Journal of Biophysical Chemistry
Vol.2 No.3(2011), Article ID:6735,4 pages DOI:10.4236/jbpc.2011.23027
The role of extracellular calcium in the effect of a snake venom Lys49-phospholipase A2 on water transport across epithelial membranes
1Unidade Acadêmica de Saúde do Centro de Educação e Saúde, Universidade Federal de Campina Grande, Cuité, Brazil; *Corresponding Author: rennerleite@yahoo.com.br
2Departamento de Ciências Fisiológicas, Universidade Federal de São Carlos, São Carlos, Brazil
Received 2 February 2011; revised 18 March 2011; accepted 3 April 2011.
Keywords: Epithelial Tissue; Membrane Permeability; Myotoxin; Lys49-Phospholipase A2; Toad Bladder; Water Transport
ABSTRACT
ACLMT is a Lys49-phospholipase A2 myotoxin isolated from the venom of the Agkistrodon contortrix laticinctus snake. This study investigated the mechanisms involved in effect of ACLMT on membrane water permeability by examining the role of extracellular calcium and strontium in this effect. Water flow across the membrane was gravimetrically measured in bladder sac preparations. The decrease in extracellular calcium promoted a higher response of epithelium to ACLMT, suggesting that the extracellular calcium protects the membrane from the action of the toxin. No alteration in the effect of the toxin on water transport was observed when calcium was replaced by strontium, indicating that this effect is independent of its enzymatic activity. These findings may bring an important contribution towards the comprehension of the mechanisms involved in the effect of Lys49-phospholipase A2 myotoxins on water permeability of epithelial membranes, with implications for the understanding of renal toxicity.
1. INTRODUCTION
Skeletal muscle necrosis is a common complication caused by the bite of different species of venomous snakes. Local myonecrosis may induce permanent tissue loss, physical disability and limb amputation; while widespread systemic myotoxicity may lead to myoglobinuria and acute renal failure, which is a frequent cause of death in snakebite victims [1]. Some mechanisms have been suggested to explain renal damage caused by snake venoms, including a direct nephrotoxic effect, disseminated intravascular coagulation, and release of vasoactive substances [2-3]. Despite such findings, the mechanisms involved in the pathogenesis of renal alterations induced by snake venoms remain unclear.
Phospholipases A2 (PLA2s) are enzymes that catalyze the hydrolysis of the phospholipid sn-2 ester bond, liberating fatty acids and lysophospholipids. Venoms from snakes of the family Viperidae contain group IIA PLA2s. This group of PLA2 is subdivided into two types: Asp49-PLA2s, which have an aspartic acid residue at position 49 and show high catalytic activity; and Lys49- PLA2s, which have a lysine residue at position 49 and show low or no catalytic activity [1]. In spite of their low enzymatic activity, Lys49-PLA2s perform several pharmacological activities, such as nephrotoxicity, myotoxicity, neurotoxicity, cytotoxicity, among others [1-3].
A hypothetical mechanism of action has been proposed for Lys49-PLA2s. The toxin would bind to an unidentified site on the cell plasma membrane, penetrating the bilayer through hydrophobic interactions. This event would destabilize the membrane, leading to impaired permeability of ions and macromolecules. The most important consequence of the membrane disturbance would be the prominent increase in cytosolic calcium. This increase would, in turn, be responsible for the onset of a variety of destructive alterations that would ultimately account for cellular damage, such as cytoskeletal and mitochondrial damage, and activation of calcium-dependent proteases and endogenous phospholipases [4]. The increase in cytosolic calcium induced by Lys49-PLA2s in muscle cells has a biphasic pattern, with an initial rapid mobilization of calcium from intracellular stores, followed by a massive calcium influx from the extracellular medium [5]. This initial increase in cytosolic calcium implies the involvement of a signaling event, therefore suggesting the participation of a membrane acceptor in the mechanism of toxicity of Lys49-PLA2s [6]. Despite the effort of researchers, the action mechanism of Lys49-PLA2s has not been conclusively determined.
