Journal of Environmental Protection
Vol. 3 No. 3 (2012) , Article ID: 17942 , 8 pages DOI:10.4236/jep.2012.33035
Dissolution of Humic Substances from Highly Humic Volcanic Ash Soil as Affected by Anionic Surfactant, Electrolyte Concentration and pH
![]()
1Graduate School of Environmental Science, Okayama University, Okayama, Japan; 2Laboratory of Soil Conservation, Research Faculty of Agriculture, Hokkaido University, Sapporo, Japan.
Email: *farookahmed12@yahoo.com, ishi-m@env.agr.hokudai.ac.jp, akae@cc.okayama-u.ac.jp
Received January 5th, 2012; revised February 8th, 2012; accepted March 1st, 2012
Keywords: surfactant; humic substances; electrolyte; volcanic Ash Aoil; pH
ABSTRACT
Dissolved humic substances separated from soils play an important role in the material cycle because they adsorb nutrients and contaminants and move with water. This study was conducted to investigate the influence of anionic surfactant, pH and electrolyte concentration on the dissolution of humic substances from a highly humic volcanic ash soil. The soil used in the experiment has a negative charge and the anionic surfactant, sodium dodecylbenzene sulfonate, has also the negative charge. The absorbance of supernatant of soil solution at different surfactant concentration and different electrolyte concentration (0.001 M, 0.01 M, 0.1 M & 0.5 M) of NaCl at pH 4.5 and 6.5 was measured at the wave-length of 400 nm; this corresponds to the relative concentration of dissolved humic substances. The surfactant adsorption and its equilibrium concentration under the same solution condition of the absorbance measurement were also measured in order to get their effect on dissolved humic substances. The zeta potential of soil particles was measured in order to evaluate the influence of electrostatic potential on dissolution of humic substances. The concentration of dissolved humic substances increased at higher surfactant concentration and adsorption, at higher pH and at lower electrolyte concentration, because the electrostatic repulsive force between the soil particles and the dissolving humic substances became larger. Therefore, surfactant concentration and adsorption, pH and electrolyte concentration are important when considering the fate of humic substances in soils.
1. Introduction
Dissolved humic substances separated from soils play an important role in the material cycle because they adsorb nutrients and contaminants [1]. Many studies have shown that surfactant concentration enhances the dissolution/ solubilisation of humic substances present in the soil [2,3]. In general, the higher the organic content of a soil the greater the degree of adsorption of hydrophobic materials and higher the surfactant concentration the higher ability to dissolve the humic substances [4].
From the above study it is clear that surfactants influence the fate of humic substances in soils. Among the anionic surfactant, Sodium dodecylbenzene sulfonate (SDBS) is widely used in domestic and industrial purposes [5-7] and it is the most common pollutant found in almost all environmental compartments [8-14]. Organic matter has been observed responsible for anionic surfactant adsorption in soil [15-17]. However, the combined study of humic substances dissolution from soil as affected by pH, electrolye concentration and different surfactant concentration is not sufficient.
In this study, influences of an anionic surfactant, pH and electrolyte concentration on dissolution of humic substances from a highly humic volcanic ash soil were investigated. For evaluating the chemical characteristics of dissolved organic matter charge condition of the soil must be considered [18]. Therefore, the zeta potential of the soil particles was measured in this research to investigate the charge characteristics of the soil. Volcanic ash soils which accumulate huge amount of organic matter in surface layer are very common in Japan and areas where volcano exists in the world. Therefore, we chose the volcanic ash soil as our experimental material.
2. Materials and Methods
The dissolution of humic substances from volcanic ash soil in presence of surfactant at different electrolyte and pH condition was systematically analyzed.
2.1. Soil
A volcanic ash soil (Highly humic, non-allophanic Andisol) of surface layer from Daisen grazing ground, Tottori Prefecture, Japan was used in this experiment. Some important characteristics of the soil are given in Table 1. The field moist soil was sieved with 2 mm-sieve.
2.2. Surfactant
Anionic surfactants, SDBS, with branched and linear carbon chains having the same chemical composition (C12H25C6H4SO3Na) and molecular weight of 348.48 g·mol–1 were purchased from Tokyo Kasei Kogyo Co. with purity of about 95% and were used without further purification.
