Smart Grid and Renewable Energy
Vol.1 No.2(2010), Article ID:2601,10 pages DOI:10.4236/sgre.2010.12015
Separation of Biomass Pyrolysis Oil by Supercritical CO2 Extraction
![]()
1School of Chemical Engineering, Shandong University of Technology, Zibo, China; 2Shandong Research Center of Engineering and Technology for Clean Energy, Shandong University of Technology, Zibo, China.
Email: cuihy@sdut.edu.cn
Received May 17th 2010; revised June 18th 2010; accepted June 23rd 2010.
Keywords: Supercritical, Extraction, Simulated Bio-Oil, Adsorbent, Carbon Dioxide
ABSTRACT
Supercritical CO2 extraction was employed to separate simulated and real bio-oils. Effects of extraction pressure, temperature and adsorbents on distribution coefficient (or enrichment coefficient) of five representative compounds were investigated using a simulated bio-oil, which was composed of acetic acid (AC), propanoic acid (PA), furfural (FR), acetylacetone (AA) and 2-methoxyphenol (MP). The distribution coefficients of AA, FR and MP between supercritical CO2 phase and liquid phase were bigger than 1.5, while those of AC and PA characteristic of relatively strong polarity were less than 1. Temperature and pressure also had impacts on the distribution coefficients of AA, FR and MP, especially remarkable for AA. The extraction of simulated bio-oil spiked on three adsorbents shows that adsorbents influence extraction efficiency and selectivity by changing intermolecular forces. High extraction pressure and relative low temperature are beneficial to reduce the water content in the extract. In addition, the feasibility of supercritical CO2 extraction of real bio-oil was examined. After extraction in the extraction fraction total ketones increased from 14.1% to 21.15~25.40%, phenols from 10.74% to 31.32~41.25%, and aldehydes from 1.92% to 3.95~8.46%, while the acids significantly dropped from 28.15% to 6.92~12.32%, and water from 35.90% to 6.64~4.90%. In view of extraction efficiency, the optimal extraction temperature was determined to be 55℃. Extraction efficiency of the real bio-oil increased with rising pressure. The maximal extraction efficiency of real bio-oil on water-free basis could reach to 88.6%. After scCO2 extraction, the calorific value and stability of the extract fraction evidently increased and the acidity slight decreased with nearly 100% volatility below 140℃, suggesting potentially applicable as substitute for engine fuel.
1. Introduction
Facing upcoming depletion of fossil fuels and increasing environmental concerns, great effort has been devoted in exploration of biomass energy in the past few decades all over the world, not only because it is recognized as one of the most attractive alternative energy resources in the current century but also it is available in abundance, renewable and environmentally friendly [1]. Biomass as an energy source is considered sustainable since it is CO2 neutral in the life cycle, causing almost zero net emissions of CO2. Moreover, it contains negligible contents of sulfur, nitrogen, and ash, and gives much lower emission of SO2, NOx, and soot, by combustion, than the conventional fossil fuels [2]. Among the various biomass utilization technologies, conversion of biomass into bio-oil by fast pyrolysis has been shown promising for internal combustion engine fuels and high value added chemicals from the viewpoint of efficiency and economics [3-5].
Bio-oil is a dark brown, crude oil-like liquid mixture, usually obtained from thermal biomass decomposition under moderate pyrolysis temperature with very high heating rate and short vapor residence time [6]. Being a non-thermodynamic equilibrium mixture, it is highly viscous, non-volatile, poor in heat value, and corrosive [7]. Moreover, it is such a complicated mixture, containing almost all kinds of oxygenated organic compounds, including alcohols, aldehydes, carboxylic acids, ketones, esters, saccharides, phenols, guaiacols, syringols, furans and multifunctional compounds [8], that severely restrains its direct application in vehicle engines as fuels. To meet the demands for substituting fossil fuels, therefore, considerable endeavors have been contributed to upgrading bio-oils, such as catalytic hydrotreatment [9,10], catalytic cracking [11], emulsification [12] and catalytic esterification [13]. Unfortunately, all of these technologies have some shortcomings due to the complexity and thermal instability of bio-oil.
Separation and refining of bio-oil possess advantages in keeping original components, simple operation, low investment, and probably obtaining valuable chemicals. Several conventional separation techniques, like distillation, molecular distillation, solvent extraction and adsorption, have been attempted, but they seemed not to be very satisfying [14,15]. Recently, supercritical technology was also introduced into this field, but mainly focused on the bio-oil upgrading by esterification and pyrolysis in supercritical alcohols [16], as well as hydrodeoxygenation of phenols in supercritical n-hexane [17]. Supercritical CO2 extraction has been demonstrated a powerful tool in separation of thermal sensitive chemicals in the past few decades because of the relatively low critical pressure (73.8 atm) and critical temperature (31.1℃) of CO2. Compared with organic solvents, carbon dioxide is nontoxic, nonflammable, noncorrosive, cheap, and readily available in abundance with high purity. Supercritical CO2 extraction has been extensively used in extracting natural products. Very recently, scCO2 extraction of bio-oil was attempted by Rout et al. [18]. Their research results clearly showed that most of the valuable compounds such as furanoids, pyranoids and benzenoids could be enriched into the extract fraction, while water could be removed effectively after extraction. As a result, the calorific value of the bio-oil was improved.
In this work, supercritical CO2 extraction of bio-oil was systemically studied. Based on the experimental estimation of two-phase distribution coefficient (or enrichment coefficient) of a simulated bio-oil, effects of extraction pressure, temperature and adsorbents on supercritical CO2 extraction of real bio-oil were investigated and the quality of the bio-oil after extraction was evaluated in comparison with the original oil.
2. Experimental
2.1 Experimental Materials
The bio-oil used in the extraction experiment was made by flash pyrolysis of pulverized corn stalk with size of 0.1 mm~0.25 mm in the temperature range of 477~480℃. 5A molecular sieve, activated carbon, silica and KarlFischer reagent were purchased from Kermel chemical Reagent Co. (Tianjin, China). 1-methoxy-2-propanol was obtained from Acros Organics Co. with purity no less than 98.5%. All other chemicals were analytic reagents with purities more than 98% and used as received. For preparation of a simulated bio-oil, five compounds were chosen to be representative compounds and directly mixed in approximate connection with the composition in the real bio-oil. Namely, it was composed of 14.24% acetylacetone, 24.87% acetic acid, 5.87% 2-methoxyphenol, 6.39% propanoic acid, 12.37% furfural and 36.25% deionized water respectively.
