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus,” Materials Letters, Vol. 61, No. 6, 2007, pp. 1413-1418. doi:10.1016/j.matlet.2006.07.042 [17] K. C. Bhainsa and S. F. D’Souza, “Extracellular Biosyn- thesis of Silver Nanoparticles Using the Fungus Aspergil- lus Fumigatus,” Colloids Surfaces B: Biointerfaces, Vol. 47, No. 2, 2006, pp. 160-164. doi:10.1016/j.colsurfb.2005.11.026 [18] S. Basavaraja, S. D. Balaji, A. Lagashetty, A. H. Rajasab and A. Venkataraman, “Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Sem- itectum,” Material Research and Bulletin, Vol. 43, No. 5, 2008, pp. 1164-1170. doi:10.1016/j.materresbull.2007.06.020 [19] N. Vigneshwaran, A. A. Kathe, P. V. Varadarajan, R. P. Nachane and R. H. Balasubramanya, “Biomimetics of Sil- ver Nanoparticles by White Rot Fungus, Phaenerochaete Chrysosporium,” Colloids Surfaces B: Biointerfaces, Vol. 53, No. 1, 2006, pp. 55-59. doi:10.1016/j.colsurfb.2006.07.014 [20] M. Tien and T. K. Kirk, “Lignin Peroxidase of Phanero- chaete Chrysosporium,” Methods in Enzymology, Vol. 161, 1988, pp. 238-249. [21] A. Leonowicz and K. Grzywnowicz, “Quantitative Esti- mation of Laccase Forms in Some White Rot Fungi Us- ing Syringaldazine as a Substrate,” Enzyme and Micro- bial Technology, Vol. 3, No. 1, 1981 pp. 55-58. doi:10.1016/0141-0229(81)90036-3 [22] M. Kuwahara, J. K. Glenn, M. A. Morgan and M. H. Gold, “Separation and Characterization of Two Extracellular H202-Dependent Oxidases from Ligninolytic Cultures of Phanerochaete Chrysosponum,” FEBS Letters, Vol. 169, 1984, pp. 247-250. [23] S. Underwood and P. Mulvaney, “Effect of the Solution Refractive Index on the Color of Gold Colloids,” Lang- muir, Vol. 10, No. 10, 1994, pp. 3427-3430. doi:10.1021/la00022a011 [24] S.A. Kumar, Y. A. Peter and J. L. Nadeau, “Facile Bio- synthesis, Separation and Conjugation of Gold Nanopar- ticles to Doxorubicin,” Nanotechnology, Vol. 19, No. 49, 2008, p. 495101. doi:10.1088/0957-4484/19/49/495101 [25] C. M. Stoscheck, “Quantitation of Protein,” In: M. P. Deutscher, Ed., Guide to Protein Purification (Methods in Enzymology, Vol. 182, 1990, pp. 50-68. [26] T. Yonezawa, T. Nomura, T. Kinoshita and K. Koumoto, “Preparation and Characterization of Poly-peptidestabilized Gold Nanoparticles,” Journal of Nanoscience and Nanotechnology, Vol. 6, No. 6, 2006, pp. 1649-1654. doi:10.1166/jnn.2006.222 [27] P. Tong, Y. Hong, Y. Xiao, M. Zhang, X. Tu and T. Cui, “High Production of Laccase by a New Basidiomycete, Trametessp,” Biotechnology Letters, Vol. 29, No. 2, 2007, pp. 295-301. [28] T.K. Kirk, S. Croan, M. Tien, K. E. Murtagh and R. L. Farrell, “Production of Multiple Ligninases by Pha- nerochaete Chrysosporium: Effect of Selected Growth Conditions and Use of a Mutant Strain,” Enzyme and Mi- crobial Technology, Vol. 8, No. 1, 1986, pp. 27-32. doi:10.1016/0141-0229(86)90006-2 [29] S. Chen, Y. Liu and G. Wu, “Stabilized and Size-Tunable Gold Nanoparticles Formed in a Quaternary Ammonium- Based Room-Temperature Ionic Liquid under Irradia- tion,” Nanotechnology, Vol. 16, No. 10, 2005, pp. 2360- 2364. doi:10.1088/0957-4484/16/10/061 [30] S. Mandal, S. Phadtare and M. Sastry, “Interfacing Biol- ogy with Nanoparticles,” Current Applied Physics, Vol. 5, No. 2, 2005, pp.118-127. doi:10.1016/j.cap.2004.06.006 [31] P. R. Selvakannan, S. Mandal, S. Phadtare, A. Gole, R.  R. SANGHI ET AL. Copyright © 2011 SciRes. ACES 162 Pasricha, S. D. Adyanthaya and M. Sastry, “Water-Dis- persible Tryptophan-Protected Gold Nanoparticles Pre- pared by the Spontaneous Reduction of Aqueous Chloro- aurate Ions by the Amino Acid,” Journal of Colloid and Interface Science, Vol. 269, No. 1, 2004, pp. 97-102. doi:10.1016/S0021-9797(03)00616-7 [32] Y. Tan, Y. Wang, L. Jiang and D. Zhu, “Thiosalicylic Acid-Functionalized Silver Nanoparticles Synthesized in One-Phase System,” Journal of Colloid and Interface Science, Vol. 249, No. 2, 2002, pp. 336-345. doi:10.1006/jcis.2001.8166 [33] J. L. Gardea-Torresdey, K. J. Tiemann, J. G. Parsons, G. Gamez and M. J. Yacaman, “Characterization of Trace Level Au (III) Binding to Alfalfa Biomass (Medicago Sa- tiva) by GFAAS,” Advances in Environmental Research, Vol. 6, No. 3, 2002, pp. 313-323. doi:10.1016/S1093-0191(01)00064-8 |

