Advances in Bioscience and Biotechnology
Vol.3 No.7(2012), Article ID:24695,8 pages DOI:10.4236/abb.2012.37106
Larvicidal and knockdown effects of some essential oils against Culex quinquefasciatus Say, Aedes aegypti (L.) and Anopheles stephensi (Liston)
![]()
1Department of Biological Sciences, AVIT, Vinayaka Missions University, Payyanoor Campus, Salem, India
2Entomology Research Institute, Loyola College, Chennai, India
3Department of Advanced Zoology and Biotechnology, Loyola College, Chennai, India
Email: *entolc@hotmail.com
Received 28 April 2012; revised 28 May 2012; accepted 4 October 2012
Keywords: Larvicidal; Knockdown; Essential Oils; Aedes aegypti; Anopheles stephensi; Culex quinquefasciatus
ABSTRACT
Efficacy of 25 essential oils was screened against filarial vector, Culex quinquefasciatus, for their larvicidal and knockdown effects in a preliminary study. Of these, 8 oils viz. calamus oil, cinnamon oil, citronella oil, clove oil, eucalyptus oil, lemon oil, mentha oil and orange oil exhibited 100% larvicidal activity at 1000 ppm and 100% knockdown effect at 10% concentration. These 8 oils were screened further against Cx. quinquefasciatus, Aedes aegypti and Anopheles stephensi for their larvicial and knockdown effects at different concentrations. Mentha oil was the most promising against An. stephensi and Ae. aegypti recording LC50 and LC90 values of 39.74 and 115.67 ppm and 46.23 and 165.36 ppm, respectively for larvicidal activity. Calamus oil was the most effective against Cx. quinquefasciatus with LC50 and LC90 values of 40.40, and 140.07 ppm, respectively for larvicidal activity. Orange oil showed the most potent knockdown effect with the KT50 and KT95 values of 27.44, 26.22 and 29.91 and 70.81, 65.33 and 68.57 min, against An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively. The results clearly indicated that mentha oil and calamus oil were the most promising larvicides and orange oil had potent knockdown effect against the tested mosquito species. These oils could be used to develop a new formulation to control mosquitoes.
1. INTRODUCTION
Man has suffered from the activities of mosquito since time immemorial and it is ranked as man’s most important insect pest. Mosquitoes belonging to the genera Anopheles, Culex and Aedes are the vectors for the pathogens of different diseases such as malaria, filariasis, Japanese encephalitis, dengue and dengue haemorrhagic fever, epidemic polyarthritis, yellow fever and chikungunya [1-3]. These diseases devastate Indian economy every year [4]. Tropical areas are more vulnerable to parasitic diseases and the risk of contracting arthropod-borne illnesses is increased due to climate change and intensifying globalization [5]. Worldwide, mosquitoes transmit diseases to more than 700 million people annually and are responsible for 1 death for every 17 people currently alive [6]. Malaria results from an infection by a protozoan carried by Anopheles stephensi. About 2.5 billion people are at risk, more than 500 million people become seriously ill with malaria every year, and more than one million people die due to malaria [7]. Culex quinquefasciatus is responsible for the transmission of lymphatic Filariasis caused by Wuchereria bancrofti. Lymphatic filariasis, disease affecting the arms, legs and genitals, is much prevalent in India. Lymphatic filariasis infects 80 million people annually of which 30 million cases exist in chronic infection. There are 45 million cases of Lymphatic filariasis in India alone [8].
Essential oils play an important role in controlling several mosquito species. In general, essential oils from plants have been considered important natural resources to act as insecticides [9,10]. Larvicidal activity of essential oils from Blumea mollis [11] and Zingifer officinalis [12] has been reported against Cx. quinquefasciatus. Larvicidal activity of essential oils from Melaleuca leucadendron, Litsea cubeba and Litsea salicifolia [13], Ocimum suave and O. kilimandscharicum [14] have been reported against Anopheles arabiensis, A. gambiae and Cx. quinqefasciatus. Larvicidal activity of essential oils form Zanthoxylum armatum [15] and Ocimum canum [16] have been reported against Cx. Quinquefasciatus, Ae. aegypti and An. stephensi. Essential oils derived from various plants not only exhibit inhibitory activity against bacteria, fungi and termites but also show strong mosquito repellent and larvicidal activities [17].
The present study was aimed to assess the larvicidal and knockdown activities of the essential oils from various plants against C. quinquefasciatus, Aedes aegypti and Anopheles stephensi.
2. MATERIALS AND METHODS
Rearing of Mosquitoes
Anopheles stephensi: Egg strips of Anopheles stephensi were obtained from Malaria Research Center (MRC), Chennai. They were placed in Petri dishes (10.5 diameter) containing dechlorinated tap water for hatching. After hatching of larvae, they were provided with powdered yeast and dog biscuits in 3:1 ratio. After attaining pupae, they were separated and kept inside the mosquito cages for emergence. The cotton soaked in 5% glucose solution was placed inside the cage for nourishment. After three days of emergence, pigeon blood meal was given to adult mosquitoes. After three days of blood meal, the eggs were laid in the Petri dishes containing tap water.