2.2 Supercritical Fluid Extraction Setup
All supercritical extraction experiments were conducted on a supercritical CO2 extraction setup as shown in Figure 1, which mainly consists of a CO2 reservior, a CO2 delivering pump, an autoclave extractor (150 mL, 316 stainless steel) equipped with electromagnetic agitation and water-bath heating system, a separator (100 mL, 316 stainless steel) and a wet gas flow meter. The temperatures both for the extractor and the separator were measured by thermal couples and the pressures were gauged by manometers. When performing extraction experiment, bio-oil or adsorbed bio-oil was firstly charged to the extractor, followed by introduction of CO2 using the CO2 delivering pump, and then heated up to the desired extraction temperature and pressure. The temperature and the pressure were controlled in accuracies of ± 1℃ and 0.1 MPa respectively.
In the case of adsorbed bio-oil equilibration experiment, 12 g bio-oil was spiked on 30 g absorbent (silica, molecular sieve or active carbon) in advance and allowed to store in a closed vessel for 24 h at room temperature. Before extraction, they were wrapped up with a piece of nylon filtration fiber and then put into the extractor. For the phase behavior experiments without absorbents, electromagnetic agitator was used to enhance transportation between scCO2 phase and liquid phase kept at constant temperature and pressure for at least 1.0 h to approaching equilibrium, while in the experiments for absorbed bio-oil where agitator couldn’t be used, the equilibration time extended to at least 3.0 h.
The sampling method is similar to that in the literature [19]. Briefly, In phase equilibration experiments when sampling from liquid phase (or scCO2 phase), as shown in Figure 1, valve 2 (or valve 4) was first turned off, and then valve 1 (or valve 3) was opened until a pressure balance between the sampling tube and the extractor was reached. Afterward, valve 1 (or valve 3) was turned off and valve 2 (or valve 4) slowly opened to depressurize and collect the sample in a flask. The sampling tubes are made of a stainless steel tube with an inner diameter of 4 mm and a length of 50 mm.
The scCO2 extraction experiments were carried in an intermittent mode. After static extraction at the required temperature and pressure for 30 min or 1.0 h, depending on the cases with or without the absorbents, CO2 effluent was depressurized into the separator. Then the releasing valve was closed and CO2 was recharged up to the original pressure. In this way, the operation ran several times. The CO2 volume was measured by a wet gas meter and calculated into volume at standard state (0℃ and 1 atm) according to the ideal gas state equation, in a unit of normal liter, NL.
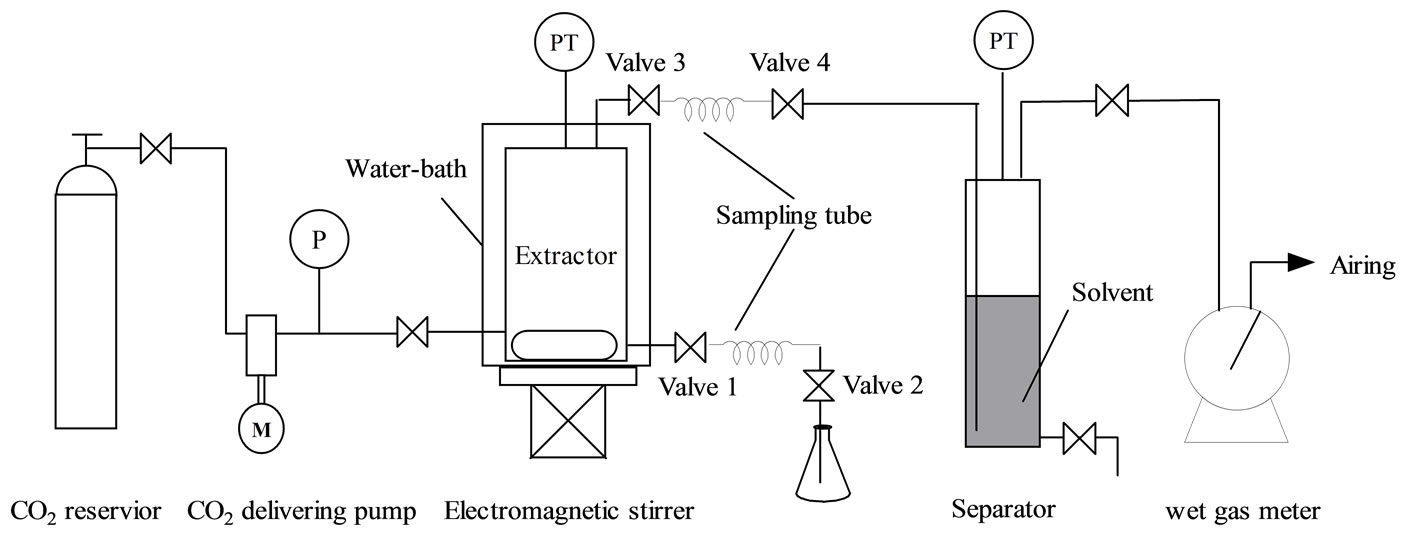
Figure 1. Schematic diagram of the experimental setup for supercritical CO2 extraction of simulated/real bio-oil
2.3 Methods of Analysis
The quantitative analysis of simulated bio-oil was acquired on a gas chromatography (Varian CP-3800), which was equipped with a 50 m × 0.25 mm × 0.33 μm PEG capillary column. Both the inlet temperature and the detector temperature were fixed at 250℃. Column temperature was controlled by a temperature program, which is: starting at 80 ℃ for 1 min, heating to 130℃ at a ramp rate of 10℃/min, followed by heating to 200℃ at a ramp rate of 30℃/min, and finally holding at 200℃ for 8 min. 1-Methoxy-2-propanol was used as an internal standard compound.
For the real bio-oil analysis, both qualitative and quantitative analyses were carried on a GC-MS system. (GC6890-MS5973N, Agilent Co.)equipped with 60 m × 0.25 mm × 0.25 μm Innowax 19091N-136 capillary column. He was used as carrier gas with a flow rate of 1mL/min and split ratio was 80. Inlet temperature was fixed at 250℃. A ramp temperature program was adopted, which started at 10℃/min from 60℃ to 120℃, then heated at 5℃/min to 200℃, and held at 200℃ for 8 min. All the acquired component compositions were based on area normalization. Water content is determined by Karl-Fischer method [20]. The calorific value was determined in a static bomb calorimeter [21].
Distribution coefficient (Di), enrichment coefficient (Ri) and percentage extraction (Ei) were calculated according to the following equation.
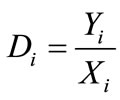
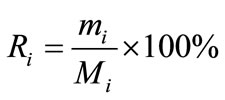
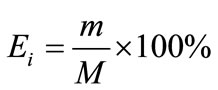
where Yi and Xi depict the mass percentage of component i in the extract and in the liquid faffinate after removal of CO2, %; mi and Mi represent the mass of component i in the extract and the total mass of component i in the simulated bio-oil respectively, g; i indicates acetic acid (AC), propanoic acid (PA), furfural (FR), acetylacetone (AA) or 2-methoxyphenol (MP); m and M describe the mass of extract and the total mass of bio-oil, g.
