Journal of Behavioral and Brain Science
Vol.3 No.5(2013), Article ID:36464,10 pages DOI:10.4236/jbbs.2013.35044
Conditioned Taste and Place Preferences Induced by Electrical Stimulation of the External Lateral Parabrachial Nucleus: A General Reinforcing Mechanism?
Department of Psychobiology, University of Granada, Granada, Spain
Email: *mjsimon@ugr.es
Copyright © 2013 Maria J. Simon et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 10, 2013; revised August 10, 2013; accepted August 20, 2013
Keywords: Electrical Stimulation; External Lateral Parabrachial Nucleus (LPBe); Reward; Conditioning; Flavor; Placement
ABSTRACT
This study examined the stimulus specificity of external lateral parabrachial (LPBe) rewarding stimulation by using two identical learning procedures that may dissociate conditioned reinforcement to either the place or the flavor stimulus. Animals were presented with two distinct flavors in two different positions (left and right) that were varied throughout the experimental sessions. In the first experiment, LPBe stimulation was associated with one or other flavor, while in the second it was conditioned to one or other place in which these flavors were offered. The results show that, despite stimulus interferences, the animals develop specific conditioned preferences for the flavor stimuli (experiment 2A), and also for the place of their presentation (experiment 2B). These data are discussed in the context of brain reward systems and the biological constraints that characterize some learning modalities.
1. Introduction
The external Lateral Parabrachial Nucleus (LPBe), located in the ventrolateral region of the Parabrachial Complex, has been related to the processing of taste [1,2] and visceral information [1,3-8] which is essential to establish specific gustatory-visceral associations. Thus, this nucleus appears to participate in the development of taste aversion learning [9,10] and in taste preferences induced by intragastric administration of rewarding foods [11].
Immunohistochemical (C-Fos) studies have confirmed that the LPBe is one of the brain areas that participate in the processing of food rewards, e.g., glucose, lactose, or sucrose [12-14], intake-related endogenous substances, e.g., cholecystokinin or leptin [15-17], and even food intake related-drugs, e.g., fenfluramine, amphetamines, or opiates [15,16,18,19].
Ongoing electrical stimulation of the LPBe induced concurrent place preferences for the associated compartment in a rectangular maze [20-22]. Likewise, conditioned taste preferences were induced by LPBe electrical stimulation in a flavor discrimination task that repeatedly presented taste-olfactory stimuli in a given place [20,23]. However, these results do not elucidate the stimulus specificity (biological constraints) of reinforcing parabrachial stimulation with respect to simultaneously present sensory indexes such as place, space, proprioceptive cues or flavor [24,25].
It appears well established that some learning modalities imply a certain biological predisposition for specific stimuli with which preference associations are established and that appear to be important for survival. Thus, aversive visceral information tends to be preferentially associated with taste stimuli, whereas noxious somatosensory signals tend to be related to exteroceptive indexes [26,27], as a probable consequence of ecological situations in which these stimuli are usually associated. In this regard, some drugs of abuse, such as morphine, can induce preference for associated environmental cues, whereas their aversive components appear to be related to taste cues [25,28-33].
It has been reported that the reinforcing effects of LPBe stimulation can be blocked by administrating opiate antagonists such as naloxone [20,22]. This nucleus may therefore be an element of an opioid reward system that some authors have related not only to the intake of appetizing products and/or reduction in a natural state of need [34-38] but also to a rewarding mechanism that does not appear to be specific to a single sensory modality [20]. This opioid system may also be involved in non-natural states of need, such as those caused by drugs of abuse [39], hence modulating the motivational value of the associated stimuli, regardless of the stimulus modality.
With this background, the main objective of this study was to examine whether reinforcing parabrachial stimulation is stimulus-specific, i.e., shows a biological constraint towards certain specific stimuli presented in the experimental context, or whether the rewarding effect is associated with stimuli of various modalities. The study objectives were: 1) to determine whether the place preferences induced by ongoing electrical stimulation of the LPBe can also be conditioned by parabrachial activation immediately after the stay of the animal in a specific place, and 2) to examine the possibility of developing specific conditioned taste preferences for flavor stimuli dissociated from any place index.
