Journal of Electromagnetic Analysis and Applications
Vol. 2 No. 6 (2010) , Article ID: 2150 , 6 pages DOI:10.4236/jemaa.2010.26049
Effect of Zn Substitution on the Magnetic Properties of Cobalt Ferrite Nano Particles Prepared Via Sol-Gel Route
![]()
1Department of Chemistry, Panjab University, Chandigarh, India; 2Department of Metallurgical and Materials Engineering, Indian Institute of Technology-Roorkee, Roorkee, India; 3Institute Instrumentation Centre, Indian Institute of Technology-Roorkee, Roorkee, India.
Email: sonal1174@gmail.com
Received February 22nd, 2010; revised April 15th, 2010; accepted April 22nd, 2010.
Keywords: Nano Particles, Saturation Magnetization, Coercivity, X-Ray Diffraction
ABSTRACT
Zinc substituted cobalt ferrite nanoparticles (CoxZn1-xFe2O4, with x = 0.0, 0.2, 0.4, 0.8 and 1.0) were prepared via sol-gel route and the effect of zinc concentration on saturation magnetization and lattice parameter were investigated. The particle sizes of the as obtained samples were found to be ~10 nm which increases upto ~92 nm on annealing at 1000oC. The frequency bands near 564-588 cm-1 and 425-442 cm-1 are assigned to the tetrahedral and octahedral clusters which confirm the presence of M-O stretching band in ferrites. The unit cell parameter ‘a’ increases linearly with increasing concentration of zinc due to larger ionic radii of Zn2+ ion . It was found that this substitution allows tunable changes in the magnetic properties of cobalt ferrite. Interestingly, saturation magnetization first increases upto x = 0.4 and then decreases for higher Zn substitution, thus tunable changes in magnetic properties of cobalt ferrite are possible. Source of such behaviour could be the variation of exchange interaction between the tetrahedral and the octahedral sites.
1. Introduction
Nanocrystalline ferrites are currently the subject of interest because a wide application in industrial as well as research areas. They are attractive because of their importance in ferrofluids, magnetic drug delivery, hyperthermia for cancer treatment, etc. [1]. An interesting example is that of CoFe2O4 which has got some peculiar properties like high saturation magnetization (Ms), high coercivity (Hc) and large anisotropy [2]. Further the substitution of Co2+ in this ferrite with Zn2+, Ni2+, Cu2+ etc. allows some tunable changes in its properties.
CoFe2O4 has inverse spinel structure with Co2+ ions in octahedral sites and Fe3+ ions equally distributed between tetrahedral and octahedral sites whereas ZnFe2O4 has a normal spinel structure with Zn2+ ions in tetrahedral and Fe3+ in octahedral sites [3]. Therefore, Zn-substitution in CoFe2O4 may have some distorted spinel structures depending upon the concentration of the precursor solutions. Effect of laser irriadiation on the cation distribution mechanism of Co0.6Zn0.4Fe2O4 ferrite was explained by Tawfik et al. [4]. It is observed that a displacement of Fe3+ ions from its original positions alters the Fe3+ -O2- bond lengths which change the IR absorption bands. Dey and Ghose prepared Co0.2Zn0.8 Fe2O4 by co-precipitation method and found decrease in magnetization with increasing particle size [5]. Arulmurugan et al. [6] suggested that substitution of Co2+ with Zn2+ lead to improved magnetic properties of nanocrystalline ferrites. They also observed a decreasing behavior of saturation magnetization and the particle size of the Co-Zn substituted ferrite nanoparticles with increasing Zn concentration.
Islam et al. [7] reported that saturation magnetization decreases with zinc concentration in cobalt zinc ferrites prepared by ceramic technique. Vaidyanathan et al. [8] also reported decrease in magnetic properties such as Ms, Hs, Hc, and Mr with increase in zinc substitution. Single phase and monodispersed nanocrystalline Zn-substituted Cobalt ferrites with grain size of 3 nm were prepared by Duong et al. [9] using forced hydrolysis method and found that the ferrites were super paramagnetic at room temperature and ferrimagnetic at lower temperatures. Recently Waje et al. [10] reported that Co0.5Zn0.5 Fe2O4 nanoparticles, prepared by mechanical alloying and sintering show a constant value of permittivity within a measured frequency range but vary with sintering temperature. However, in general the permeability values vary with both frequency and sintering temperature.
