Natural Science
Vol. 5 No. 8 (2013) , Article ID: 35579 , 5 pages DOI:10.4236/ns.2013.58110
Habitat fragmentation and the population status of rodents in Abayum forest, Ikom, Cross River State, Nigeria
![]()
1Department of Forestry and Wildlife Resources Management, University of Calabar, Calabar, Nigeria; auogogo@yahoo.com
2Department of Geography and Regional Planning, University of Calabar, Calabar, Nigeria
Copyright © 2013 Augustine Ugar Ogogo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 10 May 2013; revised 10 June 2013; accepted 17 June 2013
Keywords: Forest Fragmentation; Rodent Population; Abayum Forest; Cross River State; Nigeria
ABSTRACT
The threat to wildlife population is attributed to various anthropogenic activities. The main objective of this study was to identify the influence of fragment size on the population density of rodents in the study area. Fourteen (14) out of forty (40) fragments existing in the area were randomly sampled. The parameters used for the study were number, size of fragments and the corresponding population distribution of rodents in the study area. Fifty hunters in the area were also interviewed. The fragments were stratified into first, second and third order fragments on the basis of their sizes and randomly selected for the study. Indirect method of wildlife census was carried out through the observation of droppings, trail or tract, burrows, eating habits and noise. Fragment growth rate was 18 to 40 (87.5%) in 7 years. Anthropogenic perturbations in the form of cultivation of permanent cropland, settlement expansion, bush burning, timber exploitation and new settlements in areas previously thinly settled or not accessible to outsiders have resulted in disjointed ecosystems. The population density of rodents correlated with fragment size was highly significant ((P < 0.05) r = 0.9). It was then concluded that fragment size greatly influenced the population and diversity of rodent species. It was recommended that the remaining large fragments in the study area should be protected by law from further fragmentation.
1. INTRODUCTION
The reduction of a natural forest and the formation of forest fragments through anthropogenic activities are the main causes of wildlife population loss in tropical forests [1,2]. Abayum forest is among the most diverse and productive lands in Cross River State, Nigeria. It is however seriously fragmented [3]. Habitat fragmentation leads to a reduction of a continuous habitat into small, remnant islands and it is considered an important threat to the maintenance of wildlife population in their natural environment [4]. When a habitat size is reduced, the remaining area becomes isolated leading to a decrease in the population of wildlife [5].
Globally, habitat fragmentation reduces the population density of wildlife through the reduction of available migratory species due to accelerated habitat modification. The migratory wildlife retreat into small patches of land leading to crowding effects and increased competition [6]. Furthermore, the size of a fragment determines the number of wildlife species existing in it [7]. Smaller fragments support smaller populations which are vulnerable to extinction. In addition to size, environmental risks such as diseases, prolonged draught, fire, flood and scarcity of food which would have caused no danger in terms of extinction in a large population may be catastrophic in small isolated populations [8-10].
The objective of this study was to examine the influence of habitat fragmentation on the population density of rodents in Abayum forest, Ikom local Government area, Cross River State, Nigeria.
2. MATERIALS AND METHODS
2.1. The Study Area
The study was carried out in Abayum forest, Ikom Local Government Area, Cross River State, Nigeria. It lies between latitude 6.00˚ and 6.15˚N and longitude 8.30˚ and 8.45˚E of Greenwich meridian. The study area is demarcated by the Cross River. It was approximately 106.2 km2 in size. It is bounded in the North-West by Ogoja Local Government Area, North-East by Boki Local Government Area and South-East by Etung Local Government Area.
The area has an equatorial climate with rainy and dry seasons. It has a mean annual rainfall of 258 mm per annum with a mean temperature of 25.5˚C and relative humidity of 89 - 94 percent.
The vegetation is made up of rainforest which has been reduced to secondary forest due to intensive cultivation, bush burning and other varied human activities. Some areas still have pockets of tropical rainforest with some areas in the northern part having guinea savanna.
2.2. Data Collection
Data collected covered a total of ten villages. These were Onyenghe, Egonenkor Esaja, Ayukasa, Mile V, Njemetop, Abinti, Nto, Nyerenkpor and Ndom and Nkofap. Data on number and sizes of fragments from 2000 to 2007 were collected from satellite images obtained from Geodeve Communication Company of France. Population status of rodents was obtained through indirect census and interview of hunters.