Culex quinquefasciatus: The egg rafts of Culex quinquefasciatus were obtained from Vector Control Research Center (VCRC), Puducherry. The egg rafts were placed in Petri dishes (10.5 diameter) containing tap water. Larvae were fed with finely ground mixture of yeast and dog biscuits in 3:1 ratio. The first instar larvae developed into pupae through four stages in about 8 - 10 days. The pupae were transferred into mosquito cage for emergence. Blood meal from a pigeon was given to adult mosquitoes after three days of emergence. After 3 - 4 days of blood feeding for adult mosquitoes, the Petri dishes filled with tap water were placed inside the cage for oviposition. The egg rafts were separated and placed in glass Petri dishes for hatching.
Aedes aegypti: Aedes aegypti colony was maintained at insectary (54 cm × 45 cm × 40 cm) at 27˚C ± 2˚C and 80% ± 2% Relative humidity with a photoperiod of 12:10 hours light and dark cycles. The egg strips were obtained from Vector Control Research Center (VCRC), Puducherry to start the colony. The strips were immersed in dechlorinated tap water for hatching. To obtain the larvae of equal developmental stage, eggs were introduced by adding a stimulant such as ascorbic acid (100 mg/L) to water [18]. This started the eclosion process. The emerged larvae were maintained in Petri dishes (10.5 cm diameter) with dechlorinated tap water. Larvae were fed with a diet of yeast and dog biscuits in the ratio of 3:1. The first instar larvae developed into pupae in about 7 - 10 days through four stages. The pupae were separated by using a glass dropper into glass Petri dishes and were kept in mosquito net cages (40 cm × 45 cm × 40 cm) for emergence. The newly emerged mosquitoes were provided with 5% glucose solution soaked in cotton wool, which was placed inside the mosquito net cage for nourishment [19]. After three days of emergence, adults were given a blood meal of pigeon [20]. Glass Petri dishes of 50 mL of tap water lined with filter paper were kept inside the cage for oviposition. The eggs thus obtained were immersed in larval trays containing dechlorinated tap water for hatching.
Larvicidal Activity: For larvicidal activity, lab reared third instar larvae of the respective mosquito species were used for the present investigation. Four replicates were done with 25 larvae per replicate for each concentration [21]. For preliminary screening 1000 ppm of the respective oils were used against Cx. quinquefasciatus; the selected active oils were screened at 100, 75, 50 and 25 ppm against Cx. quinquefasciatus, Ae. aegypti and An. stephensi. The data were obtained for larvicidal activity and corrected percentage of larval mortality were calculated using Abbott’s formula [22].
Knockdown Effect: The experiments were conducted in a Peet Grandy Chamber of one cubic meter size. One hundred, two days old female mosquitoes were released into the chamber for each study. The Plant Oil Formulation was allowed to vapourize by using the vapourizer equipment [4]. The number of mosquitoes knocked down was recorded at periodic intervals of five minutes till complete knockdown. The maximum exposure period was 60 minutes. The knocked down mosquitoes were collected and placed in a recovery jar provided with 10% sugar solution to monitor mortality/recovery at 24 h period. The temperature and humidity of the chamber were maintained at 28˚C ± 2˚C and 50% - 70% respectively. The data obtained for knockdown were subjected to Finney’s method of Probit Analysis to assess the KT50 and KT95 values and they were drawn from four replicates.
Plant Oils: Some of the plant oils were extracted in the lab and some oils were purchased commercially from the authorized dealers in Chennai, Tamil Nadu, India.
3. RESULTS
Preliminary Screening: In the preliminary screening 25 essential oils were screened against Cx. quinquefasciatus in order to select effective oils on the basis of larvicidal and knock down effects for further study against Cx. quinquefasciatus, Ae. aegypti and An. stephensi. It was observed that eight oils viz. calamus oil, cinnamon oil, citronella oil, clove oil, eucalyptus oil, lemon oil, mentha oil and orange oil showed 100% larvicidal and knockdown activities. Besides these, geranium, lime, tulsi and palmarosa oils showed more than 92% larvicidal and knockdown activities. Among the 25 oils vetiver oil exhibited the least larvicidal and knockdown effects (Table 1).