3. Results and Discussion
3.1 Two-Phase Distribution of Simulated Bio-Oil Extraction
The effects of CO2 pressure on the components distribution coefficients between supercritical CO2 phase and liquid phase was investigated at 45℃ in a pressure range of 7~17 MPa (Figure 2). 24.0 g simulated bio-oil was used in each experiment.
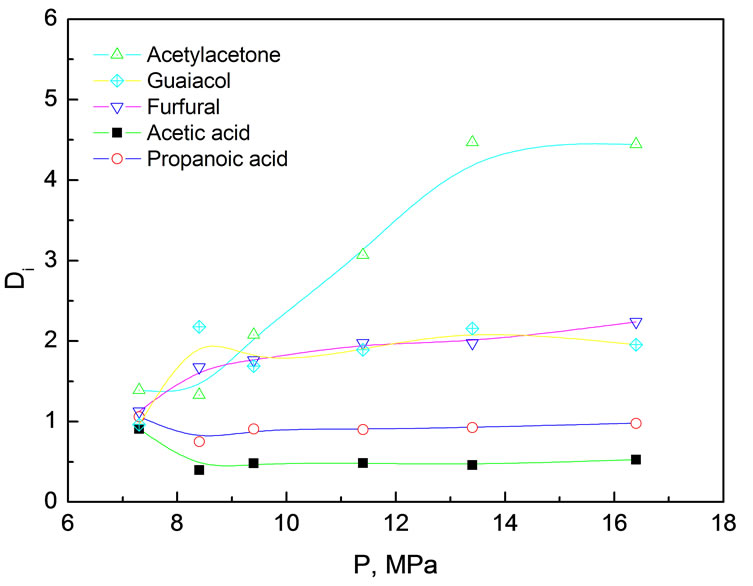
Figure 2. Distribution coefficients of various components in simulated bio-oil between supercritical CO2 phase and liquid phase as a function of pressure Extraction conditions: 45℃, pressure range from7 to17 MPa, 24.0 g simulated bio-oil
Figure 2 shows that the distribution coefficients of various components were very close to each other at low pressure (low CO2 density). Such a phenomenon might be caused by the combined effects of vaporization and scCO2 solvating power. Except for MP (b. p. 205℃), the boiling points at atmosphere of AC (118℃), PA (140.7℃), FR (162℃) and AA (140.5℃) are relatively close, suggesting they have relatively approximative partial vapor pressures. MP is weak in polarity and readily extracted by nonpolar CO2, where as AC, PA, FR and AA relatively difficult to be extracted as they are polar compounds. As the pressure rising, DAA increased remarkably from 1.5 to 4, DFR and DMP increased gradually, while DAC and DPA varied very little. Strong intermolecular interactions between homogeneous species and heterogeneous species of acetic, propanoic acid and water, capable of forming hydrogen bonds, might be a good explanation. Even under high pressure the polarity of CO2 is still very weak and hard to break up these hydrogen bonds, resulting in difficulty in extraction of AC and PA into supercritical CO2 phase. In contrast, in spite of polar compounds and capable of forming intermolecular hydrogen bonds with water, AA and FR cannot form homogeneous intermolecular hydrogen bonds. Consequently, they exhibited higher distribution coefficient under high CO2 density than under low CO2 density. Considering the fact bio-oil typically contains 20~40% water, therefore, hydrogen bonding effect might be beneficial to selective isolation or enrichment of some chemicals in supercritical CO2 extraction.
Figure 3 depicts the effect of temperature on the distribution coefficients in simulated bio-oil at constant pressure of 15.0 MPa. Likewise, 24.0 g simulated bio-oil was used in each experiment.
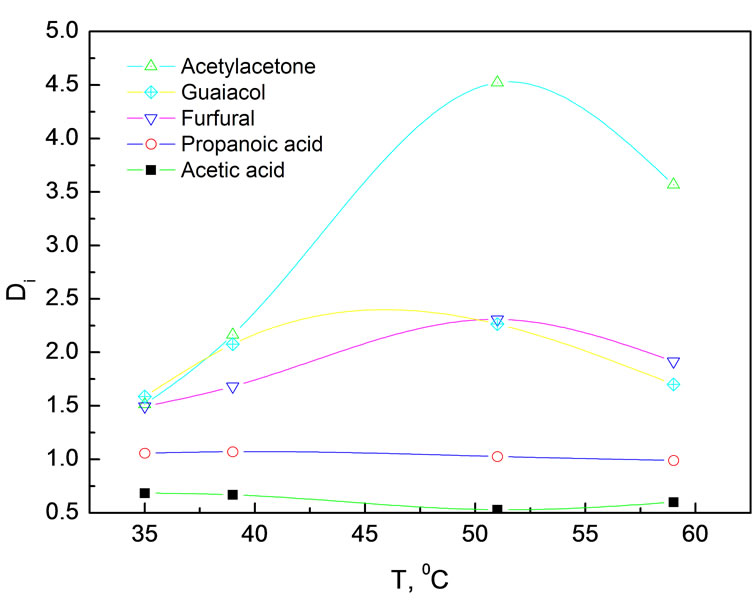
Figure 3. Effect of temperature on distribution coefficients of various components in simulated bio-oil Experimental conditions: 15.0 MPa, 24.0 g simulated bio-oil
In the experimental temperature range of 35~60℃, temperature had very weak effect on DAC and DPA, around 0.6 and 1.0 respectively. DPA is always bigger than DAC, indicating polarity played a much more important role than partial vapor pressure as the partial vapor pressure of AC is bigger than that of PA at same temperature. Low distribution coefficients both for AC and PA are attributed to the formation of hydrogen bond with water. DAA, DFR and DMP increased at the beginning and then decreased with elevating the temperature. Such an interesting tendency might be related to the CO2 density because at constant pressure CO2 density decreased and resulted in a drop in its dissolving powder when raising the temperature. On the other hand, the vapor pressures increased with temperature. As a result, the contents of low boiling point compounds in the extract tend to rise. Acetylacetone has a relative low boiling point and cannot form hydrogen bond with water. For this reason, it is not surprise that DAA showed the most evident temperature dependence.
Adding absorbents might have also effect on scCO2 extraction, in theory, because it can influence the intermolecular forces of various species. 5A molecular sieve, activated carbon and silica were chosen as the absorbents. In each experiment, 5.0 g simulated bio-oil was spiked on 20.0 g absorbent. Due to the difficulties in knowing the exact amount of the various components in the absorbents after extraction, enrichment coefficient was used instead of distribution coefficient. Figure 4 shows the enrichment coefficients at 45℃ and 18.3 ± 0.1 MPa in the presence of various absorbents.