The present work deals with the synthesis of nano particles of zinc substituted cobalt ferrite (CoxZn1-xFe2O4 where x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0) via sol-gel method and characterized using infrared spectroscopy (IR), transmission electron microscope (TEM), X-ray diffractometry (XRD) and magnetic measurements. Studies were also carried out after annealing the sample at various temperatures to see the effect of particle size on different properties. This work is an attempt to investigate the magnetic properties of zinc substituted cobalt ferrites.
2. Experimental
2.1 Preparation of Ferrites
Nanoparticles of zinc substituted cobalt ferrites Cox Zn1-x Fe2O4 (where x = 0.0, 0.2, 0.4, 0.6, 0.8 and 1.0) were prepared using sol-gel method (Figure 1). In this method each sample was prepared by taking the desired proportion of precursor nitrates, i.e., cobalt nitrate, iron nitrate and zinc nitrate were separately dissolved in minimum amount of water. After heating them at 80-90oC all the solutions were mixed. The solution thus obtained was stirred for sometimes and then citric acid followed by ethylene glycol was added. The solution was again stired till gel formation. This gel self ignites and results in nano particles of desired ferrite. These samples were then annealed at different temperatures for further characterization.
2.2 Physical Measurements
The infrared spectra of all the samples were recorded in the range 4000-400 cm-1 in FTIR instrument (PERKIN ELMER) using KBr pellets. The elemental analysis were
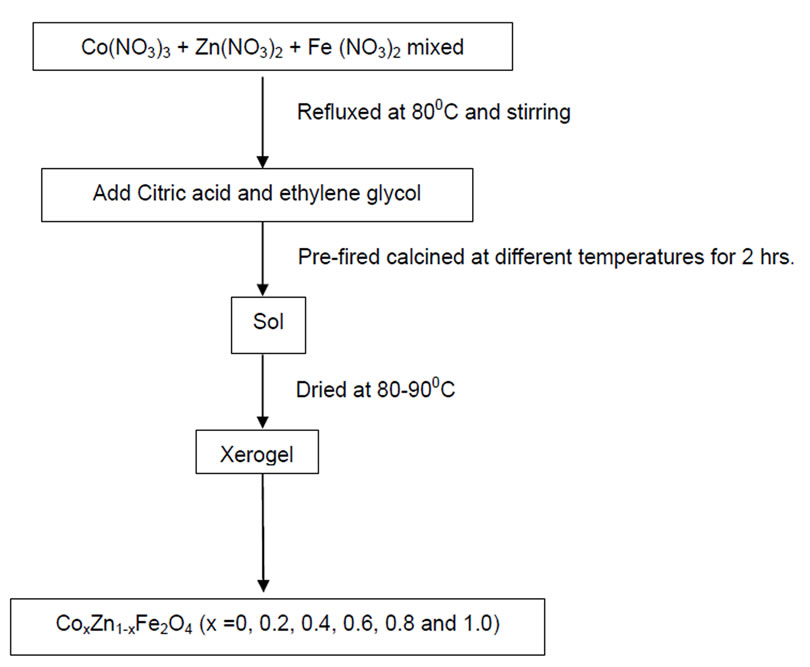
Figure 1. Sol-gel method for the preparation of ferrites
carried out by two methods viz. Electron probe micro analyzer (EPMA) (JEOL, 8600 M) and Atomic Absorption Spectrometer (AAS) (GBC, Avanta). In EPMA about 2 mm thick pellets were prepared, fixed on the sample holder and coated with the carbon to make them conducting. Analysis was done after calibrating the instrument with internal standards. Similarly analysis on AAS was also performed after calibrating the instrument with at least three standards elemental concentration. The results from two instruments were consistent and very close to the formula of the ferrite. The X-ray diffraction studies were carried out on X-ray diffractometer (Bruker AXS, D8 Advance) with FeKα radiation. Finally the magnetic measurements were made on a vibrating sample magnetometer (VSM) (PAR-155).
3. Results and Discussions
3.1 FT-IR Characterization
In the FT-IR spectra the frequency bands near 564-588 cm-1 and 425-442 cm-1 are assigned to the tetrahedral and octahedral clusters and confirms the presence of M-O stretching band in ferrites as suggested by Pradeep and Chandrasekaran [11]. The authors suggested that the vibrational mode of tetrahedral clusters is higher as compared to that of octahedral clusters, which is attributed to the shorter bond length of tetrahedral clusters.