ACLMT is a Lys49-PLA2 myotoxin isolated from the venom of the snake Agkistrodon contortrix laticinctus [7]. Leite et al. [8] demonstrated that ACLMT increases water transport and decreases AVP-stimulated water transport across toad bladder epithelium. The effect of this toxin on water transport is partially mediated by the increased cytosolic calcium, and this increase is a result of the mobilization of calcium from internal stores, as well as of calcium influx across plasma membrane. On the other hand, the effect of ACLMT on AVP-stimulated water transport is mediated by both the increase in cytosolic calcium and the stimulation of prostaglandins synthesis. In an attempt to further understand the mechanism by which Lys49-PLA2s affect water permeability of epithelial membranes, the present study aimed to investigate the role of extracellular calcium in the effect of ACLMT on water transport.
2. MATERIAL AND METHODS
The water flow across the bladder epithelium was measured by the gravimetric technique described by Leite et al. [8]. All experiments were performed in bladder sacs isolated from Bufo marinus toads, which were kept in an animal room with plenty of food and water, at room temperature, in accordance with the Guide for Care and Use of Laboratory Animals. The bladders were quickly excised from the abdominal cavity of the animals and incubated in phosphate Ringer’s solution (110 mM NaCl, 3.5 mM KCl, 2.0 mM Na2HPO4, 1.8 mM CaCl2, 0.5 mM MgCl2 and 10 mM glucose), with total osmolality of 250 mOsm/l, and pH 7.4 at 23˚C. The bladders were separated in two different lobes (one serving as control and the other as experimental). They were mounted so that the mucosal surface was inside and the serosal one was outside. The bladder sacs were filled with 3 ml of diluted phosphate Ringer's solution (50 mOsm/l) and immersed in a bathing with 40 ml of phosphate Ringer’s solution (250 mOsm/l). The bathing solution was fully aerated with compressed air and continuously stirred. After a 60 min equilibration period, the water flow was gravimetrically measured by weighing the bladder sacs before and after a 30 min period of incubation in the presence of the ACLMT. The difference in weight of the bladder sacs represented the amount of water that was transported. The variation in weight (mg) was expressed as variations of volume (ml) and presented in terms of mean ± standard error. Student’s t-test for paired and unpaired samples was used to verify the level of significance (p). Three experiments were conducted, each one using bathing solution at a particular concentration of calcium, namely 1.8 mM, 0.9 mM and 0.45 mM. These concentrations are greater than the minimum needed for tissue integrity [9]. To investigate whether the effect of ACLMT on water transport is dependent on its enzymatic activity, calcium was quantitatively replaced with strontium. This manipulation has been used as a means to investigate the effect of enzymatic activity on toxicity of Lys49-PLA2s [10].