In the presence of absorbent, the interaction force between the component and the absorbent depends on not only the component but also the type and internal texture of the absorbent [22,23]. Competitive adsorption between species occurred on the surface of the absorbent after spiking. For this reason, the intermolecular forces between different components could be changed and thus influence their extractability by supercritical CO2. In contrast to the case in absence of absorbent, the enrichment coefficients of FR, AA and PA declined, whereas those for MP and AC rose. Among all of the five compounds, AC is the strongest and MP is the weakest in polarity. All of the other three compounds whose enrichment coefficients declined in the presence of absorbents are moderate in polarity. Therefore, extraction selectivity might be linked to the surface polarity of the absorbents. AA, FR and MP are high value chemicals. Thus it is reasonable to expect much more of these compounds could be preferentially extracted into the scCO2 phase. In this context, except for MP, all three adsorbents seemed not to be very satisfying. Therefore, functionalized adsorbents should be taken into consideration in the future research work.
Water content in the bio-oil is of significance since it
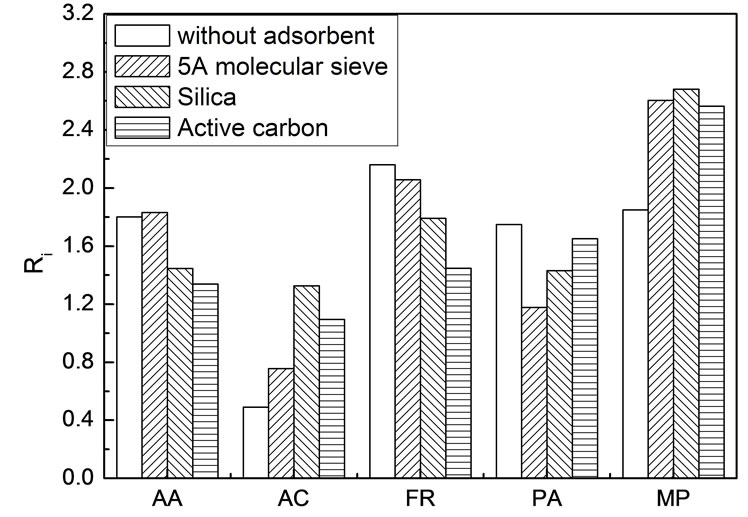
Figure 4. Enrichment coefficients of various components in simulated bio-oil in the presence of adsorbents Experimental conditions: 45℃ and 18.3 ± 0.1 MPa, 5.0 g simulated bio-oil, 20.0 g absorbents
influences both the heat value and combustibility in the internal combustion engines. Effects of extraction pressure and temperature on the water content in the extract were also surveyed (Figure 5 and Figure 6).
It can be seen in Figure 5, water content in the extract decreased with raising the pressure. For example it was 13.28% at 9.4 MPa and dropped drastically to only 5.52% at 18.4 MPa. This was in accordance with the explanation in the section of pressure effect on distribution coefficients. The volatility of a compound at low pressure is a dominant factor while the dissolving capacity of CO2 becomes dominant at high pressure. High water content in the extract is attributed to the lowest boiling point and strongest polarity of water among all of the components in the simulated bio-oil.
According to the above explanation, it is expectable to see in Figure 6 that water content in the extract gradually
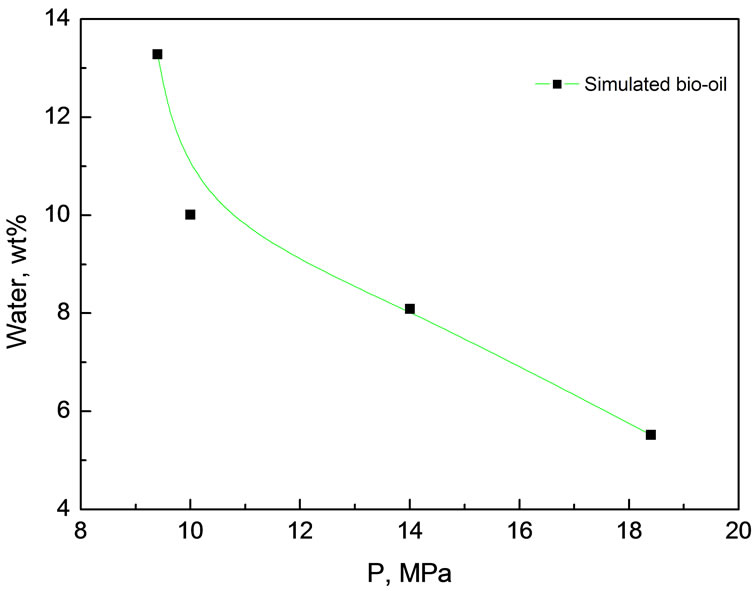
Figure 5. Effect of extraction pressure on the water content in the extract at 45℃ Experimental conditions: 45℃, 5.0 g simulated bio-oil

Figure 6. Variation of water content in the extract as a function of extraction temperature under 12.0 MPa pressure Experimental conditions: 12.0 MPa, 5.0 g simulated bio-oil
increased with elevating the temperature at 12.0 MPa. At 75℃, the water content could go up to as high as 28.3%. Elevating temperature creates two effects on the extraction: 1) reducing the density of CO2 and thus its dissolving power, resulting in poorer extraction efficiency when rising the temperature; 2) increasing the saturated vapor pressure of extractable compounds, as a result, leading to higher extraction efficiency. As a compromise, dissolving power of CO2 plays a dominant role at low temperature, while vapor pressure turns to be a dominant factor at high temperature. Given a fact that the polarity of water is very strong, its solubility is still very low in CO2 phase even though at low temperature. Therefore, the tendency that the water content increased with temperature is understandable.
3.2 Supercritical CO2 Extraction of a Real Bio-Oil
For the extraction of real bio-oil, pyrolysis bio-oil of corn stalk powder was selected and subjected to extraction by supercritical CO2, whose composition acquired by GCMS was summarized in Table 1. It could be clearly seen that large amount of water (35.9%) and organic acids (24.51% acetic acid, 2.07% propanoic acid, 1.57% acryl acid) were contained in the original bio-oil, which were dominantly responsible for the corrosiveness of bio-oils. Phenols which resulted from the pyrolysis of the lignin in corn stalk occupied about 10% in bio-oil, mainly existing in the form of phenol, methyl phenol, methoxy phenol and ethyl phenol. Except for the compounds listed in Table 1, other kinds of compounds, like saccharides and charcoals, might also be contained in the bio-oil, but could not be measured by GC-MS and weren’t taken into account in this study.