2.3. Adsorption Experiment
A batch experiment was conducted with SDBS (both branched and linear carbon chain) to get the dodecylbenzene sulfonate (DBS) adsorption amount of the soil. The experiment was conducted at room temperature (25˚C ± 1˚C). The soil (2.5 g dry weight basis) was taken in 50 cm3 centrifuge tube. It was equilibrated with a different electrolyte concentration of NaCl solution (0.001 M, 0.01 M, 0.1 M and 0.5 M NaCl) and the solution pH was adjusted to 4.5 and pH 6.5 by adding diluted HCl and NaOH, respectively. After the soil solution was centrifuged, the supernatant was discarded and 25 ml of SDBS solutions of different concentrations (0.001, 0.005, 0.01, 0.05, 0.10, 0.70, 1.0, 2.0, 3.0, 5.0 and 10.0 mmol·L–1) at different electrolyte concentration of NaCl were added to the soil and remaining solution in the tube. The tube was shaken well for 24 hours. After the elapsed time the soil solutions were centrifuged for 10 min at 8000 rpm. The supernatants of the tubes were collected and the surfactant concentrations were measured by anionic surfactant selective electrode [19] that was assembled by the authors. The concentration cell was constructed as follows:
Ag/AgCl electrode | Agar bridge | reference solution (C0) | functional membrane | test solution (C1) | Agar bridge | Ag/AgCl electrodewhere, C0 and C1 are the concentrations of the surfactant in the reference solution and that in the test solution. The electromotive force (EMF) was measured using a digital voltmeter with high input impedance at 25˚C ± 1˚C. The EMF (E) can be expressed with the following equation:
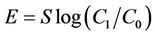 (1)
(1)
where S is the experimental slope. The theoretical value of S is 59.2 mV at 25˚C (Nernstian slope). The measured values for the standard solutions ranged from 54.35 mV to 59.00 mV. The electrode was carefully washed before each measurement and continuously checked with standard solutions in order to get proper result.
The adsorbed amount of surfactant in the soil was obtained using the Equation (2)where V (L) is the remaining water volume in the soil after discarding the supernatant and before adding the SDBS solution.
2.4. Measurement of Absorbance of Supernatant
The absorbance of supernatant that was sampled after mixing SDBS solution and the soil in the adsorption experiment was measured by using spectrophotometer (HITACHI U-1100). A one-hundredth volume of 10 W/V% NaOH was added to the collected supernatant and the absorbance of the mixed solution at 400 nm was measured. The solution condition in the absorbance measurement was the same as that of the adsorption experiment. The absorbance corresponds to the relative concentration of dissolved humic substances.
2.5. Zeta Potential Measurement
Zeta potential of the soil particles was measured under the same solution condition of the adsorption experiment. In order to get a good measurement result, the ratio of

Table 1. Physical and chemical characteristics of the soil used in the experiment.
 (2)
(2)
soil to water in weight was fixed at 1:20000. Zeta potential of the soil particles was obtained by measuring the electrophoretic mobility of the soil particles (Model 502, Nihon Rufuto).
3. Results and Discussion
3.1. Absorbance of Supernatant and DBS Concentration/Adsorption
The relationships between the measured absorbance of supernatant which corresponds to the relative concentration of dissolved humic substances and the equilibrium surfactant concentration for the DBS-adsorbed soil at different pHs are shown in Figures 1(a) to 4(a).
The relationships between the absorbance and DBS adsorption at different pHs are also shown in Figures 1(b) to 4(b). The result shows that when the pH is lower (pH 4.5), the absorbance becomes lower. When the pH is higher (pH 6.5), the absorbance becomes higher. The difference between the absorbance at pH 4.5 and that at pH 6.5 are very clear for each electrolyte concentration, especially those at higher electrolyte concentration. As the negative charge of the soil and that of the dissolving humic substances increases with an increase of pH due to the pH dependent charge, those increases is likely to have affected the dissolution of the humic substances from the
 (a)
(a)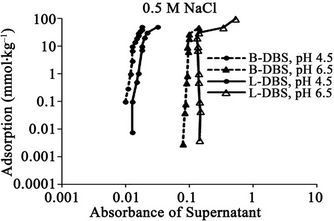 (b)
(b)
Figure 1. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at 0.5 M NaCl. The absorbance corresponds to relative concentration of dissolved humic substances. B-DBS indicates DBS with branched carbon chain and L-DBS with linear carbon chain.