In the first experiment, the LPBe was activated in a concurrent/ ongoing electrical stimulation place preference task (experiment 1). In the second experimental series (procedures 2A and 2B), a discriminative learning task was used in which the animals were simultaneously presented with two different indexes from distinct stimulus modalities: taste-olfactory (strawberry or coconut flavors) and place of flavor presentation (left or right), which were changed throughout the experimental sessions. LPBe electrical stimulation was associated with one of the two flavors, regardless of its position (experiment 2A), or this reinforcing effect was conditioned with one of the two places (left or right) in which the taste stimuli were presented, regardless of the specific flavor (experiment 2B).
We hypothesized that electrical stimulation of the LPBe would produce an increase in the preference for the associated taste stimulus (Procedure 2A) or for the taste stimulus presented in the stimulated place (Procedure 2B) in animals for which the LPBe stimulation was rewarding (experiment 1), while control (non-stimulated) animals would be randomly distributed between the two taste stimuli.
2. Materials and Methods
2.1. Subjects and Surgery
Sixty-two male Wistar rats weighing 260 - 420 g at the time of surgery were used in this study. Upon their arrival at the laboratory, animals were housed individually in methacrylate cages (15 × 30 × 15 cm) that also served as training chambers. Room temperature was maintained at 21˚C - 24˚C, under a 12 h:12 h light-dark cycle, with lights on at 8:30 a.m. All experimental procedures complied with guidelines established by the European Union (2010/63/EU) and Spanish Law (1201/2005). This study was approved by the Ethics Committee for animal research of the University of Granada.
Out of these 62 animals, 46 were implanted in the LPBe nucleus with a monopolar 00 stainless steel electrode insulated except at the tip (Coordinates: AP = −0.16 V = 3.0 and L = ±2.5 according to the atlas by Paxinos and Watson 2005 [40]), whereas the remaining 16 animals were used as unimplanted neurologically intact control groups. Two groups of 23 implanted animals and two groups of 8 intact animals were formed.
Surgery for chronic implants was performed under general anesthesia (sodium pentothal, 50 mg/kg., Braun Medical S.A. Barcelona, Spain) using a stereotaxic device (Stoelting Co. Model Stereotaxic 51.600, USA), as previously described [20]. After surgery, animals were returned to their cages where they remained for recovery period of ≥7 days with water and food ad libitum (Laboratory Food, Aa-04 rat-mouse maintenance, Panlab Diets S.L., Barcelona, Spain).
2.2. Apparatus
Electrical stimulation was delivered by a CS-20 stimulator connected to an ISU-165 isolation unit (both from Cibertec, Madrid, Spain), monitoring the current on an oscilloscope (Model HM 507, Hameg Instruments, Frankfurt, Germany). Before the start of behavioral procedures, the optimal current intensity for each animal was ascertained by increasing the current until reaching a behaviorally observable level of response without producing escape behaviors, jumping, or vocal reactions. We applied a current range from 50 to 110 µA (mean 83.5 µA) with rectangular cathodic pulses at 66.6 Hz and 0.1 ms pulse duration.
The concurrent stimulation-induced Place Preference test used to classify animals according to the effect induced by the electrical stimulation was conducted in a rectangular maze (50 × 25 × 30 cm) with two open compartments separated by a narrow (8 × 25 cm2) neutral area in which each animal was placed at the beginning of the experimental session. The lateral compartments differed in the texture and design of floor and walls. The floor was brown cork or synthetic black and white painted cork. Walls were painted with horizontal or vertical black and white 1-cm wide stripes. The central area had a white methacrylate floor and natural wood walls, as described elsewhere [20-22].
As noted above, the remaining experimental procedures were conducted in methacrylate chambers that served as home cages. The sides of these cages were black and opaque, and the front and back panels were transparent. The front side of each cage had two 1.6 cm holes at the same distance from the center and edges and at the same height above the floor. Through those orifices, the animal had access to spouts attached to graduated burettes in which the flavors were offered [41].
2.3. Behavioral Procedures
Implanted and neurologically intact animals were initially subjected to a concurrent stimulation-induced place preference test to classify them (Procedure 1) for the subsequent conditioning tasks (Procedure 2A or 2B, see below) that were similar in the structure and stimulus cues used but differed in the stimulus (flavor vs. placement) associated with electrical stimulation of the LPBe.
2.4. Concurrent Electrical Stimulation-Induced Place Preference (Procedure 1)
After the recovery period, all animals (implanted and neurologically intact controls) underwent two 10-min sessions of an unbiased, counterbalanced concurrent stimulation-induced place preference procedure, separated by a 24-h interval. Unilateral electrical stimulation of the LPBe was administered concurrently with the voluntary stay of the animal in one of the two lateral compartments of the maze, previously selected at random and used for both sessions. The total time that each subject remained in the stimulated compartment was recorded for each session as the dependent variable. The process was identical for rats in the intact control group, except that there was no brain stimulation.