3.2 X-Ray Diffraction Studies
Typical X-Ray diffraction pattern for the sample Co0.6Zn0.4Fe2O4 after annealing at 400, 600, 800, 1000oC are shown in Figure 2. The diffraction pattern did not show any peaks for the as prepared ferrite samples thereby showing the amorphous nature of the samples. However for the annealed samples regular peaks were observed, which confirmed that particle size increases with increase in temperature and the intensity of the peaks grew stronger with the grain size growth. The samples were found to be face centered cubic with Fd-3m space group.
Figure 3 represents the X-Ray powder diffraction pattern for the samples CoxZn1-xFe2O4 (where x = 0.0, 0.2, 0.4, 0.6, 0.8 and 1.0) annealed at 1000oC. The lattice parameters were calculated using Powley and Le-Bail refinement methods. It is observed that the lattice parameter ‘a’ increases linearly with increase in zinc concentration as shown in Figure 4. The reason for this increase of lattice parameter values may be due to the larger ionic radii of Zn2+ (88 pm) as compared to Co3+ (83.8 pm). The crystallite size was calculated using Debye Scherrer equation [12].
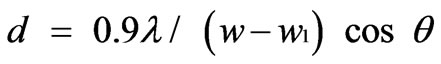
where d is the grain diameter, w is the half intensity
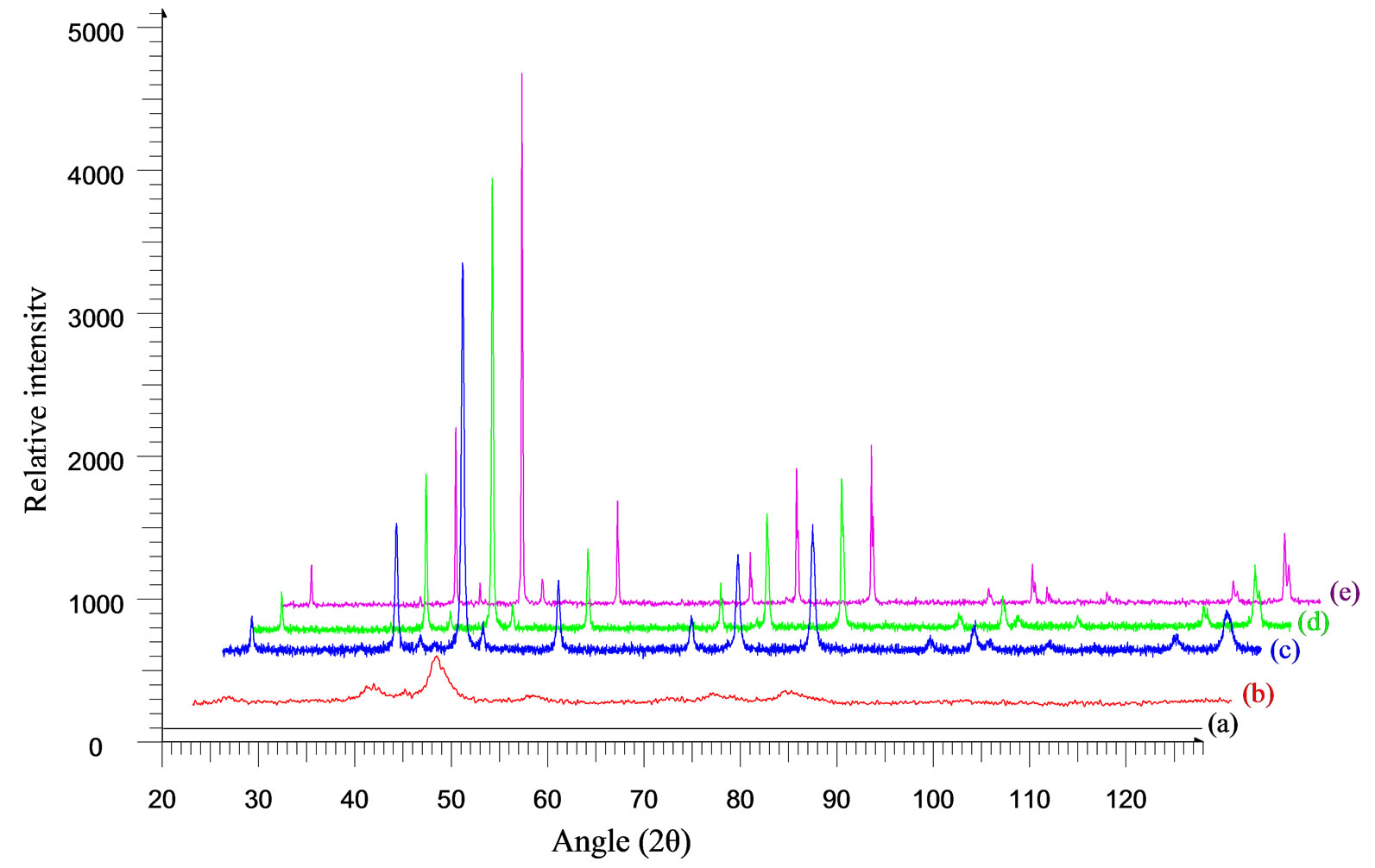
Figure 2. X-ray diffractographs of Co0.6Zn0.4Fe2O4 (a) as obtained and after annealing at (b) 400; (c) 600; (d) 800 and (e) 1000 ℃.