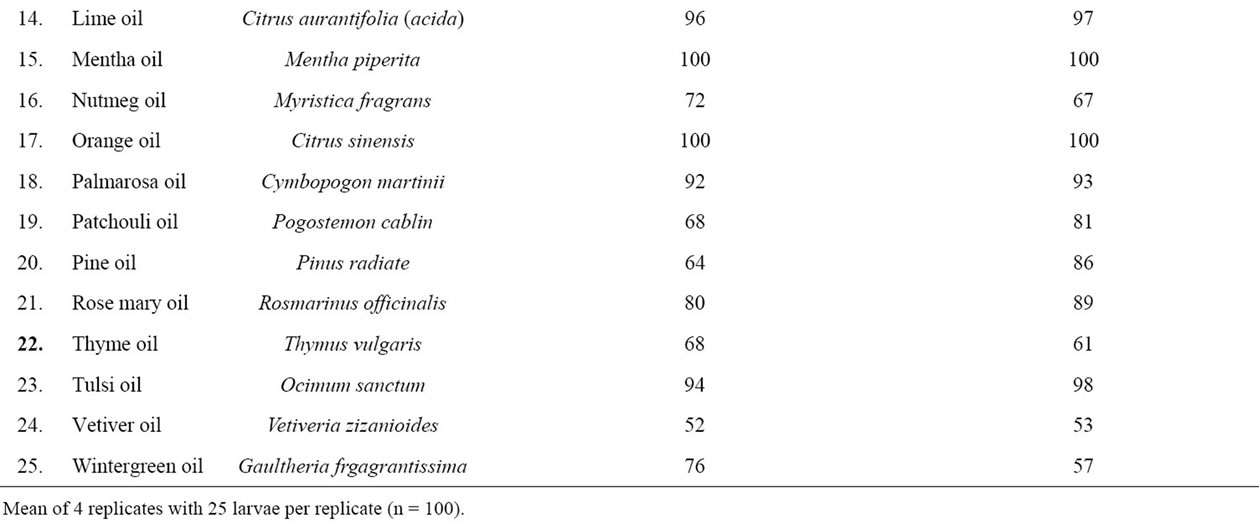
Table 1. Preliminary screening of selected essential oils against Culex quinquefasciatus.
Larvicidal Activity: The oils which exhibited cent percent larvicidal and knockdown effects were evaluated against Cx. quinquefasciatus, Ae. aegypti and An. Stephensi for their larvicidal and knowdown activities. Among the oils tested against An. stephensi, the most promising oils were mentha, clove and calamus oils which recorded low LC50 and LC90 values of 39.74, 39.80, 40.79 ppm and 115.67, 149.81 and 126.05 ppm, respectively with 95% confidence lower limits of 30.64, 20.98 and 31.14 ppm and upper limits of 50.21, 52,10 and 51.94 ppm, respectively for larvicidal activity. Eucalyptus oil showed the least larvicidal activity with LC50 and LC90 values of 68.45 and 247.18 ppm, respectively (Table 2).
Among the oils tested against Cx. quinquifasciatus, the most promising oils were calamus, mentha and lemon oils which recorded LC50 and LC90 values of 40.40, 42.25, 43.79 ppm and 140.07, 132.41, 146.94 ppm, respectively with 95% confidence lower limits of 30.04, 32.25 and 33.07 ppm and upper limits of 52.26, 53.89 and 56.30 ppm, respectively for larvidial activity. Citronella oil showed least larvicidal activity with LC50 and LC90 values of 91.23 and 327.00 ppm, respectively (Table 3).
Among the oils against Ae. aegypti, Mentha, citronella and clove oils showed the most potent larvicidal activity and recorded LC50 and LC90 values of 46.23, 47.21 and 50, 54 ppm and 165.36, 181.70 and 177.52 ppm, respectively with 95% confidence lower limits of 34.64, 34.54 and 38.27 ppm and upper limits of 60.07, 62.49 and 65.55 ppm, respectively. Eucalyptus oil showed the least effective larvicidal activity with LC50 and LC90 values of 80.93 and 339.01 ppm, respectively (Table 4).
The results of the present investigation clearly indicated that mentha oil was the most promising one to con-
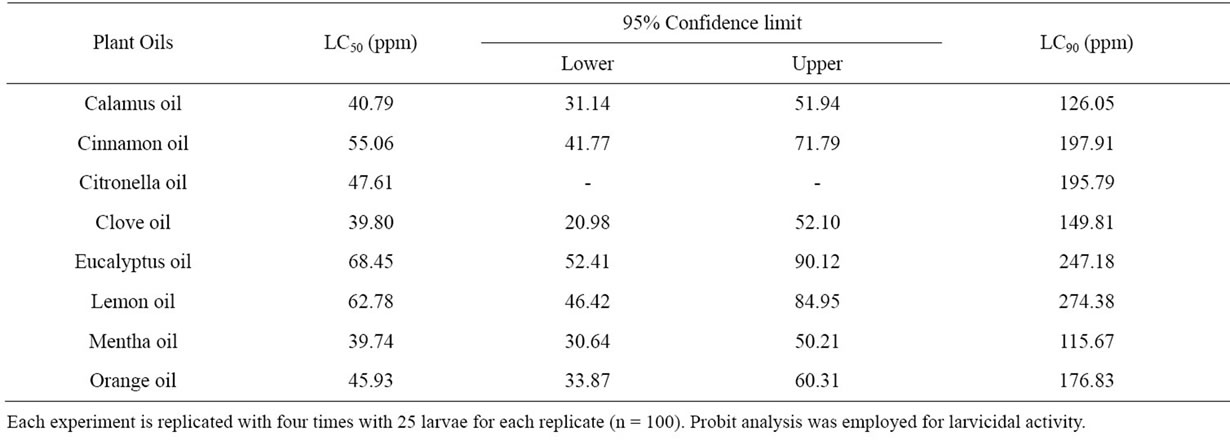
Table 2. Larvicidal activity of the selected plant oils against Anopheles stephensi—24 h exposure.