All electrically stimulated were classified as positive, negative, or neutral according to behavioral criteria used in previous studies {positive = stay in stimulated compartment for >50% of session time; negative group = stay for <30% of the session time in stimulated compartment; and neutral = stay between 30% - 50% of the time in the stimulated compartment [20-22]}. Animals included in the neutral group were no longer stimulated and used as implanted control groups in subsequent procedures (2A and 2B).
2.5. Conditioned Taste Discrimination with Simultaneous Flavor and Placement Cues (Procedure 2A)
Two pre-training sessions were carried out with animals that were deprived of water for 23 h 50 min and then allowed to drink tap water for 10 min from graduated burettes. The burettes were placed alternatively in the left or right holes of the front panel of the cage to avoid the development of place preferences. Next, after withdrawal of the water, animals had access to 20 g of food.
Subsequently, all animals in this group (23 implanted and 8 neurologically intact rats) were subjected to a conditioning task in which one of the taste stimuli was associated with electrical stimulation of the LPBe. The initially neutral stimuli “strawberry” (S) and “coconut” (C) (McCormick, San Francisco, CA), diluted in 0.5% water, were presented for 14 min on alternate days in graduated burettes. At 7 minutes after starting to drink one of these flavors (previously selected), the subjects were electrically stimulated for 7 minutes, while the flavor remained available for animals through the burette. The position of each burette in the left or right orifice was changed throughout the tests (see Table 1).
Half of the animals were stimulated in association with S and half with C-flavored solution intake. At the end of the acquisition phase, each subject would have undergone two trials with one of the flavors associated with electrical stimulation of the LPBe, although in a different position (left/right) each time. Animals in the control groups did not receive brain stimulation in any case.
A two-bottle free choice test (test I) was conducted on day 5 by placing two burettes in the cage at the same time, each containing one of the two flavored stimuli previously used during the training sessions. During this phase, animals were allowed to freely drink the flavored solutions for 7 minutes and the total intake of each solution was recorded. The animals were connected to the stimulator throughout the test but no current was administrated. This procedure was repeated in two additional trials, and a second choice test was then conducted (Test II). We calculated the percentage preference, i.e., the consumption of taste stimulus associated with the electrical stimulation of the LPBe with respect to the total amount of liquid consumed by each animal in each test.
After ending each experimental session, the animals
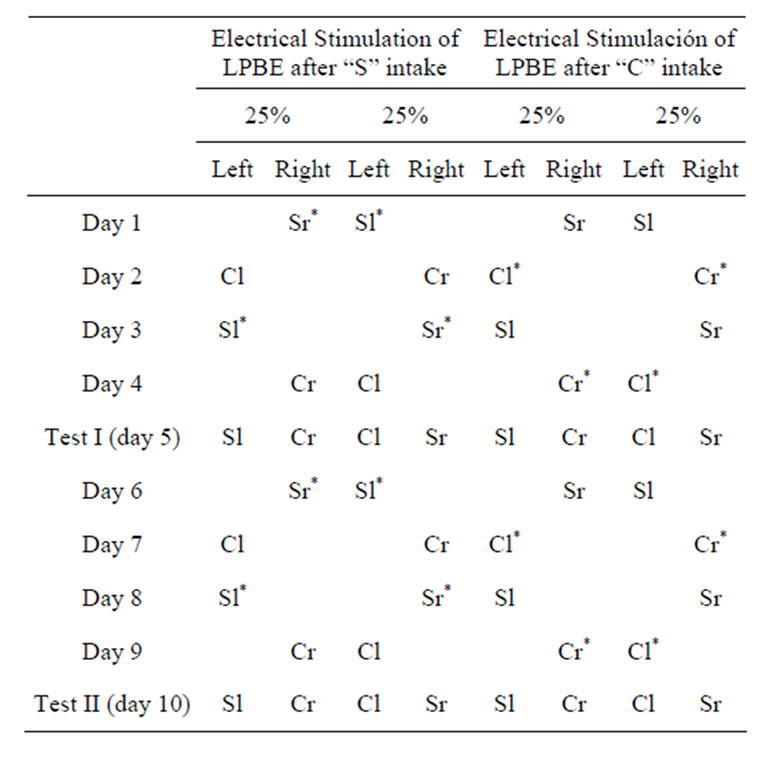
Table 1. Experimental design (Procedure 2A) used in the rewarding flavor discrimination tests (“S”: strawberry, “C”: coconut, “l”: left side, “r”: right side; *Stimulated flavor).
received 20 g of solid food in their home cages. White noise was used to mask any possible sounds that could alter experimental conditions.