The forty fragments were stratified into first, second and third order fragments. The first order fragments were greater than or equal to 10 km2, second order fragments were greater than or equal to 1 km2 and third order fragments, <0.03 km2. Apart from the first order fragments that were few and were all taken, the second and third order fragments were randomly sampled at 30 percent sampling intensity based on the number of fragments in each order. Three plots, two plots and one plot each were selected from first, second and third order fragments. Each of the plots measured 50 m × 50 m with transects of 5 m intervals established for the researchers to carefully observe the foot prints, droppings, trails or tracts, burrows, eating habits and noise of wildlife [11,12]. A total of 21 plots were sampled. Data on population distribution were collected from the plots and were summed up fragment by fragment and divided by the size of the fragment to obtain population density of rodents. Both young and old hunters were interviewed. Questions asked included the type of rodents common in the area, various hunting methods and apparatus employed in exploiting wildlife for instance, guns, traps and baits used. The frequency of catching a particular species and the species of rodents most preferred were asked. Also the type of fragment that contains more rodents, why some wildlife species were preferred to others and the number of bush meat vendors who come to the area in search of meat were asked.
Five persons were interviewed in each of the ten villages visited, bringing the total number of persons interviewed to 50.
2.3. Data Analysis
Correlation and regression analysis were used to analyse data.
Frequency and percentage tables were used in the presentation of results. Each table contained information on the research questions asked following the objectives of the study.
3. RESULTS AND DISCUSSION
Following the presence of grassland and pockets of forest around this area, the following rodents were found to be present. Grass cutter (Thryonomys swinderianus), porcupine (Cristeris cristata), bush mouse (Myomys daltoni) and ground squirrel (Xerus erythropus). The intense exploitation of porcupine, grass cutters and land squirrels for food assisted to reduce the population of these species of animals in the study area.
In Table 1, the number of fragments increased from 18 in the year 2000 to 40 in the year 2007. The percentage increase was 87.5 percent in seven years.
As shown in Table 2, 40% of the anthropogenic activities were settlement expansion comprising of building of private houses, churches, schools and health centres,
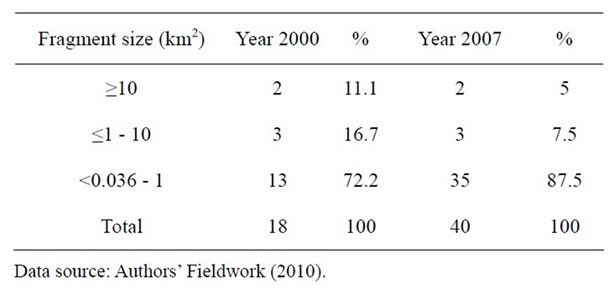
Table 1. Number and sizes of fragments between 2000 and 2007.
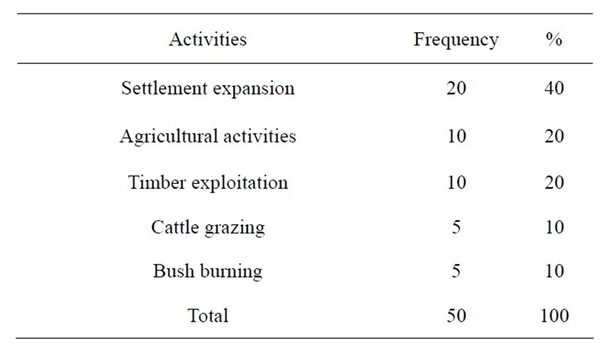
Table 2. Anthropogenic activities leading to fragmentation.
20 percent was agricultural activities and, the remaining 10 percent was timber exploitation including logging.
Excessive hunting was given by 50% of the respondents as the main reason for the fall in wildlife population. This was followed by farming activities that lead to the destruction of wildlife habitats which was given by 30% of the respondents. The use of chemicals and forest fragmentation were named by 10% of the respondents each (Table 3).