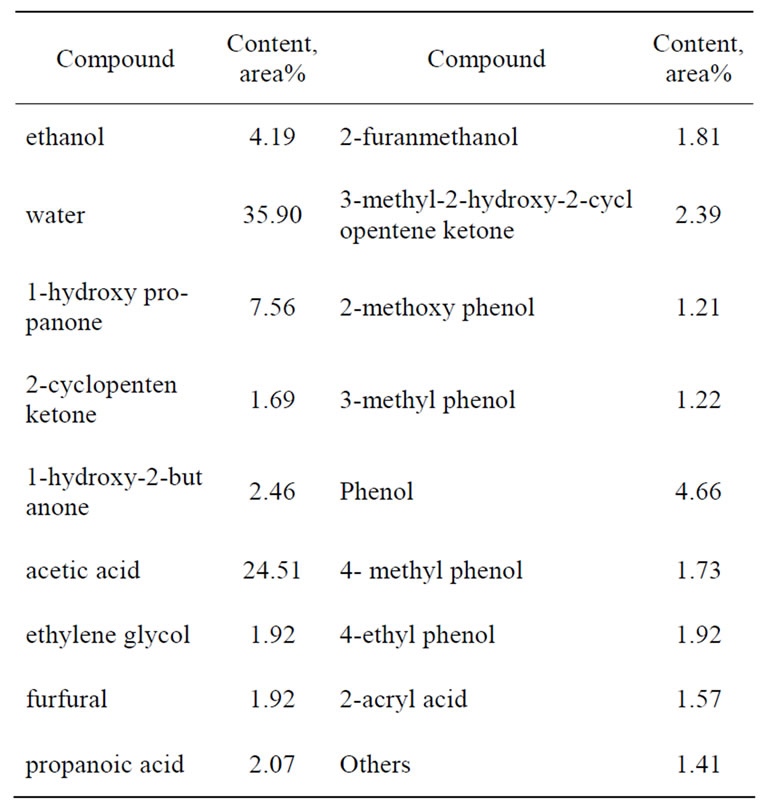
Table 1. Composition of the pyrolysis bio-oil of corn stalk
3.2.1 Changes of Various Categories of Compounds in the Extracts after scCO2 Extraction
For scCO2 extraction of the real bio-oil, 12.0 g bio-oil was spiked on 30.0 g adsorbents (5A molecular sieve or silica) and then subjected to extraction at 45℃ and 26.0 MPa. Typical profiles for various compound categories were given in Table 2 when the CO2 volume was 50NL and extraction efficiency was 20%.
Given a fact that the composition of bio-oil varies with the biomass resources, pyrolysis processes, and the operation conditions, and most of the compounds in the bio-oil are in very low concentration, our experiments focused on the extractability of various categories of compounds rather than individual compound. Similar to the extraction of simulated bio-oil, intermittent operation mode was used for the extraction of the real bio-oil. The extraction pressure swing range was between 26.0 MPa and 10.0 MPa. All of compounds identified by GC-MS were classified into 8 groups. They are: 1) alcohols including ethanol, methanol, ethanediol, isobutyl alcohol furfuryl alcohol, etc; 2) ketones including 2-propanone- 1-hydroxy, 2-cyclopentanone, 1-hydroxy-2-butanone, 2- hydroxy-cyclopentanone, 3-hydroxy-2-butanone, 4-methyl-cycloheptanone, acetyl-acetone, furan acetophenone, 2,2-dimethyl-1-propeneketo-1-cyclohexenyl, etc; 3) acids including acetic acid, propanoic acid, butyric acid, vinyl acid, crotonic acid, 9,10-diene stearic acid, etc; 4) phenols including 4-ethyl-2-methoxyphenol, 2,5-dimethylphenol, 4-methyl phenol, 3-methoxy-phenol, 3-methylphenol, 3-methyl-4-ethyl phenol, 4-ethyl phenol, 2,5-dimethyl-phenol, 2,4-dimethyl-3-dimethyl-etherbase-4-methoxy-phenol, 2,6-dimethyloxy-phenol, 2-me-thoxy-phenol, 2-ethoxy-phenol, phenol, etc; 5) aldehydes including furfural, 2-furyl glyoxal, 4-ethoxy-benzaldehyde, 3-hydroxy-4-methoxybenzaldehyde, etc; 6) esters including methyl acetate, 4-methyl-amyl methyl ester, 1-propylene glycol acetate, 2-methyl ethylene, etc; 7) water, and 8) the others which refer to all compounds which were detectable by GC but unidentified by MS.
It has been reported that hundreds of compounds might be contained in bio-oils. However, for our original bio-oil only 17 compounds could be identified by GCMS analysis. Besides the nonvolatile compounds, there were still a variety of compounds whose contents were so low that they couldn’t be detected out by the GC-MS. After scCO2 extraction the compound species detected in the extract rose up to 80, suggesting that scCO2 extraction can be used as a useful pretreatment tool for qualitative and quantitative analysis of bio-oils. Table 2 shows the enrichment of all categories of compounds when the extraction efficiencies on water-free basis were about 20%.
Compared with the original bio-oil, there was only a little change of alcohols in the extract fraction after extraction which might be attributed to the low content alcohols in original bio-oil. Water content decreased significantly, about one fifth of that in the original bio-oil (35.90%) suggesting that scCO2 extraction was very effective in reducing the water content and thus boosting up the heat value of bio-oil. The total ketones in bio-oil increased from 14.1% to 21.15~25.40%, phenols from 10.74% to 31.32~41.25%, aldehydes from 1.92% to 3.95~8.46%, while the acids were significantly reduced from 28.15% to 6.92~12.32%. These tendencies are in
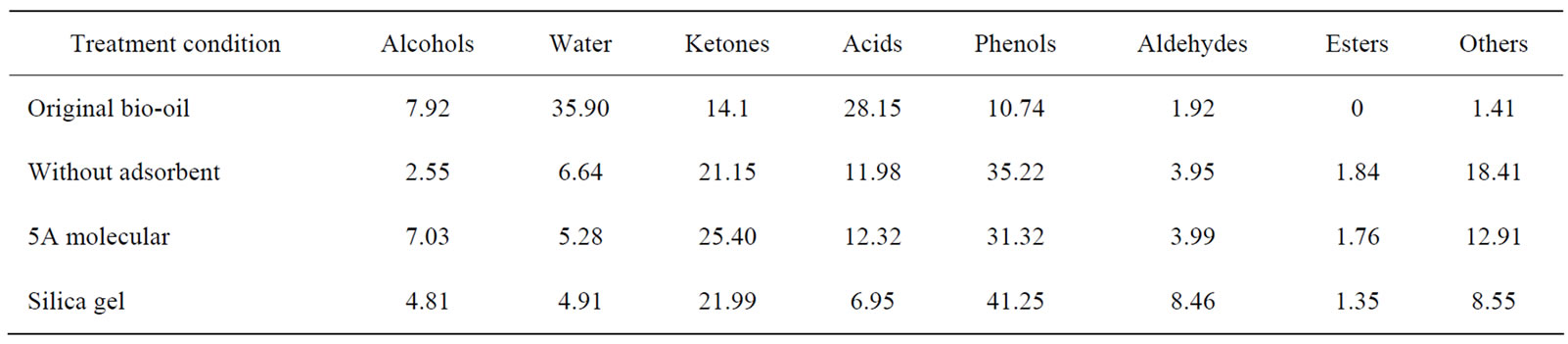
Table 2. Typical profiles for various categories of compounds in the extracts after scCO2 extraction
accordance with the experimental results of the simulated bio-oil.