2.6. Conditioned Placement Discrimination with Simultaneous Flavor and Place Cues (Procedure 2B)
One group of 23 implanted animals and another of 8 neurologically intact animals underwent a procedure that was identical to Procedure 2A above, except that the left or right position of the taste/olfactory stimulus and not the flavor stimulus itself was associated with electrical stimulation of the LPBe.
After two pre-training days (see above), the experimental phase began, in which the burettes with the neutral taste stimuli S and C were presented in left or right position in association with electrical stimulation of the LPBe, regardless of the flavor ingested. In tests I and II, we recorded the amount of flavor stimuli ingested from each of the two positions (Table 2) and calculated the percentage preference for the taste stimulus associated with the stimulated place with respect to the total amount of liquid consumed by each animal in each test.
2.7. Histology
After concluding the behavioral tests, animals were deeply anesthetized with a sodium pentothal overdose and intracardially perfused with isotonic saline and 4% formaldehyde. Localization of the electrodes in the LPBe was tested with a small electrolytic lesion (0.3 mA of cathodic current for 5 s). The brains were then removed
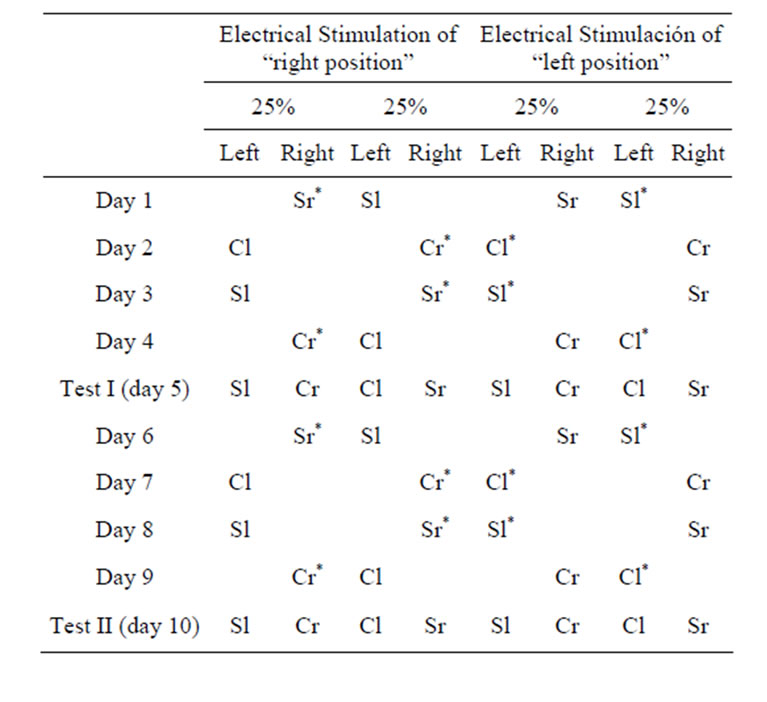
Table 2. Experimental design (Procedure 2B) used in the rewarding place discrimination tests (“S”: strawberry, “C”: coconut, “l”: left side, “r”: right side; *Stimulated placement).
and stored in formaldehyde for at least 1 week before their subsequent lamination in 40-μ sections with a cryostat (Microm HM 550, Microm International GmbH, Walldorf). Sections were mounted, stained with Cresyl violet, and photographed (SZ-61 microscope and Altra 20 Soft Imaging System, Olympus, Tokyo, Japan). Figure 1 depicts the results of the histological study.
2.8. Statistical Analysis
Statistical version 6.0 (Statsoft Inc., OK) was used for the statistical analyses. Pearson’s correlation coefficient contrast was used to confirm the within-subject consistency of the preferences/aversions of the animals towards the stimulated compartment (Procedure 1).
Two 2-factor ANOVAs (group x test) were performed to analyze the effects of conditioning with taste and place cues, respectively (Procedures 2A and 2B). Pairwise planned comparisons were carried out in order to determine whether the stimulation generates modifications in preferences for the taste stimulus associated with the LPBe activation or for the stimulated place in experimental versus control animals.