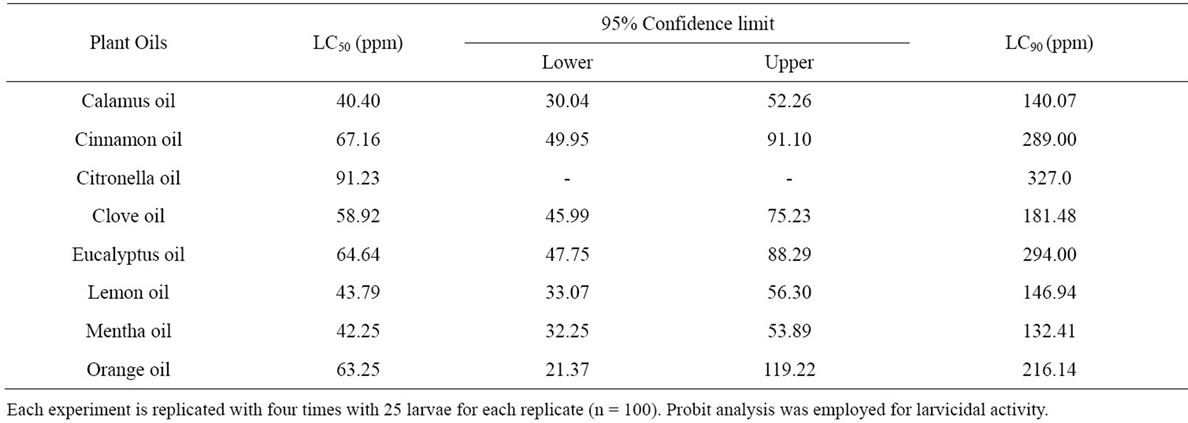
Table 3. Larvicidal activity of the selected plant oils against Culex quinquefasciatus—24 h exposure.
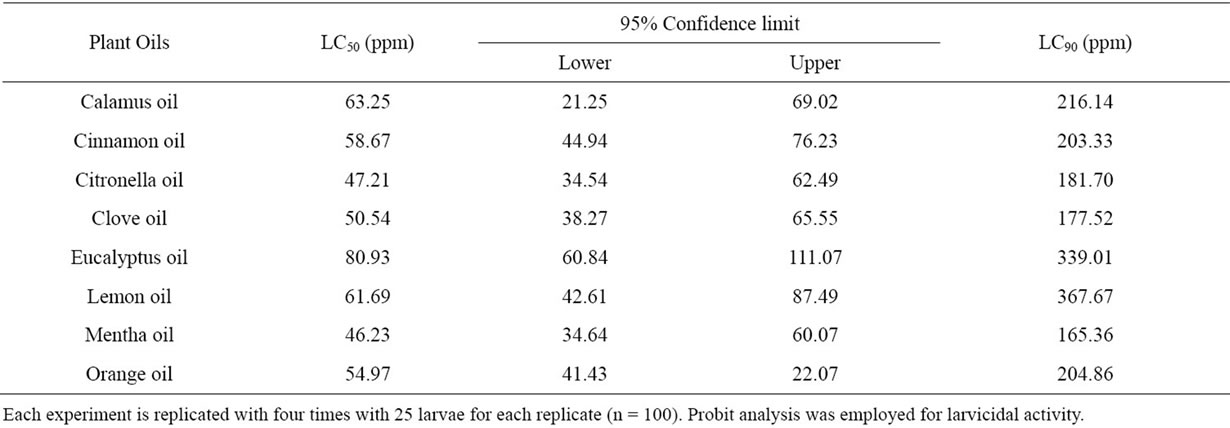
Table 4. Larvicidal activity of the selected plant oils against Aedes aegypti—24 h exposure.
trol the larvae of all the mosquito species. Calamus oil was able to control An. stephensi and Cx. quinquefasciatus larvae. Citronella oil had the potential to control Ae. aegypti larvae only.