In summary, although the selectivity differed from one compound to another, depending on the types of absorbents, water and acids can be greatly reduced after scCO2 extraction, indicating significant heat value increment and corrosiveness reduction (lower pH) from the viewpoint of upgrading bio-oil. In addition, selective extraction or enrichment of high value-added chemicals might be probable by selecting appropriate adsorbent.
3.2.2 Effect of Pressure on Extraction Efficiency
Effect of pressure on extraction of real bio-oil was studied at fixed temperature of 45℃. Figure 7 shows the pressure dependence as a function of CO2 consumption in standard volume, NL. In each experiment, 30.0 g original bio-oil was used.
As shown in Figure 7, extraction pressure had strongly positive influence on the extraction efficiency of real bio-oil. The higher the extraction pressure at same CO2 volume, the higher extraction efficiency was. When the CO2 volume was 560 NL, for example, extraction efficiencies of the bio-oil were respectively 8.00% at 7.0 MPa, 24.20% at 10.0 MPa, 36.53% at 15.0 MPa, 47.59% at 20.0 MPa, 58.85% at 25.0 MPa and 77.58% at 30.0 MPa. Since the dissolving power of CO2 mainly depended on by its density and was tunable by modifying the pressure, it is easy to understand the pressure effect on extraction efficiency. In addition, pressure also affects the polarity of CO2. The polarity of scCO2 has been confirmed to be increased with elevating the pressure at constant temperature. Therefore, the composition of various compounds in the extraction fraction varied with the pressure. It is reasonable to expect that the compounds with weak polarity are preferentially extracted at low pressure since CO2 is a nonpolar compound, while the compounds with strong polarity can be extractable at
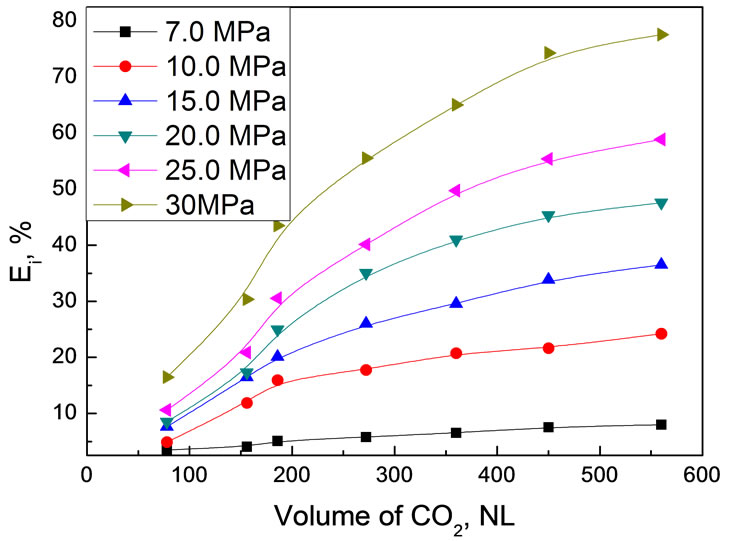
Figure 7. Extraction efficiency of bio-oil as a function of CO2 volume under different pressure Experimental conditions: 45℃, 30.0 g bio-oil
high pressure although their solubility in scCO2 might still be very low. According to this, the solubility of water in scCO2 phase should also be increased. However, the experimental results showed that the water content in the extract decreased with elevating the pressure at constant temperature. To explain this, the competitive extraction between various compounds and the phase behavior in such a complicated system need to be taken into account.
3.2.3. Effect of Temperature
The effect of extraction temperature on extraction efficiency of bio-oil was surveyed at 30.0 MPa using 30.0 g bio-oil. Figure 8 shows the variation of extraction efficiency at different temperature as a function of CO2 volume.
Extraction temperature didn’t show remarkable impact on the extraction efficiency in the experimental temperature range of 45~65℃. As discussed in Subsection 3.1, temperature in scCO2 extraction has two adverse effects. Therefore, it is not surprised to see that the extraction conducted at 55℃ afforded the highest extraction efficiency, which might be a compromise of these two effects.
It is well known that modified CO2 using low molecular weight organic compounds like methanol or acetic acid, has been shown to be more powerful in extracting polar substances than pure CO2. Considering that ethanol and methanol are available from conversion of biomass and can be used directly as engine fuels, they were employed in the investigation of the impacts of modifiers on the extraction. When performing the experiment, certain amount of methanol or ethanol was directly poured into 30.0 g bio-oil. The experimental results at constant consumption of CO2, 156NL, showed that extraction efficiency could be increased about 20% and 10% respec tively compared to the case without modifiers when 3.0 g
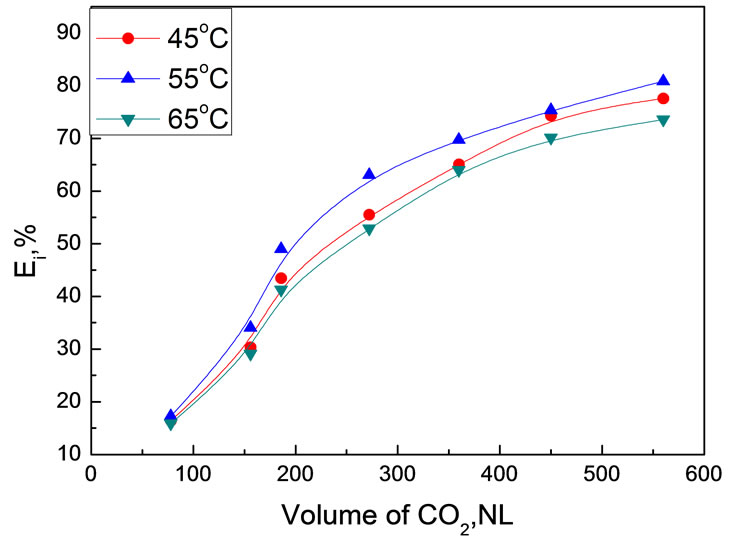
Figure 8. Temperature dependence of bio-oil extraction efficiency as a function of CO2 volume at 30MPa Experimental conditions: 30.0 MPa, 30.0 g bio-oil
modifier was used in 30.0 g bio-oil. Evidently, methanol is more eligible as a modifier than ethanol in enhancing the dissolving power of scCO2. It should be cautious that, however, the water content in the extract was significant higher when excess methanol was used than that in the case of pure CO2 extraction.