 (a)
(a)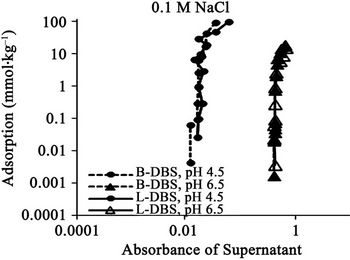 (b)
(b)
Figure 2. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at 0.1 M NaCl. The absorbance corresponds to relative concentration of dissolved humic substances. B-DBS indicates DBS with branched carbon chain and L-DBS with linear carbon chain.
soil. Increase of the negative charge density on the surface of the humic substances promotes dissolution into water, because the humic substances become more hydrophilic. Increases of the negative charge densities on the soil surface and the humic substances generate more electrostatic repulsive force between the soil and the humic substances. Therefore, the dissolution of the humic substances are promoted at higher pH.
Figures 1 to 4 shows the influence of the surfactant on dissolution of the humic substances. When the surfactant concentration or surfactant adsorption increases, the absorbance increases, except those at 0.001 M NaCl at pH 6.5. The absorbance at 0.001 M NaCl at pH 6.5 takes constant value at all DBS concentration or adsorption, because the measured absorbance value reaches the maximum for the apparatus. The concentration of dissolved humic substances increases with increase of the surfactant concentration or surfactant adsorption. It has been reported that dissolved organic substances increases as surfactant concentration increases [3]. It has also been reported that above the critical micelle concentration (CMC) the surfactant increases the dissolved humic substances [4]. In our experiments, the absorbances at 0.1 M and 0.5 M NaCl showed increase near CMC; the CMC for SDBS with branched carbon chain were 0.33 mmol·L–1 at 0.1 M NaCl and 0.11 mmol·L–1 at 0.5 M NaCl, and those with linear carbon chain were 0.09 mmol·L–1 at 0.1 M NaCl and 0.04 mmol·L–1 at 0.5 M NaCl. However, the absorbances at 0.001 M NaCl at pH 4.5 and at 0.01 M NaCl showed increase before CMC; the CMC for SDBS with branched carbon chain were 2.8 mmol·L–1 at 0.001 M NaCl and 1.8 mmol·L–1 at 0.01 M NaCl, and those with linear carbon chain were 1.3 mmol·L–1 at 0.001 M NaCl and 0.21 mmol·L–1 at 0.01 M NaCl. Probably, that is because the electrostatic repulsive potential near the soil particles became larger under low electrolyte concentration.
As a surfactant has an ability to dissolve the hydrophobic substances, it adsorbs on the hydrophobic part of the humic substances and dissolves some of them. Moreover, as DBS is an anionic surfactant, DBS adsorption in the soil increases the negative charge of the soil. The increase of the negative charge also promotes the dissolution of the humic substances by the electrostatic repulsive force.
 (a)
(a) (b)
(b)
Figure 3. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at 0.01 M NaCl. The absorbance corresponds to relative concentration of dissolved humic substances. B-DBS indicates DBS with branched carbon chain and L-DBS with linear carbon chain.
 (a)
(a)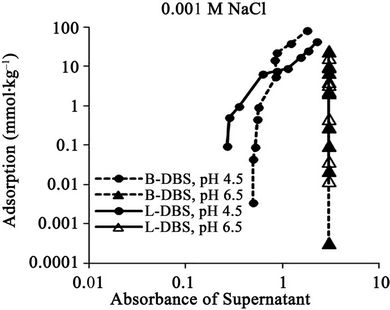 (b)
(b)
Figure 4. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at 0.001 M NaCl. The absorbance corresponds to relative concentration of dissolved humic substances. B-DBS indicates DBS with branched carbon chain and L-DBS with linear carbon chain.
The relationships between the absorbances and the DBS concentrations at different electrolyte concentrations are shown in Figures 5(a) to 8(a). The relationships between the absorbances and the DBS adsorptions at different electrolyte concentrations are also shown in Figures 5(b) to 8(b). The influence of the electrolyte concentration on the absorbance is clear in Figures 5-8.
When the electrolyte concentration decreases, the absorbance increases. That is, when the electrolyte concentration increases, the concentration of dissolved humic substances decreases. At higher electrolyte concentration, the electric fields near the surface of soil and humic substances are shielded by the electrolyte. Then, the humic substances are flocculative and remain in the soil. As the soil charge is shielded, repulsive electrostatic force between the soil and the humic substances is small. Therefore, the concentration of dissolved humic substances is low at higher electrolyte concentration.