3. RESULTS AND DISCUSSION
Table 1 shows that the effect of ACLMT significantly increased in the presence of decreased extracellular calcium. At 1.8 mM calcium, ACLMT increased basal water flow by 50% in relation to control bladder sacs. When calcium was reduced to 0.90 mM and 0.45 mM, the toxin increased water transport by 66% and 58%, respectively. Significant differences were observed when comparing concentration of 1.8 mM Ca2+ with both 0.9 mM Ca2+ and 0.45 mM (p < 0.01; unpaired t test). However, the comparison between 0.9 mM Ca2+ and 0.45 mM Ca2+ (unpaired t-test) showed that difference was not statistically significant. Thus, the decrease in extracellular calcium seems to promote a higher response of the epithelium to ACLMT, suggesting that extracellular calcium may protect the membrane from the action of the toxin. These results are in agreement with Villalobos et al. [11] and Heluany et al. [12]. The former demonstrated that, in skeletal muscle cell culture, the decrease in extracellular calcium increases the cytotoxicity of Lys49-PLA2 of the snake Bothrops asper; the latter showed that, in skeletal muscles, the increased extracellular calcium antagonizes contractures, twitch depression and membrane despolarization induced by Lys49-PLA2 of the snake Bothrops jararacussu. Indeed, calcium plays a stabilizing role in plasma membrane in a number of cell types, thus protecting the membrane from the action of a variety of cytolytic agents [13]. Table 2 shows that the substitution of calcium for strontium did not alter the effect of ACLMT on water transport, suggesting that the intracellular pool of calcium has a prominent role in this effect, as well as that the extracellular calcium is not required for the initial response of the epithelium to the toxin. Moreover, the effect of ACLMT is shown to be independent of the enzymatic activity (Table 2). These findings are in accordance with Rodrigues-Simioni et al. [14], who demonstrated that the replacement of calcium by strontium decreased the enzymatic activity of Lys49-PLA2 of the snake Bothrops jararacussu, but had no effect on its pharmacological
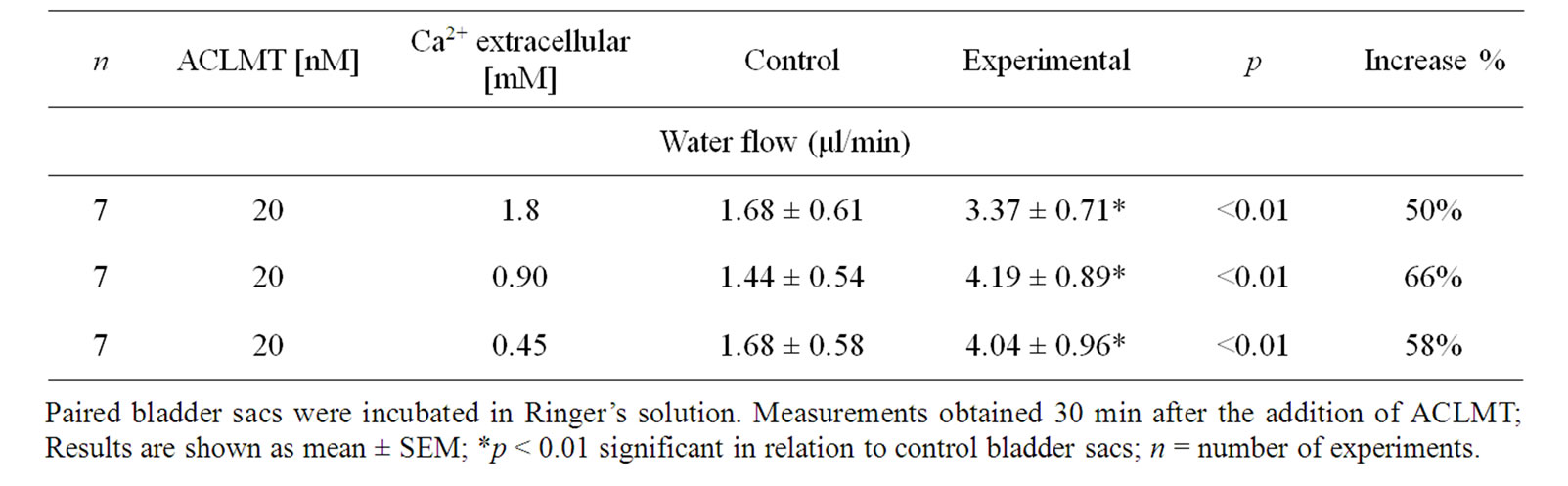
Table 1. The effect of decreasing the concentration of extracellular calcium on water flow induced by ACLMT.
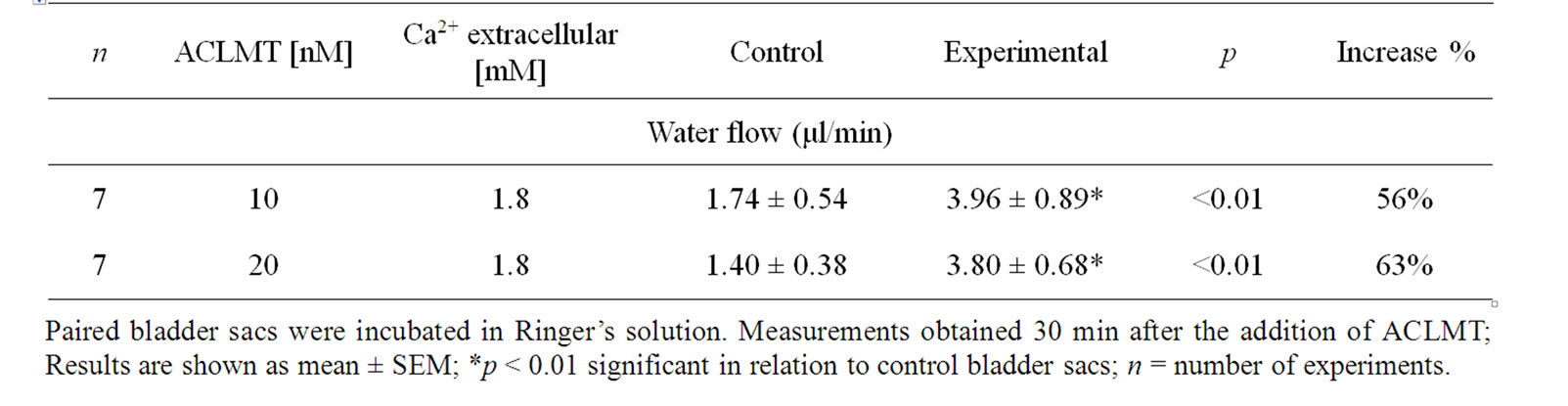
Table 2. The effect of replacing calcium with strontium on water flow induced by ACLMT.