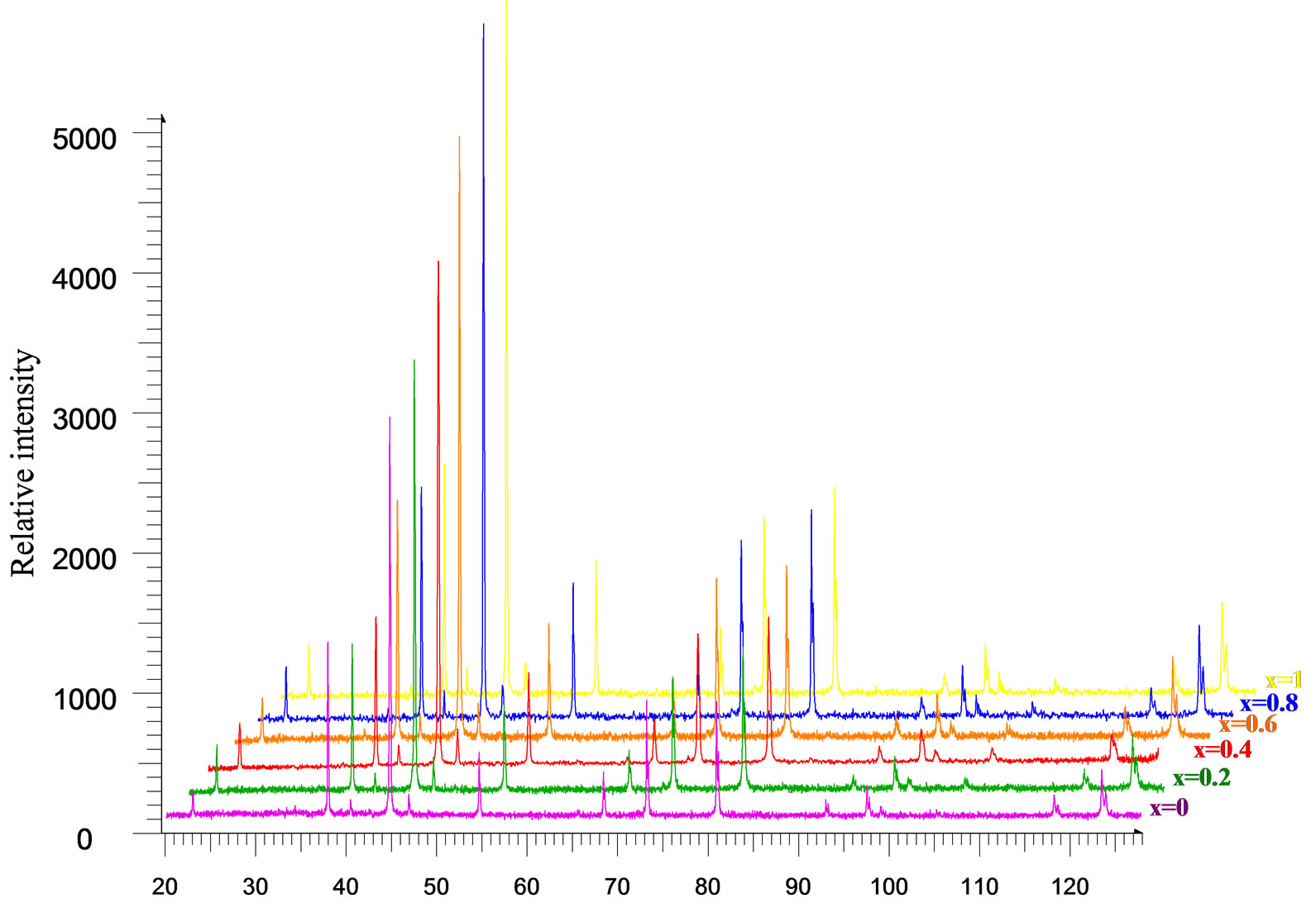
Figure 3. X-ray diffractographs of CoXZn1-XFe2O4 after annealing at 1000℃
widths of the relevant diffraction peak and w1 is the instrumental broadening, λ is the X-Ray wavelength and θ is the angle of diffraction. A linear increase in the particle size of the samples was observed with increasing annealing temperature.