From Table 4, it is clear that the population of rodents was decreasing in the area as the number of fragments increased. For instance the smallest fragment with size 0.12 km2 had a population of 0 squirrels 47 giant rat, 141 porcupines, 94 grass cutters and 0 bush mice, while a larger fragment of size 19.52 km2 had 25,770, 31,232, 33,574, 15,616 and 2577 of the same species respectively. However, there were few exceptions where small frag-
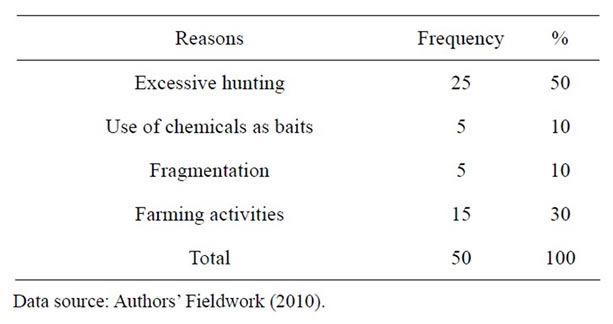
Table 3. What hunters perceive as the main reasons for the fall in wildlife population.
ments contained a high population of rodents and some did not have at all.
The result was in conformity with the species area relationship in line with the theory of island biogeography developed by [6]. It states that large fragments have larger numbers of species while small fragments have fewer numbers of species.
This was confirmed by the fact that correlation of fragment size with all rodent populations was highly significant (P < 0.05, Table 5).
The correlation also revealed a highly significant relationship between bush mouse and grass cutter but a low relationship between bush mouse and porcupine as well as between grass cutter and porcupine. This may be due to the fact that the former two are found in the same habitat which is grassland, while the porcupine is found in a different habitat which is high forest. Squirrels however appeared to inhabit all the habitats studied. Prediction equation of fragment size and rodent population is given in Table 6.
Fifty percent of respondents in the study area attributed the decrease in wildlife population to excessive hunting while 30 percent attributed it to farming activities.
The general decline in wildlife population could be attributed to the fact that 90 percent of the rural populace prefer bush meat to other sources of protein resulting in high wildlife hunting pressure in the area. The preference to bush meat is not limited to the rural population alone, urban dwellers prefer bush meat as well. This may ex-
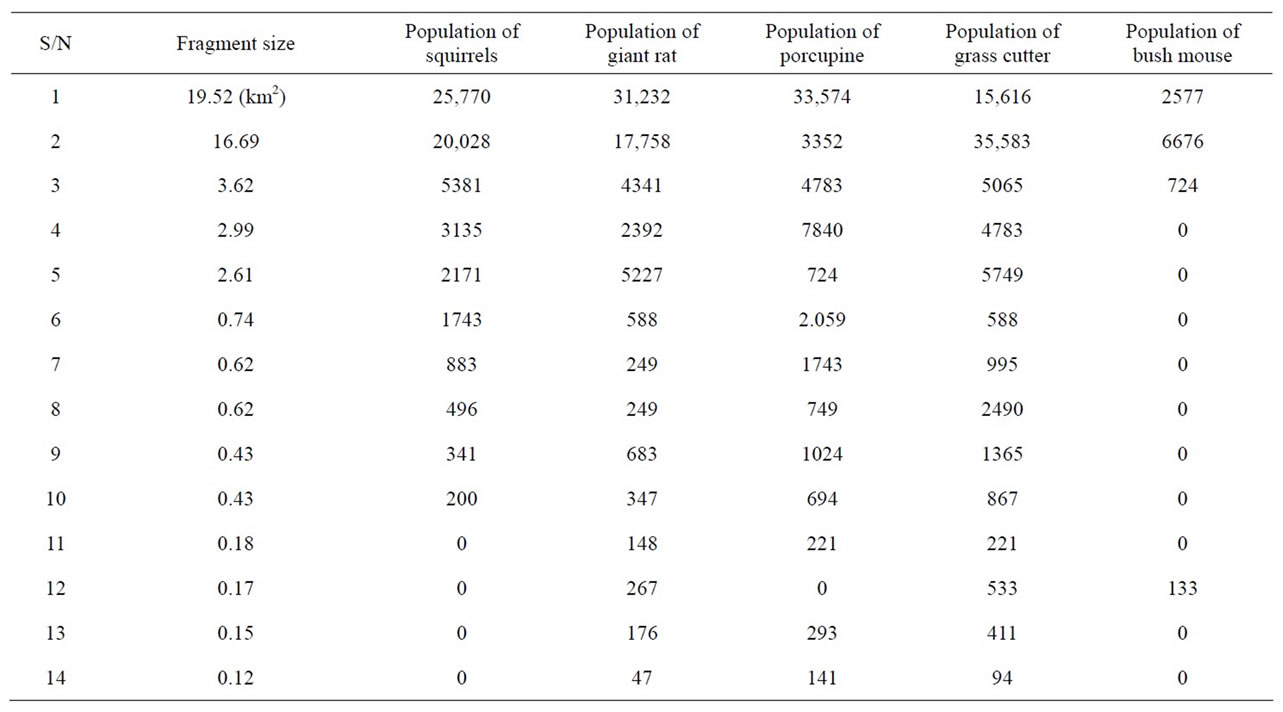
Table 4. Fragment size and population distribution of rodents.