activities. Interestingly, Barbosa et al. [15] demonstrated that the myotoxins isolated from Bothrops jararacussu venom, BthTx-I (Lys49-PLA2) and BthTx-II (Asp49- PLA2), promote changes in renal physiology, such as increases in perfused pression (PP), resistence vascular renal (RVR), urinary flow (UF) and rate filtration glomerular (GFR), as well as decreases in sodium, potassium and chloride tubular transports [15]. Indomethacin inhibited renal effects induced by BthTx-I and partially reduced the effects induced by BthTx-II, pointing to the participation of the eicosanoids in renal damage caused by these myotoxins. Barbosa et al. [3] also showed that BmTx-I, a Lys49-PLA2 myotoxin of the snake Bothrops moojeni, promotes renal alterations similar to those promoted by BthTx-I. Tezosentan inhibited renal alterations induced by BmTx-I in UF, GFR and sodium, potassium and chloride tubular transports, suggesting a role for endotelin in renal pathophysiologic changes induced by this toxin [3]. These findings are particularly relevant to the present study, since toad bladder is a structure whose transport characteristics and response to hormones and drugs resemble those from the mammalian distal nephron [16-17]. Thus, it is possible to suggest that, in addition to the previously well-described effects of Lys49-PLA2s myotoxins on renal hemodynamics [3-15], the direct epithelial actions of these myotoxins may also contribute to their diuretic effect. However, research focusing on the effect of Lys49- PLA2s myotoxins on water transport across membranes of mammalian renal epithelial cells would be necessary to confirm this hypothesis.
The findings of this study may contribute to shed light on the mechanisms involved in the effect of Lys49- PLA2s myotoxins on water transport across epithelial membranes, with implications for the understanding of renal toxicity. Further studies in amphibian epithelium would improve the understanding of the mechanisms involved in the pathogenesis of renal alterations induced by this family of toxins.
4. ACKNOWLEDGEMENTS
This research was supported by CAPES (Brazil). The authors are grateful to Solange Maimoni Gonçalves for the English review.
REFERENCES
- Lomonte, B., Angulo, Y. and Calderón, L. (2002) An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon, 42, 885-901. doi:10.1016/j.toxicon.2003.11.008
- Boer-Lima, P.A., Gontijo, J.A.R. and Cruz-Hofling, M.A. (1999) Histologic and functional renal alterations caused by Bothrops moojeni snake venom in rats. The American Journal of Tropical Medicine and Hygiene, 61, 698-706.