The different influences of surfactant on absorbance are observed among under different electrolyte concentrations as shown in Figures 5 to 8. The increase of absorbance with increase of surfactant concentration or surfactant adsorption is larger at lower pH (pH 4.5). As the charge density of the soil and the humic substances are smaller at pH 4.5 because of the effect of pH dependent charge, addition of surfactant affects much stronger because the surfactant adsorption increases the charge density. Especially, the influence of surfactant is most significant at 0.01 M NaCl at pH 4.5, because the condition is just on the border of dissolution of the humic substances. On the other hand, the influence of surfactant on absorbance is smaller at higher pH (pH 6.5). As the charge density of the soil and humic substances are larger, increase of surfactant charge after the adsorption does not affect much. The absorbance at pH 6.5 shows almost constant value except those at 0.5 M NaCl at larger than 20 mmol·kg–1 DBS adsorption.
The results for DBS with branched carbon chain are compared with those with linear carbon chain in Figures 1 to 4. The trend is almost same. The clear difference is not observed. The absorbance for that with linear chain is slightly larger at 0.5 M NaCl.
 (a)
(a) (b)
(b)
Figure 5. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at pH 4.5 for DBS with branched carbon chain. The absorbance corresponds to relative concentration of dissolved humic substances.
 (a)
(a)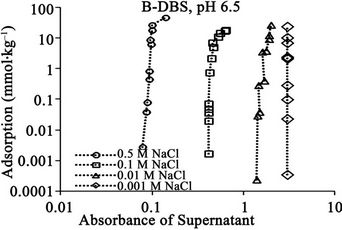 (b)
(b)
Figure 6. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at pH 6.5 for DBS with branched carbon chain. The absorbance corresponds to relative concentration of dissolved humic substances.
 (a)
(a)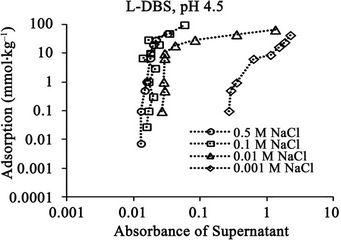 (b)
(b)
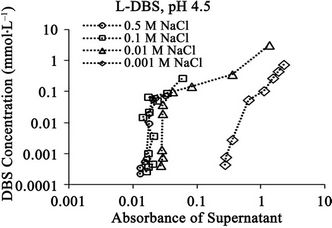
Figure 7. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at pH 4.5 for DBS with linear carbon chain. The absorbance corresponds to relative concentration of dissolved humic substances.
 (a)
(a) (b)
(b)
Figure 8. Relationships between absorbance of supernatant at 400 nm and (a) DBS concentration of supernatant; (b) Adsorption of DBS at pH 6.5 for DBS with linear carbon chain. The absorbance corresponds to relative concentration of dissolved humic substances.
3.2. Zeta Potential and Absorbance
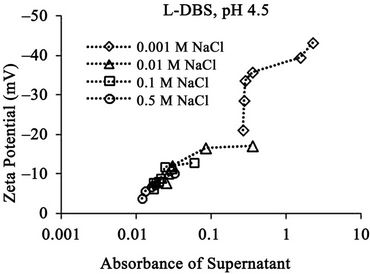
The relationships between the absorbance of supernatant and the zeta potential of the soil particles in the suspension at different pH are shown in Figure 9. The result shows the general trend that the absorbance increases with the increase of zeta potential for each condition except those at 0.001 M at pH 6.5. This result indicates that the zeta potential relates to the concentration of dissolved humic substances. The absorbance increases when DBS adsorption increases as mentioned in the above section. The DBS adsorption increases the negative charge amount of the soil particle. Thus, the zeta potential of the soil particles increases. Therefore, the zeta potential increases when the absorbance increases. The absorbance takes the maximum constant value at 0.001 M NaCl at pH 6.5 although the zeta potential increases, because the absorbance value reaches the maximum limit value in the measurement as mentioned in the above section.
The absorbance is larger at higher pH (pH 6.5) than that at lower pH (pH 4.5) as mentioned in the above section. The zeta potential is comparatively larger at higher pH, although the difference is not so larger than that for
 (a)
(a) (b)
(b)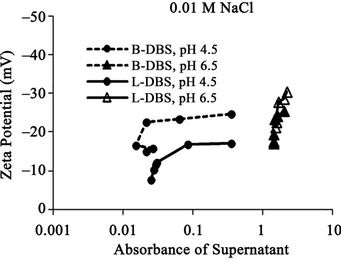 (c)
(c) (d)
(d)
Figure 9. Relationships between absorbance of supernatant and zeta potential of the soil particles in suspension at electrolyte concentration of (a) 0.5 M NaCl and (b) 0.1 M NaCl, (c) 0.01 M NaCl and (d) 0.001 M NaCl for both branched and linear DBS at pH 4.5 and 6.5. The absorbance corresponds to the relative concentration of dissolved humic substances. B-DBS indicates DBS with branched carbon chain and L-DBS with linear carbon chain.
the absorbance. This result shows that the zeta potential is not directly related to the concentration of dissolved humic substances at different pH, although there is a trend of correlation. This weak relation probably comes from the difference of soil surface condition at different pH. The differences between that with branched carbon chain and that with linear carbon chain are not large. They show similar trend as a whole.