The X-ray densities (dx) for all the annealed samples are tabulated in Table 1 which was calculated using the equation dx = 8M/Na,3 where M is the molecular mass of the sample, N is Avogadro’s number and ‘a’ is the lattice parameter. It is clearly seen from Figure 4 that the X-ray density increases linearly with increasing zinc concentration which should be due to the heavier weight of zinc atom as compared to that of cobalt atom.
3.3 Magnetic measurements
Typical hysteresis loops of CoFe2O4 as obtained and after annealing at 400, 600 and 1000 oC are shown in Figure 5. Typical hysterises loops for the samples Co0.2Zn0.8 Fe2O4, Co0.4Zn0.6Fe2O4, Co0.8Zn0.2Fe2O4 are also shown in Figure 6. The hysteresis loop for the as obtained sample exhibits no hysteresis, which may be attributed to superparamagnetic relaxation. The saturation magnetization of the annealed samples of CoFe2O4 at 1000℃ is ~84 emu/g, which is in good agreement with the reported values [13,14].
In a cubic system of ferromagnetic spinels, the magnetic order is mainly due to a super exchange interaction mechanism occurring between the metal ion in the A and B sublattices. The substitution of nonmagnetic ion such as zinc, which has a preferentially A site occupancy results in the reduction of the exchange interaction between A and B sites. Hence, by varying the amount of zinc substitution, it should possible to vary magnetic properties of the samples. According to Neel’s two sublattice model of ferrimagnetism, the magnetic moment per formula unit in mB, nBN(x) is expressed as:
nBN(x) = MB(x) – MA(x)
where MB and MA are the Band Asublattice magnetic moment in mB respectively.
The saturation magnetization for all the ferrites after annealing at 1000℃ is listed in Table 1. From the Table 1 it is clear that for the samples CoFe2O4, Co0.8Zn0.2Fe2O4, and Co0.6Zn0.4Fe2O4, the saturation magnetization increases from 84.5-91.6 emu/g. This could be due to Zn2+ (with zero magnetic moment) replace ion on the tetrahedral A–sites, causing the decrease of magnetic moment in the sublattice MA, resulting in the increase of total magnetic moment as discussed earlier. On further increase of zinc substitution in Co0.4Zn0.6Fe2O4, Co0.2Zn0.8Fe2O4 and ZnFe2O4 the saturation magnetization decreases. This could be due to further increase in the concentration of Zn2+ (more than 0.4), the exchange interaction between A and B sites gets lowered resulting in strengthening of B-B interaction and weakening of A-B interaction, which leads to decrease of saturation magnetization.
The value of coercivity (Hc), reaches a maximum value and then decreases as the grain size increases as shown in Figure 5. This variation of Hc with grain size can be explained on the basis of domain structure, critical diameter and the anisotropy of the crystal [15,16]. In the single domain region as the grain size decreases the coercivity decreases because of the thermal effects. The coercivity Hc in the single domain region is expressed as Hc = g – h/D2 where g and h are constants. In the multi domain region the variation of coercivity with grain size can be expressed as Hc = a + b/D where; a’ and ‘b’ are constants and ‘D’ is the diameter of the particle [17]. Hence in the multi domain region the coercivity decreases as the particle diameter increases. A decrease in coercivity as observe in Figure 6, with increase in zinc concentration may be attributed to the decrease in anisotropy field, which in turn decreases the domain wall energy [18,19].