- Barbosa, P.S.F., Martins, A.M.C., Alves, R.S., Amora, D.N., Martins, R.D., Toyama, M.H., Havt, A., Nascimento, N.R.F., Rocha, V.L.C., Menezes, D.B., Fonteles, M.C. and Monteiro, H.S.A. (2006) The role of indomethacin and tezosentan on renal effects induced by Bothrops moojeni Lys49 myotoxin I. Toxicon, 47, 831- 837. doi:10.1016/j.toxicon.2006.01.012
- Montecucco, C., Gutiérrez, J.M. and Lomonte, B. (2008) Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cellular and Molecular Life Sciences, 65, 2897-2912. doi:10.1007/s00018-008-8113-3
- Cintra-Francischinelli, M., Pizzo, P., Rodrigues-Simioni, L., Ponce-Soto, L.A., Rossetto, O., Lomonte, B., Gutiérrez, J.M., Pozzan, T. and Montecucco, C. (2009) Calcium imaging of muscle cells treated with snake myotoxins reveals toxin synergism and presence of acceptors. Cellular and Molecular Life Sciences, 66, 1718-1728. doi:10.1007/s00018-009-9053-2
- Cintra-Francischinelli, M., Pizzo, P., Angulo, Y., Gutiérrez, J. M., Montecucco, C. and Lomonte, B. (2010) The C-terminal region of a Lys49 myotoxin mediates Ca2+ influx in C2C12 myotubes. Toxicon, 55, 590-596. doi:10.1016/j.toxicon.2009.10.013
- Johnson, E.K. and Ownby, C.L. (1993) Isolation of a myotoxin from the venom of Agkistrodon contortrix laticinctus (broad-banded copperhead) and pathogenesis of myonecrosis induced by it in mice. Toxicon, 31, 243-255. doi:10.1016/0041-0101(93)90143-7
- Leite, R.S., Franco, W., Ownby, C.L. and Selistre-deAraujo, H.S. (2004) Effects of ACL myotoxin, a Lys49 phospholipase A2 from Agkistrodon contortrix laticinctus snake venom, on water transport in the isolated toad urinary bladder. Toxicon, 43, 77-83. doi:10.1016/j.toxicon.2003.10.024
- Hardy, M.A. and Dibona, D.R. (1982) Extracellular Ca2+ and the effect of antidiuretic hormone on the water permeability of the toad urinary bladder: An example of flow induced alteration of flow. Journal of Membrane Biology, 67, 27-44. doi:10.1007/BF01868645
- Fletcher, J. E. and Jiang, M. S. (1998) Lys49 phospholipase A2 myotoxins lyse cell cultures by two distinct mechanisms. Toxicon, 36, 1549-1555. doi:10.1016/S0041-0101(98)00147-0
- Villalobos, J.C., Mora, R., Lomonte, B., Gutiérrez, J.M. and Angulo, Y. (2007) Cytotoxicity induced in myotubes by a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops asper: Evidence of rapid plasma membrane damage and a dual role for extracellular calcium. Toxicology in Vitro, 21, 1382-1389. doi:10.1016/j.tiv.2007.04.010
- Heluany, N.F., Homsi-Brandeburgo, M.L., Giglio, J.R., Prado-Fransceschi, J. and Rodrigues-Simioni, L. (1992) Effects induced by bothropstoxin, a component from Bothrops jararacussu snake venom, on mouse and chick muscle preparations. Toxicon, 30, 1203-1207. doi:10.1016/0041-0101(92)90436-9
- Bashford, C.L., Alder, G.M., Graham, J.M., Menestrina, G. and Pasternak, C. (1988) Ion modulation of membrane permeability: Effect of cations on intact cells and on cells and phospholipid bilayers treated with pore-forming agents. Journal Membrane Biological, 103, 79-94. doi:10.1007/BF01871934
- Rodrigues-Simioni, L., Prado-Franceschi, J., Cintra, A.C.O., Giglio, J.R., Jiang, M.S. and Fletcher, J.E. (1995) No role for enzymatic activity or dantrolene-sensitive Ca2+ stores in the muscular effects of bothropstoxin, a Lys49 phopholipase A2 myotoxin. Toxicon, 33, 1479-1489. doi:10.1016/0041-0101(95)00089-5
- Barbosa, P.S.F., Martins, A.M.C., Havt, A., Toyama, D.O., Evangelista, J.S.A.M., Ferreira, D.P.P., Joazeiro, P.P., Beriam, L.O.S., Toyama, M.H., Fonteles, M.C. and Monteiro, H.S.A. (2005) Renal and antibacterial effects induced by myotoxin I and II isolated from Bothrops jararacussu venom. Toxicon, 46, 376-386. doi:10.1016/j.toxicon.2005.04.024
- Hays, R. and Leaf, A. (1962) Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. Journal of the American Society of Nephrology, 45, 905-919.
- Leaf, A. (1982) From toad bladder to kidney. American Journal of Physiology, 242, 103-111.