The relationships between the absorbance of supernatant and the zeta potential at different electrolyte concentration are shown in Figure 10 for SDBS with branched carbon chain and in Figure 11 for SDBS with linear carbon chain.
The zeta potential increases with the increase of absorbance at each pH. Both the zeta potential and the absorbance increase with the decrease of electrolyte concentration and the increase of DBS adsorption. This result shows that under the same pH condition, the relationship between the zeta potential and the concentration of dissolved humic substances correlate well. Because
 (a)
(a)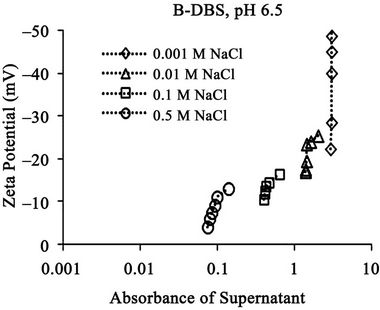 (b)
(b)
Figure 10. Relationships between absorbance of supernatant and Zeta Potential (a) at pH 4.5 and (b) pH 6.5 for branched DBS at different electrolyte concentration. The absorbance corresponds to the relative concentration of dissolved humic substances.
 (a)
(a) (b)
(b)
Figure 11. Relationships between absorbance of Supernatant and Zeta Potential (a) at pH 4.5 and (b) pH 6.5 for linear DBS at different electrolyte concentration. The absorbance corresponds to the relative concentration of dissolved humic substances.
the negative increase of zeta potential increases the electrostatic repulsive force between the soil particles and the humic substances. Therefore, some part of the humic substances dissolves to the solution and the absorbance increases when the zeta potential negatively increases. The decrease of the absolute value of zeta potential with the increase of electrolyte concentration clearly shows the shielding effect of electric field of the soil particles by electrolytes.
4. Conclusions
In this research the relative concentration of dissolved humic substances were measured as influenced by SDBS concentration, pH and electrolyte concentration for the highly humic volcanic ash soil. The humic substances in the soil dissolves more into the solutions at higher surfactant concentration, higher pH, and lower electrolyte concentration due to the increase of electrostatic repulsive force between the soil particles and the dissolving humic substances. Those relations were well evaluated by measuring the zeta potential of the soil particles.
As dissolved humic substances adsorb nutrients and contaminants and flow into the water environment, their fate in the environment is important when considering material cycle and environmental protection. Because the dissolution of humic substances is strongly influenced by surfactant concentration, pH and electrolyte concentration, the soil condition must be carefully observed for the good environmental management.
5. Acknowledgements
We thank Prof. K. Shirahama (Saga Univ.) for his instructions regarding the preparation of surfactant-selective membrane and Du Pont for supplying Elvaroy 742. This research was supported by Grants-in-Aid for Scientific Research (No. 22380130) from the Japan Society for the Promotion of Science.
REFERENCES
- J. Buffle, “Complexation Reactions in Aquatic Systems,” Ellis Horwood Ltd., Chichester, 1988. doi:10.1002/aheh.19890170220
- D. E. Kile and C. T. Chiou, “Water Solubility Enhancements of DDT and Trichlorobenzene by Some Surfactants below and above the Critical Micelle Concentration,” Environmental Science & Technology, Vol. 23, 1989, pp. 832-838. doi:10.1021/es00065a012
- K. D. Pennell, L. M. Abriola and W. J. Weber, “Surfactant-Enhanced Solubilization of Residual Dodecane in Soil Columns. 1. Experimental Investigation,” Environmental Science & Technology, Vol. 27, No. 12, 1993, pp. 2332-2340. doi:10.1021/es00048a005
- S. D. Haigh, “A Review of the Interaction of Surfactants with Organic Contaminants in Soil,” The Science of the Total Environment, Vol. 185, No. 1, 1996, pp. 161-170. doi:10.1016/00489697(95)05049-3
- A. Fachini, M. A. Mendes, I. Joekes and M. N Eberlin, “Oxidation of Sodium Dodecylbenzenesulfonate with Chrysotile: On-Line Monitoring by Membrane Introduction Mass Spectrometry,” Journal of Surfactants and Detergents, Vol. 10, No. 4, 2007, pp. 207-210. doi:10.1007/s11743007-1032-8
- K. Inoue, K. Kaneko and M. Yoshida, “Adsorption of Dodecylbenzenesulphonates by Soil Colloids and Influence of Soil Colloids on Their Degradation,” Soil Science & Plant Nutrition, Vol. 24, 1978, pp. 91-102. http://ci.nii.ac.jp/naid/110001719265/en
- W. S. He, R. F. Wang and J. J. Chen, “Erythrocyte Micronucleus of Tadpole (Bufobufo Andrewsi) by Synthetic Detergent Powder,” Acta Scientiae Curcumstaniae, Vol. 11, No. 3, 1991, pp. 351-357.