Knockdown Activity: Eight effective oils selected from preliminary screening were evaluated for their knockdown effect (expressed in minutes) against An. stephensi, Cx. quinquefasciatus and Ae. aegypti. Orange oil was the most promising one showing KT50 and KT95 values of 27.44 and 70.81 min, respectively against An.
stephensi with the 95% fiducial lower limit of 20.36 min and upper limit of 34.99 min. The remaining oils showed good knockdown activity with KT50 values ranging from 30.53 to 33.92 min and the KT95 values ranging from 65.59 to 91.19 (Table 5).
Orange oil was the most potent one against Cx. quinquefasciatus showing KT50 value of 26.22 min and KT95 value of 65.33 min with 95% fiducial lower limit of 20.67 min and upper limit of 31.90 min. The remaining oils also had notable knockdown activity with KT50 values ranging from 30.37 to 32.70 min and KT95 values ranging from 60.93 to 76.42 min (Table 6).
Orange oil was the most promising one against Ae. aegypti with KT50 value of 29.91 min and KT95 value of 68.57 min with 95% fiducial lower limit of 20.83 min and upper limit of 40.13 min. Rest of the oils had also exhibited good knockdown activity with KT50 values ranging from 30.32 to 32.54 min and KT95 values ranged from 62.96 to 76.25 min against Ae. aegypti (Table 7).
From the results of knockdown activity, it was inferred that orange oil was the most promising one against all the three mosquito species tested.
4. DISCUSSION
Using essential oils to control mosquitoes is a better and environmentally safe option than the use of synthetic chemical pesticides. In the preliminary investigation, calamus oil, cinnamon oil, citronella oil, clove oil, eucalyptus oil, lemon oil, mentha oil and orange oil showed 100% larvicidal and knockdown activities against Cx. quinquefasciatus. Amer and Mehlhorn [23] reported that camphor, thyme, lemon, cedorwood, cinnamon, eucalyptus, menthe, citronella, geranium, lemongrass and some other oils showed larvicidal activity against Ae. aegypti.
In the present study, lemon oil recorded LC50 value of 43.79 ppm against Cx. quinquefasciatus after 24 h exposure for larvicidal activity. Similar observation was
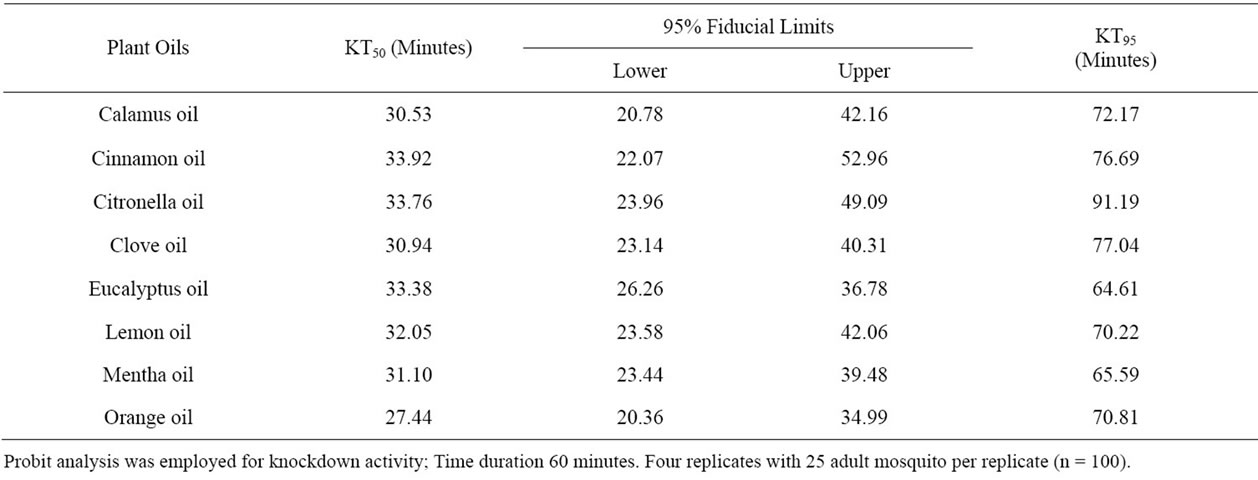
Table 5. Knockdown effect of the selected plant oils against the adult Anopheles stephensi.