3.2.4 Property Comparison between Original Bio-Oil and Extracted Oil
One can see that in Table 3, pH value increased from 2.10 to 4.1~4.5 and the calorific value from 13.95 to 18.59~25.41 kJ/kg in different treatment cases, while the density decreased from 1.15 to 0.92~0.98 and the moisture from 35.9% to 4.29~6.64%. The stability of the bio-oils was judged by after 6 month storage under room temperature to observe whether phase splitting or noticeable change in viscosity occurred. The original biooil was dark brown opaque liquid with high viscosity, increased noticeably in viscosity, and phase splitting happened after storage, while the extracts from scCO2 extraction were light brown transparent liquids with relatively low viscosities and hadn’t noticeable changes in viscosity and phase splitting, implying relatively high stability.
3.2.5 Bio-oil Volatility
Volatility is vital in evaluating transport fuels since it relates to the atomization and combustion performance. To estimate the volatility of bio-oil, 2.0 g bio-oil was accurately weighed and allowed to temperature programmed evaporating in an open dish in air drying oven. The mass variation was determined by analytical balance. The extract and the faffinate used were obtained from the scCO2 extraction under 35℃ and 30.0 MPa with extraction efficiency of 88.6% (water-free basis). Figure 9 shows temperature dependence of bio-oil volatility as a function of evaporation time.
As shown in Figure 9, the extract fraction showed evidently higher in volatility than the original bio-oil, while the faffinate showed significantly lower. For example, at 60℃ for 15 min, the volatility percentages of original bio-oil, extract and faffinate were 50.32%, 68.98% and 45.34%, respectively. When elevating temperature to 140℃, more than 97.69% of extract became volatile, whereas only 89.56% and 83.92% for original bio-oil and faffinate. If taking the fact that most of water was left in the faffinate into account, the volatility experiments strongly suggested that the quality upgrading of the extract faction in view of potential application in combustion engine since the nonvolatile compounds were mainly left in the faffinate after extraction.
4. Conclusions
Supercritical CO2 extraction was an effectively powerful tool in separation and upgrading of bio-oil. By adjustment of operation temperature, pressure, and using absorbents, weakly polar compounds are capable of selective separation and enrichment into scCO2 phase. The
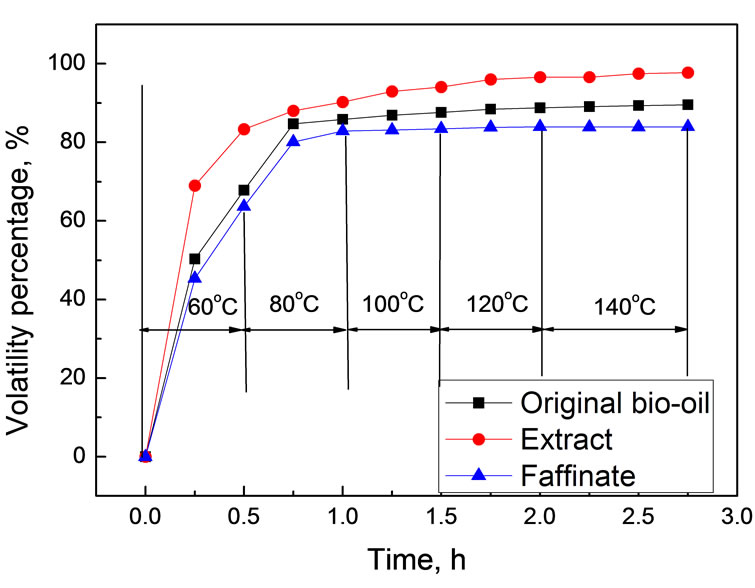
Figure 9. Variation of volatile as a function of time at different temperatures
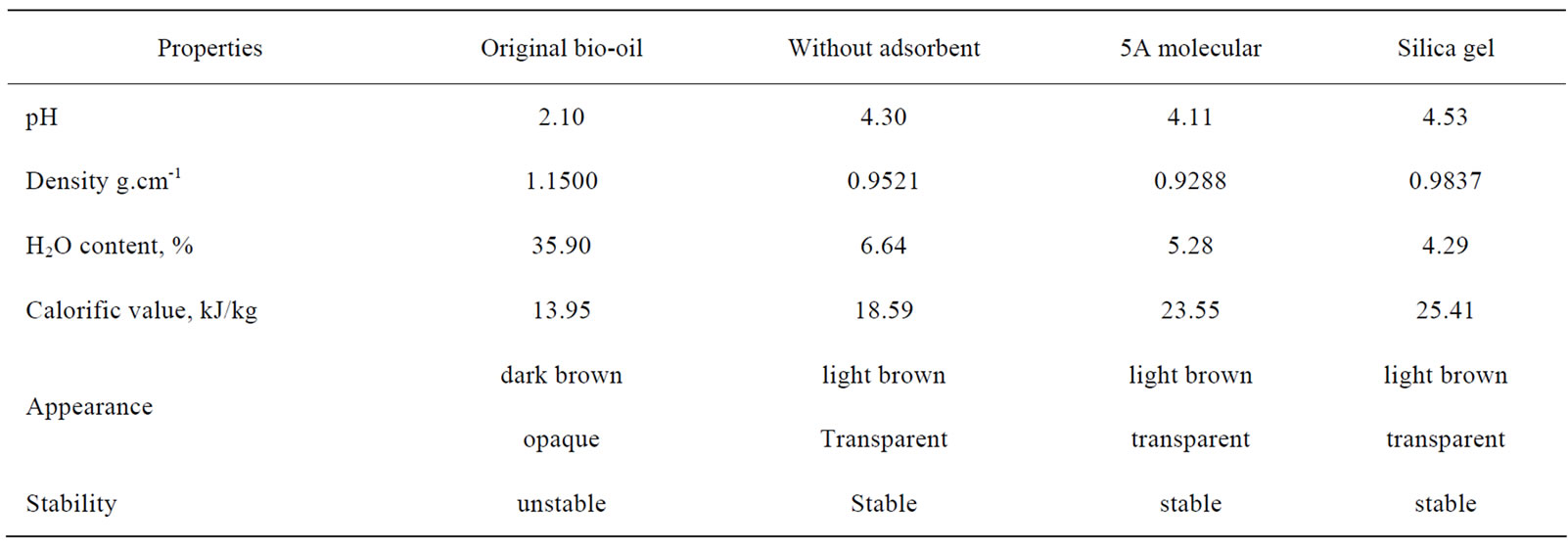
Table 3. Properties of bio-oil and extracts at different conditions
adsorbent can affects the intermolecular forces between different components. Therefore, valuable chemicals might be expected to be isolated from bio-oil by selecting appropriate adsorbent. High extraction pressure and relative low extraction temperature favor in effectively reducing the water content in extracted bio-oil. Extraction efficiency of real bio-oil increased with raising the pressure. The maximum extraction efficiency of supercritical CO2 extract bio-oil reached to 88.6% on water-free basis at 30.0 MPa and 35℃. After scCO2 extraction, the quality of bio-oil can be improved significantly from the viewpoint of water content reduction, acidity, calorific value, stability and appearance. Water content could be reduced to one fifth of that of original bio-oils. Calorific value could increase to be doubled. pH value went up from 2.1 to above 4.1. The extract fraction showed nearly 100% volatility below 140℃ since most of the nonvolatile compounds was left in the faffinate.