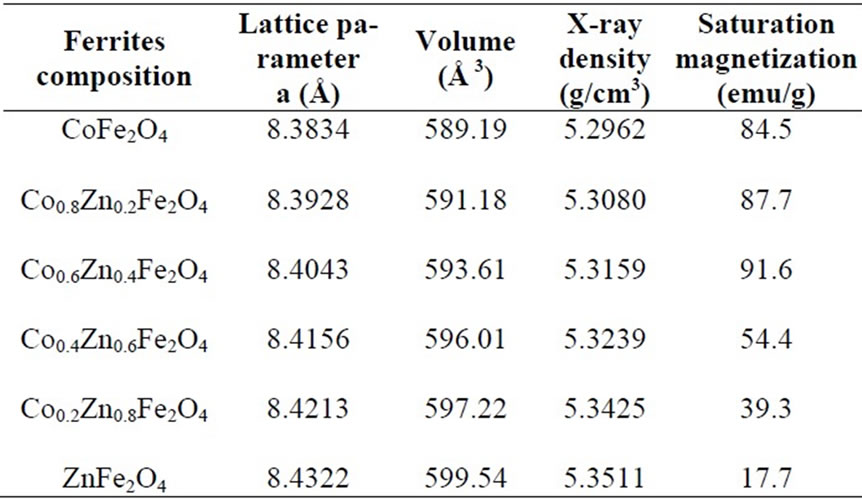
Table 1. Lattice parameters derived from X-ray diffraction pattern and saturation magnetization of the ferrites after annealing at 1000℃
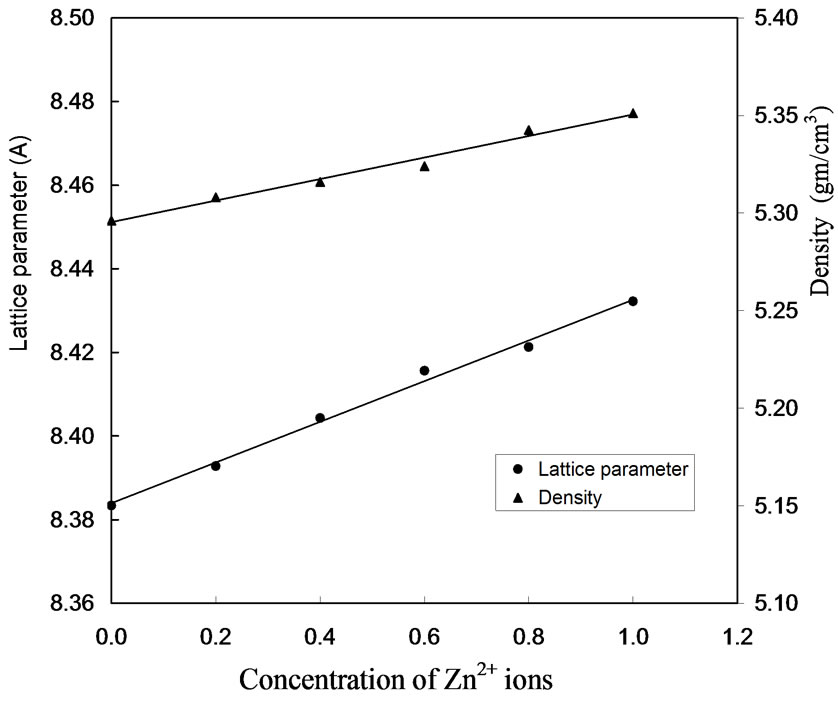
Figure 4. Variation of lattice parameters and density with the zinc concentration
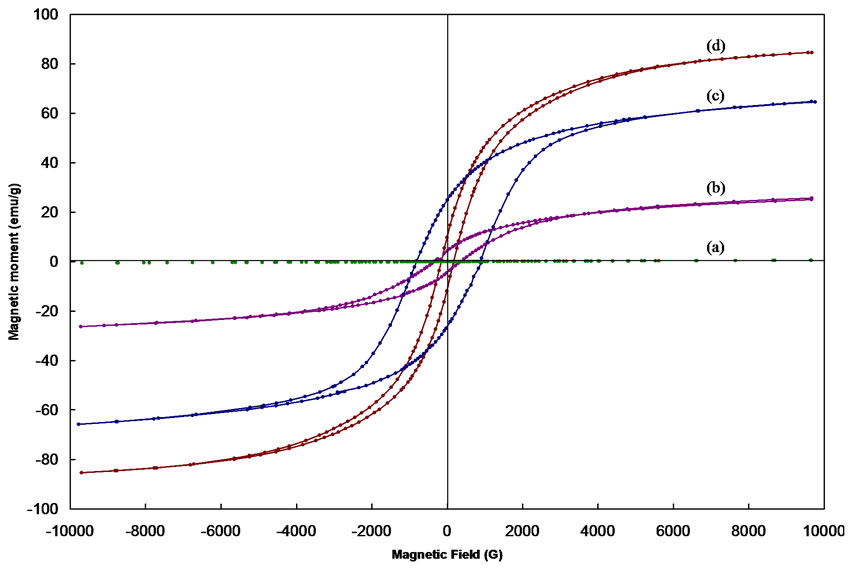
Figure 5. Hysteresis loops for the CoFe2O4 (a) as obtained and after annealing at (b) 400, (c) 600 and (d) 1000 ℃
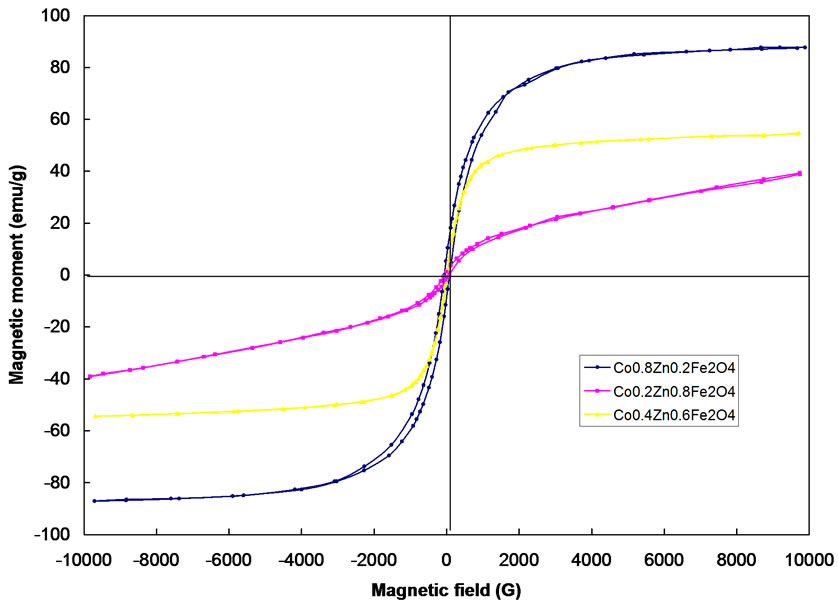
Figure 6. Hysterisis loop of Co0.2Zn0.8Fe2O4, Co0.4Zn0.6Fe2O4, Co0.8Zn0.2Fe2O4 after annealing at 1000℃
4. Conclusions
Sol-gel method has been used to synthesize Zn substituted cobalt ferrite samples at nanometer scale. A slight change in the concentration of the nitrate precursor salts changes the lattice parameter and magnetic properties. The vibrational mode of tetrahedral clusters (564-588 cm-1) is higher as compared to that of octahedral clusters (425-442 cm-1), which is attributed to the shorter bond lengths of tetrahedral clusters. An increasing growth of grain size is also observed with increasing annealing temperature and formation of sharp peaks at 1000oC in X-ray powder diffraction pattern is in conformity with this result. The lattice parameter and the X-ray density, increases with increasing Zn concentration. The saturation magnetization first increases from CoFe2O4 to Co0.6Zn0.4Fe2O4 and then shows a decreasing behavior till ZnFe2O4.
REFERENCES
- K. Raj, R. Moskowitz and R. Casciari, “Advances in Ferrofluid Technology,” Journal of Magnetism and Magnetic Materials, Vol. 149, No. 1-2, 1995, pp. 174-180.
- R. Valenzuela, “Magnetic Ceramics,” Cambridge University Press, Cambridge, 1984, pp. 191-212.