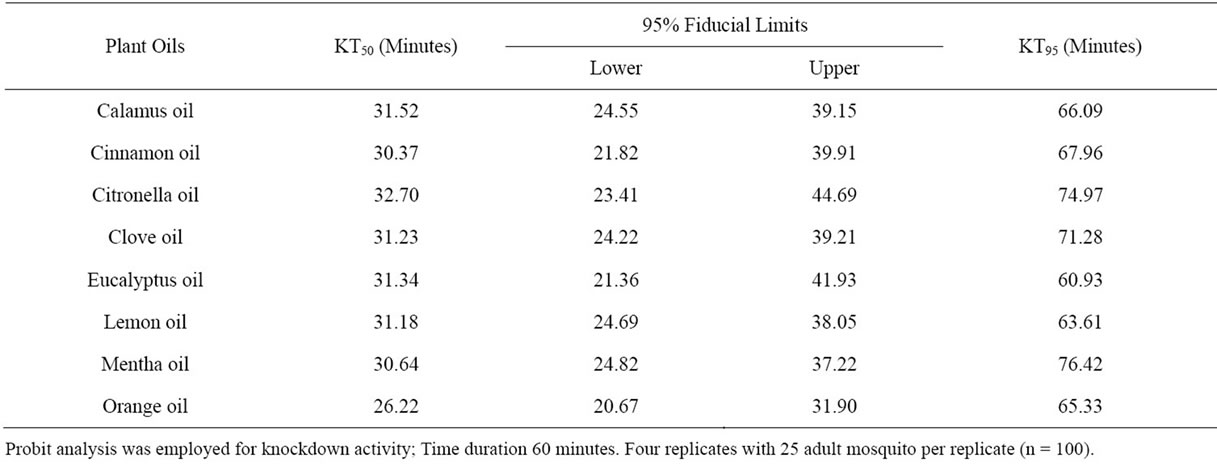
Table 6. Knockdown effect of the selected plant oils against the adult Culex quinquefasciatus.

Table 7. Knockdown effect of the selected plant oils against the adult Aedes aegypti.
recorded by Amer and Mehlhorn [23] with lemon oil; LC50 value of 50.2 ppm was noted against Cx. quinquefasciatus at 24 h exposure. Orange oil in the present study recorded the LC50 values of 54.97, 63.25 and 45.93 ppm against Ae. aegypti, Cx. quiquefasciatus and An. stephensi respectively. This result corroborates with the findings of Michaelakis et al. [24] who reported the LC50 value of 51.5 ppm against Cx. pipiens. Essential oil from Zingiber officinalis exhibited LC50 value of 50.78 ppm after 24 h exposure against Cx. quinquefasciatus [12]. Citronella oil in the present study showed LC50 value of 47.21 ppm against Ae. aegypti at 24 h exposure period. Nataya Sutthanont et al. [25] observed that Zingiber zerumbet showed LC50 value of 48.88 ppm at 24 hr exposure period against Ae. aegypti. Clove oil in the present study showed LC50 value of 39.80 ppm against Anopheles stephensi. Essential oil derived from Syzygium aromaticum showed the LC50 value of 169 ppm against Ae. aegypti [25]. Cinnamon oil in the present study exhibited the LC50 value of 67.16 ppm and 55.06 ppm against C. quinquefasciatus and An. stephensi, respectively at 24 h exposure. Zhu et al. [26] reported that LC50 value of 84 and 66 ppm against A. albopictus and C. pipiens pipiens, respectively. Eucalyptus oil in the present study showed LC90 value of 247.18 and 294.00 ppm against An. stephensi and C. quinquefasciatus, respectively at 24 h exposure. At 24 h exposure, eucalyptus oil showed LC90 value of 274.00 and 264 ppm against A. albopictus and C. pipiens pipiens, respectively [26].
In our study Cymbopogon nardus (Citronella oil) exhibited LC50 value of 47.21 and 47.62 ppm against Ae. aegypti and An. stephensi, respectively, which was lower than the LC50 value of 69 ppm reported by Sukumar et al. [27] for Cymbopogon citratus and 63 ppm for Lippia sidoides by Carvalho et al. [28] against Ae. aegypti. The LC50 values of lemon and Eucalyptus oil in our study were 43.79 and 64.64 ppm, respectively against Cx. quinquefasciatus. Traboulsi et al. [29] reported that LC50 values for oils from Citrus sinensis and Eucalyptus spp were 60.0 and 120.0 ppm, respectively against Culex pipiens larvae. Senthilkumar et al. [11] reported LC50 values of 71.79 ppm against Cx. quinquefasciatus for the essential oil extracted from Blumea mollis. Mentha oil in our study recorded the LC50 value of 42.25 ppm and LC90 value of 132.41 ppm against the larvae of Cx. quinquefasciatus at 24 h exposure. Koliopoulos et al. [30] reported that the LC50 values of 4 Mentha species ranged from 47.88 to 74.28 ppm and LC90 values ranged from 64.34 to 107.45 ppm against Cx. pipiens biotype molestus after 48 h exposure.