5. Acknowledgements
This research is financial supported by Natural Science Foundation of Shandong Province (Grant No. ZR2009 BL023) and National High Technology Plan (863) Project No. 2009AA05Z401 Granted by the Ministry of Science and Technology of the People’s Republic of China.
REFERENCES
- M. Baratieri, P. Baggio, L. Fiori and M. Grigiante, “Biomass as an Energy Source: Thermodynamic Constraints on the Performance of the Conversion Process,” Bioresource Technology, Vol. 99, No. 15, 2008, pp. 7063- 7073.
- J. L. Zheng and W. M. Yi, N. N. Wang, “Bio-Oil Production From Cotton Stalk,” Energy Conversion and Management, Vol. 49, No. 6, 2008, pp. 1724-1730.
- A. V. Bridgwater and G. V. C. Peacocke, “Fast Pyrolysis Processes for Biomass,” Renewable and Sustainable Energy Reviews, Vol. 4, No. 1, 2000, pp. 1-73.
- C. David, O. Anja and S. Yrjö, “Power Generation Using Fast Pyrolysis Liquids from Biomass,” Renewable and Sustainable Energy Reviews, Vol. 11, No. 6, 2007, pp. 1056-1086.
- A. V. Bridgwater, D. Meier and D. Radlein, “An Overview of Fast Pyrolysis of Biomass,” Organic Geochemistry, Vol. 30, No. 12, 1999, pp. 1479-1493.
- M. Peter, “Energy Production from Biomass (Part 2): Conversion Technologies,” Bioresource Technology, Vol. 83, No. 1, 2002, pp. 47-54.
- Q. Lu, W. Z. Li and X. F. Zhu, “Overview of Fuel Properties of Biomass Fast Pyrolysis Oils,” Energy Conversion and Management, Vol. 50, No. 5, 2009, pp. 1376- 1383.
- T. A. Milne, F. Agblevor, M. Davis, et al., “A Review of Chemical Composition of Fast Pyrolysis Oils,” In: A. V. Bridgwater, Ed., Developments in Thermochemical Biomass Conversion, London, Blackie Academic & Professional, 1997, pp. 409-424.
- V. A. Yakovlev, S. A. Khromova, O. V. Sherstyuk, et al., “Development of New Catalytic Systems for Upgraded Bio-Fuels Production from Bio-Crude-Oil and Biodiesel,” Catalysis Today, Vol. 144, No. 3-4, 2009, pp. 362-366.
- Y. Xu, T. J. Wang, L. L. Ma, et al., “Upgrading of Liquid Fuel from the Vacuum Pyrolysis Of Biomass over the Mo–Ni/γ-Al2O3 Catalysts,” Biomass and Bioenergy, Vol. 33, No. 8, 2009, pp. 1030-1036.
- L. Garcia, R. French, S. Czernik, et al., “Catalytic Steam Reforming of Bio-Oils for the Production of Hydrogen: Effects of Catalyst Composition,” Applied Catalysis A: General, Vol. 201, No. 2, 2000, pp. 225-239.
- I. Michio, S. Maria and H. Ed, “Emulsification of Pyrolysis Derived Bio-Oil in Diesel Fuel,” Biomass and Bioenergy, 2003, Vol. 24, No. 3, pp. 221-232.
- Y. Xu, J. Chang, Q. Zhang, et al., “Upgrading of Bio-Oil by Esterification over Solid Base Catalyst,” Petrochemical Technology, Vol. 35, No. 1, 2006, pp. 615-618.
- F. Mahfud, H. Melia′n-Cabrera, I. R. Manurung and H. J. Heeres, “Upgrading of Flash Pyrolysis Oil by Reactive Distillation Using a High Boiling Alcohol and Acid,” Trans IChemE, Part B, Process Safety and Environmental Protection, Vol. 85, No. B5, 2007, pp. 466-472.
- B. Tuya, M. Chaala, C. R. Garcia-Perez, et al., “Colloidal Properties of Bio-Oils Obtained by Vacuum Pyrolysis of Softwood Bar Characterization of Water-Soluble and Water-Insoluble Fraction,” Energy Fuels, Vol. 18, No. 3, 2004, pp. 704-712.
- J. Peng, P. Chen, H. Lou and X. M. Zheng, “Catalytic Upgrading of Bio-Oil by HZSM-5 in Suband Super-Critical Ethanol,” Bioresource Technology, Vol. 100, No. 13, 2009, pp. 3415-3418.
- H. H. A. Hussain, “Supercritical Hexane Extraction: a New Solvent System for Extracting Bio-Oil,” Division of Fuel Chemistry, Vol. 53, No. 2, 2008, pp. 862-864.
- P. K. Rout, M. K. Naik, S. N. Naik, et al., “Supercritical CO2 Fractionation of Bio-oil Produced from Mixed Biomass of Wheat and Wood Sawdust,” Energy & Fuels, Vol. 23, No. 12, 2009, pp. 6181-6188.
- H. Y. Cui, T. Wang, F. J. Wang, et al., “Kinetic Study on One-pot Synthesis of Dimethyl Carbonate in Supercritical CO2 Conditions,” Industrial & Engineering Chemistry Research, Vol. 43, No. 24, 2004, pp. 7732-7739.
- H. Wang, R. H. Liu, C. M. Zhang and J. D. Luan, “An Experimental Study on Determination of the Water Content in Bio-Oil by Karl-Fischer Titration,” Renewable Energy, Vol. 3, No. 1, 2005, pp. 17-20.
- C. M. Jiao and F. He, “Heating Value Determination of Liquid Product from Corn Stalk Pyrolysis,” Journal of Shandong University of Technology, Vol. 20, No. 2, 2006, pp. 11-13.
- Y. K. Matsui, Y. S. Fukuda and T. K. Inoue, “Effect of Natural Organic Matter on Powdered Activated Carbon Adsorption of Trace Contaminants: Characteristics and Mechanism of Competitive Adsorption,” Water Research, Vol. 37, No. 18, 2003, pp. 4413-4424.
- C. Pelekani and V. L. Snoeyink, “Competitive Adsorption between Atrazine and Methylene Blue on Activated Carbon: The Importance of Pore Size Distribution,” Carbon, Vol. 38, No. 10, 2000, pp. 1423-1436.