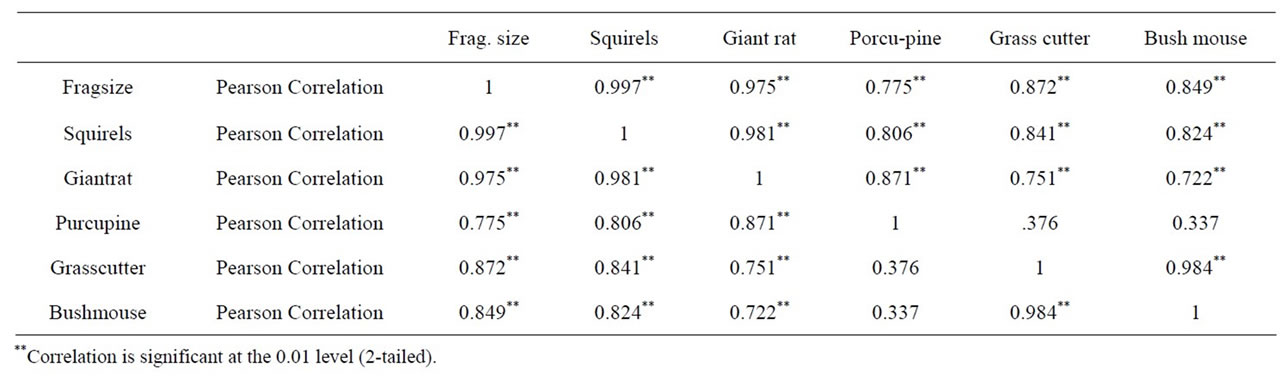
Table 5. Pearson correlation coefficient between fragment size and rodent population.
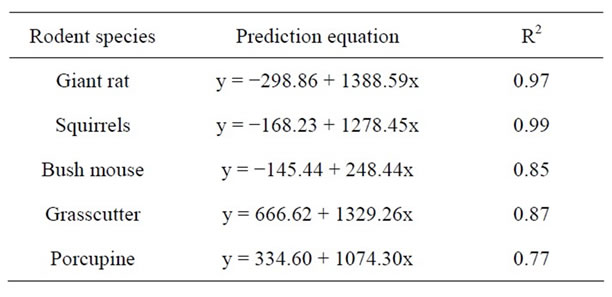
Table 6. Prediction equation of rodent population (y) from fragment size (x).
plain why drinking spots and restaurants that serve bush meat receive high patronage from customers in view of the pleasure they derive from the combination of bush meat with their food or drinks. There is therefore a booming trade in bush meat with the traders transporting large quantities of various species of smoked bush meat to the townships for sale. Another reason that could account for the decline in the population of wildlife was that bush meat was a source of income to hunters. The income generated was used for the payment of school fees of hunter’s children, building of houses and as a protein source to members of the hunters’ family. Furthermore, meat from the red deer was used for traditional marriage and as an additive to herbal and traditional medicine [12].
The fundamental cause of population decline and extinction observed through studies and long term wildlife census by [13,14] was that a small population size is the strongest determinant of vulnerability to extinction thereby confirming theoretical predictions by most researchers. Researchers like [15] stated that large bodied species like elephants were more vulnerable to extinction because their population is small due to low reproduction rates and long gestation period, slow growth rate and long life span. On the other hand, small bodied species especially rodents like bush mice, grass cutter, squirrels and purcupine were less at risk because of their fast population growth rate which enables them to recover from severe reduction in number due to demographic or environmental perturbations.
Animals with long life span apparently enable species in that group to spread their reproductive efforts for a long period, thus avoiding the catastrophic consequences of a few bad moments, which may drive animals with short life span to extinction.
Apart from body size, [15] reported that migratory behaviour is also responsible for species extinction because of failure of wildlife to return to their original habitat but could fall victims to human hunters who could use sophisticated weapons to exploit them for their economic gains.