- W. T. Sullivan and R. D. Swisher, “Methylene Blue Active Substancesand Linear Alkylate Sulfonate Surfactants in Illinois River, 1968,” Environmental Science & Technology, Vol. 3. 1969, pp. 481-483. doi:10.10021/es60028a002
- J. McAvoy and W. Giger, “Determination of Linear Alkylbenzenesulfonates in Sewage Sludge by High-Resolution Gas Chromatography/Mass Spectrometry,” Environmental Science & Technology, Vol. 20, No. 4, 1986, pp. 376-383. doi:10.1021/es00146a009
- H. Takada and R. Ishiwatari, “Linear Alkylbenzenes in Urban Riverine Environments in Tokyo: Distribution, Source and Behavior,” Environmental Science & Technology, Vol. 21, No. 9, 1987, pp. 875-883. doi:10.1021/es00163a005
- A. Yediler, Y. Zhang, J. Cai and F. Korte, “Effects of the Microbial Population Size on the Degradation of Linear Alkylbenzenesulfonate in Lake Water (Dong Hu = East Lake, Wuhan, Hubei, P. R. China),” Chemosphere, Vol. 18, No. 7-8, 1989, pp. 1589-1597. doi:10.1016/0045-6535(89)90049-0
- R. A. Rapaport and W. S. Eckhoff, “Monitoring Linear Alkylbenzene Sulfonate in the Environment: 1973-1986,” Environmental Toxicology & Chemistry, Vol. 9, 1990, pp. 1245-2565. doi:10.1002/etc.5620091003
- D. C. McAvoy, C. E. Whik, B. L. Moore and R. A. Rapaport, “Chemical Fate and Transport in a Domestic Septic System: Sorption and Transport of Anionic and Cationic Surfactants,” Environmental Toxicology & Chemistry, Vol. 13, No. 2, 1994, pp. 213-221. doi:10.1002/etc.5620130205
- D. C. McAvoy, W. S. Eckhoffand and R. A. Papaport, “Fate of Linear Alkylbenzene Sulfonate in the Environment,” Environmental Toxicology & Chemistry, Vol. 12, 1993, pp. 977-987. doi:10.1002/etc.5620120604
- K. Figge, J. Klahn and J. Koch, “Kinetic Distribution Model for Chemicals Based on Results from a Standard Environmental System,” Ecotoxicology and Environmental Safety, Vol. 11, No. 3, 1986, pp. 320-338.
- E. Mathijs and H. DeHenau, “Adsorption and Desorption of LAS,” Tenside Surfactants Detergents, Vol. 22, 1985, pp. 299-304.
- M. Krishna, G. Voil and M. Jackson, “Soil Adsorption of LSA,” Soil Science America Proceedings, Vol. 30, 1966, pp. 685-688.
- T. H. Boyer, P. C. Singer and G. R. Aiken, “Removal of Dissolved Organic Matter by Anion Exchange: Effect of Dissolved Organic Matter Properties,” Environmental Science & Technology, Vol. 42, No. 19, 2008, pp. 7431- 7437. doi:10.1021/es800714d
- H. Fukui, A. Kaminaga, T. Maeda and K. Hayakawa, “Preparation of Surfactant Anion-Selective Electrodes with Different Selectivity Coefficients and a Trial to Determine Each Component in Binary Surfactant Mixtures,” Analytica Chimica Acta, Vol. 481, No. 2, 2003, pp. 221- 228. doi:10.1016/S0003-2670(03)00118-1
NOTES
*Corresponding author.