In the present investigation orange oil showed the most potent knockdown activity of 27.44, 26.22 and 29.91 min against An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively. Tarelli et al. [31] reported that the knockdown time (KT50) values obtained for orange oil was 10.1 minutes against the housefly, Musca domestica. KT50 values of 14, 20 and 18 min against An. stephensi, Cx. quinquefasciatus and Ae. aegypti, respectively [32] were recorded for an essential oil isolated from Lantana camara. Eucalyptus oil in the present study showed the KT50 values of 33.38, 31.34 and 32.33 min against An. stephensi, Cx. quiquefasciatus and Ae. aegypti, respectively. This is in agreement with Tolaza et al. [33] who recorded KT50 value of 31.39 min against head lice for Eaculyptus oil. Citronella oil in the present study showed KT50 values of 33.76 and 32.70 min against An. stephensi and Cx. quinquefasciatus. This value was much lower than 45.02 min which was observed in the same oil against Ae. aegypti by Zaridah et al. [34].
In short, through the larvicidal and knockdown effects, our study clearly demonstrated that mentha oil, clove oil and orange oil had high potency to control three species of vector mosquitoes. These essential oils can be used to develop herbal formulations with larvicidal and knockdown effects against the vector mosquitoes.
5. ACKNOWLEDGEMENTS
We are grateful to Entomology Research Institute for financial assistance.
![]()
![]()
REFERENCES
- Rahuman, A.A., Bagavan, A., Kamaraj, C., Saravanan, E., Zahir, A.A. and Elango, G. (2009) Efficacy of the larvicidal botanical extracts against Culex quinquefasciatus Say (Dipetera: Culicidae). Parasitology Research, 104, 1365- 1372. doi:10.1007/s00436-009-1337-9
- Fradin, M.S. (1998) Mosquitoes and mosquito repellents: A clinician’s guide. Annals of Internal Medicine, 128, 931-940.
- Jaswanth, A., Ramanathan, P. and Ruckmani, K. (2002) Evaluation of mosquitocidal activity of Annona squamosa leaves against filarial vector mosquito, Culex quinquefasciatus. Indian Journal of Experimental Biology, 40, 363-365.
- Borah, R., Kalita, M.C., Kar, A. and Talukdar, A.K. (2010) Larvicidal efficacy of Toddalia asiatica (Linn.) Lam against two mosquito vector Aedes aegypti and Culex quinquefasciatus. African Journal of Biotechnology, 9, 2527-2530.
- Karunamoorthy, K., Ilango, G. and Murugan, K. (2010) Laboratory evaluation of traditionally used plant-based insect repellents against the malaria vector Anopheles arabiensis Patton. Parasitology Research, 106, 1217-1223. doi:10.1007/s00436-010-1797-y
- WHO (2005) Guidelines for laboratory and field testing of mosquito larvicides.
- WHO (2007) 10 facts on malaria. www.who.inf/factfiles/malaria/en/index.html
- Bowers, W.S., Sener, B., Evans, H., Bingal, F. and Erdogon, I. (1995) Activity of Turkish medicinal plants against mosquitoes Aedes aegypti and Anopheles gambiae. Insect Science and its Application, 16, 339-342.
- Gbolade, A.A., Dyedele, A.D., Sosan, M.B., Adewayin, F.B. and Soyela, O.I. (2000) Mosquito repellent activities of essential oils from two Nigerian Ocimum species. Journal of Tropical Medicinal Plants, 1, 146-148.
- Adebayo, T.A., Gbolade, A.A. and Olaifa, J.J. (1999) Comparative study of toxicity of essential oils to larvae of three mosquito species. Nigerian Journal of Natural Products and Medicine, 3, 74-76.
- Senthilkumar, A., Kannathasan, K. and Venkatesalu, V. (2008) Chemical constituents and larvicidal property of the essential oil of Blumea mollis (D. Don) Merr. against Culex quinquefasciatus. Parasitology Research, 103, 959- 962. doi:10.1007/s00436-008-1085-2
- Pushpanathan, T., Jebanesan, A. and Govindarajan, M. (2008) The essential oil of Zingiber officinalis Linn (Zingiberaceae) as a mosquito larvicidal and repellent agent against the filarial vector Culex quinquefasciatus say (Diptera: Culicidae). Parasitology Research, 102, 1289- 1291. doi:10.1007/s00436-008-0907-6
- Noosidum, A., Prabaripai, A., Chareonviriyaphap, T. and Chandrapatya, A. (2008) Excito-repellency properties of essential oils from Melaleuca leucadendron L., Litsea cubeba (Lour.) persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) mosquitoes. Journal of Vector Ecology, 33, 305. doi:10.3376/1081-1710-33.2.305
- Kweka, E.J., Mosha, F., Lowassa, A., Mahande, A.M., Kitau, J., Matowo, J., Mahande, M.J., Massenga, C.P., Tenu, F., Feston, E., Lyatuu, E.E., Mboya, M.A., Mndeme, R., Chuwa, G. and Temu, E.A. (2008) Ethnobotanical study of some mosquito repellent plants in North Eastern Tanzania. Malaria Journal, 7, 152. doi:10.1186/1475-2875-7-152
- Tiwary, M., Naik, S.N., Tewary, D.K., Mittal P.K. and Yadav, S. (2007) Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. Journal of Vector Borne Diseases, 44, 198-204.