- D. S. Mathew and R.-S. Juang, “An Overview of Structure and Magnetism of Spinel Ferrite Nanoparticles and their Synthesis in Microemulsions,” Journal of Chemical Engineering, Vol. 129, No. 1-3, 2007, pp. 51-65.
- A. Tawfik, I. M. Hamada, O. M. Hemeda, “Effect of Laser Irriadiation on the Structure and Electromechanical Properties of Co-Zn Ferrite,” Journal of Magnetism and Magnetic Materials, Vol. 250, 2002, pp. 77-82.
- S. Dey and J. Ghose, “Synthesis, Characterization and Magnetic Studies on Nanocrystalline Co0.2Zn0.8Fe2O4,” Materials Research Bulletin, Vol. 38, No. 11-12, 2003, pp. 1653-1660.
- R. Arulmurugan, B. Jeyadevan, G. Vaidyanathan and S. Sendhilnathan, “Effect of Zinc Substitution on Co-Zn and Mn-Zn Ferrite Nanoparticles Prepared by Co-Precipitation,” Journal of Magnetism and Magnetic Materials, Vol. 288, 2005, pp. 470-477.
- M. U. Islam, M. U. Rana and T. Abbbas, “Study of Magnetic Interactions in Co-Zn-Fe-O System,” Materials Chemistry and Physics, Vol. 57, No. 2, 1998, pp. 190- 193.
- G. Vaidyanathan, S. Sendhilnathan, R. Arulmurgan, “Structural and Magnetic Properties of Co1-xZnxFe2O4 Nanoparticles by Co-Precipitation Method,” Journal of Magnetism and Magnetic Materials, Vol. 313, No. 2, 2007, pp. 293-299.
- G. V. Duong, N. Hanh, D. V. Linh, R. Groessinger, P. Weinberger, E. Schafler and M. Zehetbauer, “Monodispersed Nanocrystalline Co1-xZnxFe2O4 Particles by Forced Hydrolysis: Synthesis and Characterization,” Journal of Magnetism and Magnetic Materials, Vol. 311, No. 1, 2007, pp. 46-50.
- S. B. Waje, M. Hashim, W. D. W. Yousoff and Z. Abbas, “Sintering Temperature Dependence of Room Temperature Magnetic and Dielectric Properties of Co0.5Zn0.5 Fe2O4 Prepared Using Mechanically Alloyed Nanoparticles,” Journal of Magnetism and Magnetic Materials, Vol. 322, No. 6, 2010, pp. 686-691.
- A. Pradeep, G. Chandrasekaran, “FTIR Study of Ni, Cu and Zn Substituted Nanoparticles of MgFe2O4,” Material Letters, Vol. 60, No. 3, 2006, pp. 371-374.
- H. P. Klug and L. E. Alexender, “X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials,” Chapter 9, 2nd Edition, Wiley, 1974.
- N. Hanh, O. K. quy, N. P. Thuy, L. D. Tung and L. Spinu; “Synthesis of Cobalt Ferrite Nanocrystallites by Forced Hydrolysis Method and Investigation of their Magnetic Properties,” Physica B, Vol. 327, No. 2, 2003, pp. 382- 384.
- Y. Shi, J. Ding and H. Yin, “CoFe2O4 Nanoparticles Prepared by the Mechanical Method,” Journal of Alloys and Compounds, Vol. 308, No. 1, 2000, pp. 290-295.
- B. D. Cullity, “Introduction to Magnetic Materials,” Addison-Wesely Publishing Company Inc., Reading MA, 1972.
- S. Chikazumi, “Physics of Magnetism,” Wiley, New York, 1959.
- M. George, A. M. John, S. S. Nair, P. A. Joy and M. R. Anantharaman, “Finite Size Effects on the Structural and Magnetic Properties of Sol-Gel Synthesized NiFe2O4 Powders,” Journal of Magnetism and Magnetic Materials, Vol. 302, No. 1, 2006, pp. 190-195.
- Y. M. Yakovlev, E. V. Rubalikaya and N. Lapovok, “Ferromagnetic Resonance in Lithium Ferrite,” Soviet Physics-Solid State, Vol. 10, 1969, pp. 2301-2303.
- H. K. Jun, J. H. Koo and T. J. Lee, “Study of Zn-Ti Based H2S Removal Sorbents Promoted Co and Ni Oxides,” Energy Fuels, Vol. 18, No. 1, 2004, pp. 41-48.