Furthermore, vulnerability to extinction is common with species that are highly specialized to particular habitats and breeding sites. For instance, the alligator and water turtle are found more in lakes and stagnant water or food sources than in terrestrial habitats. The occupation of insular habitats or ranges, where populations are often small, species may be induced to be restricted to highly distinctive environmental conditions thereby increasing the risk of extinction when the environment is disturbed by way of habitat modification and fragmentation.
4. CONCLUSION
Fragmented ecosystems cause extinction of local animal populations. The biodiversity is also reduced. Anthropogenic perturbations in the form of cultivation of permanent cropland, settlement expansion, bush burning, timber exploitation and new settlements in areas previously thinly settled or not accessible to outsiders have resulted in disjointed ecosystems. Furthermore, the hunting of large animals with the use of sophisticated weapons and the use of chemicals in choice food offered to the animals as baits have seriously placed the wildlife population at risk of extinction today more than any other time in the past. This pressure on land leading to the extinction of the wildlife population is due to extreme poverty, population pressure and migration of visitors to the study area due to the fertile soil found in the region leading to a tremendous pressure on the natural resources. Forest fragmentation has resulted in the transformation of large portions of forest land into isolated patches of vegetated terrains that harbour few species thereby decreasing their probability of persistence.
5. ACKNOWLEDGEMENTS
We wish to acknowledge the co-operation of the Vice-Chancellor, University of Calabar, Professor James Epoke for the permission granted us to use the GIS laboratory to carry out some components of the work. Our sincere thanks go to Mr. Marcus Idoko who handled the interpretation of the data obtained from Geodeve Communication Company of France. We also sincerely appreciate retired chief Ranger Attep and his dear wife for their cooperation and assistance during the field work.
![]()
![]()
REFERENCES
- Janzen, D.H. (1988) Tropical dry forest: The most endangered major tropical ecosystem. In: Wilson, E.O., Ed., Biodiversity, National Academy Press, Washington, D.C. 130-137.
- Quesada, M. and Stoner, M.K. (2004) Threats to the conservation of tropical dry forest in Costa Rica. In: Frankie, G.W., Amata and Vincon, S.B., Eds., Biodiversity Conservation in Costa Rica: Learning the Lessons from a Seasonal Dry Forest, University of California Press, Berkeley.
- Tawo, A.N. (2011) Effects of habitat fragmentation on biodiversity of rodents and wild ruminants in Ikom Forest Cross River State of Nigeria. Ph.D. Thesis, University of Calabar, Calabar.
- Saunders, D.A.R., Hobbs, J. and Margules, C.R. (1991) Biological consequences of ecosystem fragmentation: A review. Conservation Biology, 5, 18-32. doi:10.1111/j.1523-1739.1991.tb00384.x
- Ellstrand, J.A. and Ellam, M.F.T. (1993) Zoogeography: The geographical distribution of animals. New York.
- MacArthur and Wilson (1963) The theory of island biogeography. Princeton University Press, Princeton.
- Rosenzweig, M.L. (1995) Species diversity in space and time. Cambridge University Press, Cambridge. doi:10.1017/CBO9780511623387
- Root, R.B. (1973) Organization of plant anthropoid association in simple and diverse habitats: The fauna of collards (Brassica oleracea) Ecological Monographs, 43, 95-120. doi:10.2307/1942161
- Raupp, M.J. and Denno, R.F. (1979) The influence of patch size on a guild of sap-feeding insects that inhabit the salt march grass (Spartina patterns). Environmental Entomology, 8, 412-417.
- Connor, E.E., Courtney, A.C. and Yoder, J.M. (2000) Individual area relationship: The relationship between animal population density and area. Ecology, 81, 234-748
- Dasman, R.E. (1964) Wildlife biology. John Willey and Sons, Hoboken, 231 p.
- Ogogo, A.U. (2004) Wildlife Management in Nigeria, Objectives, Principles and Procedures. 1st Edition, Median Communications, Calabar.
- Diamond, J.M. (1984) “Normal” extinction in isolated populations. In: Niteck, M.H., Ed., Extinction, University of Chicago, New York, 191-245.
- Pimm, S.L., Johes, H.L. and Diamond (1988) On the risk of extinction. American Naturalist, 132, 257-785.
- Leigh, E.G. (1981) The average lifetime of a population in a varying environment. Journal of Theoretical Biology, 90, 213-239