- Singh, N.P., Kumari V. and Chauhan, D. (2003) Mosquito larvicidal properties of the leaf extract of a herbaceous plant, Ocimum canum (Family: Labitae). Journal of Communicable Diseases, 35, 43-45.
- Cheng, S.S., Liu, J.Y., Tsai, K.H., Chen, W.J. and Chang, S.T. (2004) Chemical composition and mosquito larvicidal activity of essential oils form leaves of different Cinnamonum osmophloem provenances. Journal of Agricultural and Food Chemistry, 52, 4395-4400. doi:10.1021/jf0497152
- WHO (1987) Report of an informal consultation of the detection, isolation, identification and ecology of biocontrol of disease vectors. WHO report—TDR/BCV/IC-CE/87.3.
- Verma, K.V.S. and Rahman, S.J. (1986) Development of knockdown resistance (KDR) against fenvelarate in a DDT—resistant strains of Anopheles stephensi. Current Science, 55, 914-916.
- Reuben, R. (1987) Feeding and reproduction in mosquitoes. Proceedings of the Indian Academy of Sciences, 96, 275-280.
- WHO (1996) Report of the WHO informal consultation on the evaluation and testing of insecticides, CTD/WHOPES/ IC/96.1. Control of Tropical Diseases Division, World Health Organization, Geneva.
- Abbott, W.S. (1925) A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265-267.
- Amer, A. and Melholrn, H. (2006) Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitology Research, 99, 478-490. doi:10.1007/s00436-006-0184-1
- Michaelakis, A., Papachristos, D., Kimbaris, A., Koliopoulos, G., Giatropoulos, A. and Polissiou, M.G. (2009) Citrus essential oils and four enantiomeric pinenes against Culex pipiens (Diptera: Culicidae). Parasitology Research, 105, 769-773. doi:10.1007/s00436-009-1452-7
- Sutthanont, N., Choochote, W., Tuetun, B., Junkum, A., Jitpakdi, A., Chaithong, U., Riyong, D. and Pitasawat, B. (2010) Chemical composition and larvicidal activity of edible plant-derived essential oils against the pyrethroidsusceptible and -resistant strains of Aedes aegypti (Diptera: Culicidae). Journal of Vector Ecology, 35, 106-115. doi:10.1111/j.1948-7134.2010.00066.x
- Zhu, J., Zeng, X., Yan, T.L., Qian, K., Yuhua, H., Suqin, X., Brad, T., Gretchen, S., Joel, C., Wayne, R. and Aijun Z. (2006) Adult repellency and larvicidal activity of five plant essential oils against mosquitoes. Journal of the American Mosquito Control Association, 22, 515-522. doi:10.2987/8756-971X(2006)22[515:ARALAO]2.0.CO;2
- Sukumar, K., Perich, M.J. and Boobar, L.R. (1991) Botanical derivatives in mosquito control: A review. Journal of the American Mosquito Control Association, 7, 210- 237.
- Carvalho, A.F.U., Melo, V.M.M., Craveiro, A.A., Machado, M.I.L.M., Bantim, M.B. and Rabelo, E.F. (2003) Larvicidal activity of the essential oil from Lippia sidoides Cham. against Aedes aegypti Linn. Memórias do Instituto Oswaldo Cruz, 98, 569-571. doi:10.1590/S0074-02762003000400027
- Traboulsi, A.F., El-Haj, S., Tueni, M., Taoubi, K., Nader, N.B. and Mrad, A. (2005) Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Management Science, 61, 597-560. doi:10.1002/ps.1017
- Koliopoulos, G., Pitarokili, D., Kioulos, E., Michaelakis, A. and Tzakou, O. (2010) Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitology Research, 107, 327-335. doi:10.1007/s00436-010-1865-3
- Tarelli, G., Zerbam, E.N. and Raúl, A.A. (2009) Toxicity to vapor exposure and topical application of essential oils and monoterpenes on Musca domestica (Diptera: Muscidae). Journal of Economic Entomology, 102, 1383-1388. doi:10.1603/029.102.0367
- Dua, V.K., Pandey, A.C. and Dash, A.P. (2010) Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian Journal of Medical Research, 131, 434-439.
- Toloza, A.E., Lucia, A., Zerba, E., Masuh, H. and Picallo, M.I. (2010) Eucalyptus essential oil toxicity against permethrin-resistant. Parasitology Research, 106, 409-414. doi:10.1007/s00436-009-1676-6
- Zaridah, M.Z., Nor Azah, M.A. and Rohani, A. (2006) Mosquitocidal activities of malaysian plants. Journal of Tropical Forest Science, 18, 74-80.
NOTES
*Corresponding author.