3. Results
One animal in Group A was excluded because the implant became detached, and two animals in Group A and three animals in Group B were excluded for circling behavior. Therefore, the final study sample comprised 20 implanted and 8 neurologically intact animals in each of the two experimental procedures.
3.1. Ongoing Stimulation-Induced Place Preferences (Procedure 1)
The performance of the implanted animals showed a sig-
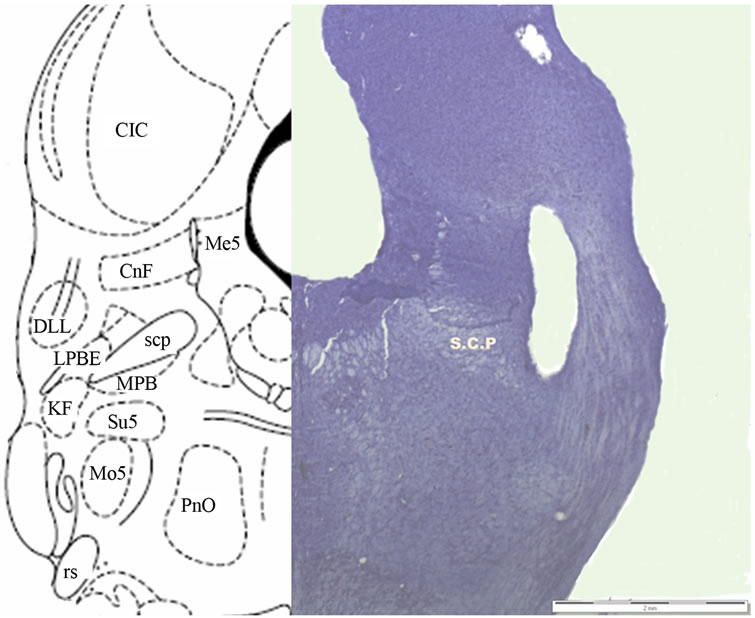
Figure 1. Histological localization of the electrode in the LPBe.
nificant correlation between the two cCPP trials [r = 0.467, p < 0.038* for group A (Figure 2) and r = 0.798 p < 0.00002* for Group B (Figure 3)], suggesting a consistent preference or rejection behavior towards the stimulated compartment. In contrast, animals that had not received stimulation (neurologically intact control groups) moved randomly between the two compartments of the maze [r = −0.462, p < 0.2480 for unimplanted animals in Group A and r = 0.553, p < 0.1550 for unimplanted animals in Group B]. According to the behavioral criterion established in Concurrent electrical stimulation-induced Place Preference (Procedure 1) section, 11 of the stimu-
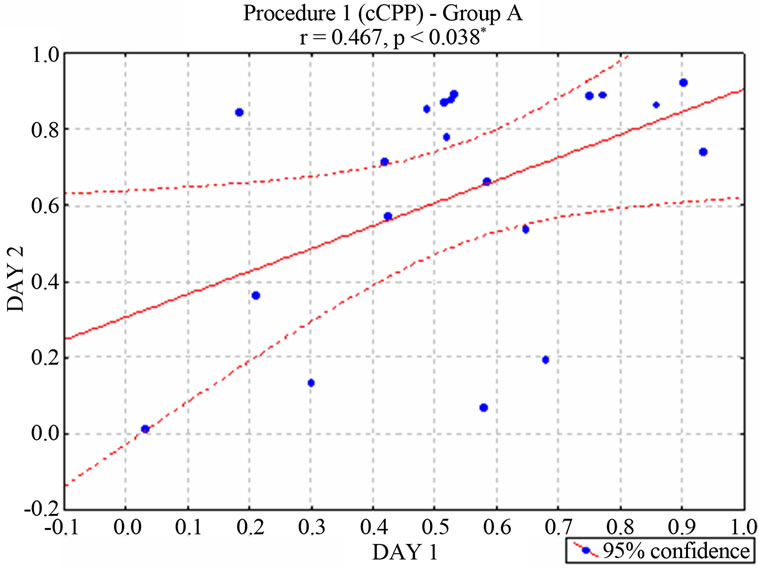
Figure 2. Correlation between the two ongoing stimulationinduced Place Preference trials–percentage of preferencein rats of the Group A. (According with the behavioral criterion defined in Procedure 1, 11 animals were considered positive, 3 negative and 6 neutral. Subjects of this neutral group were no longer stimulated).
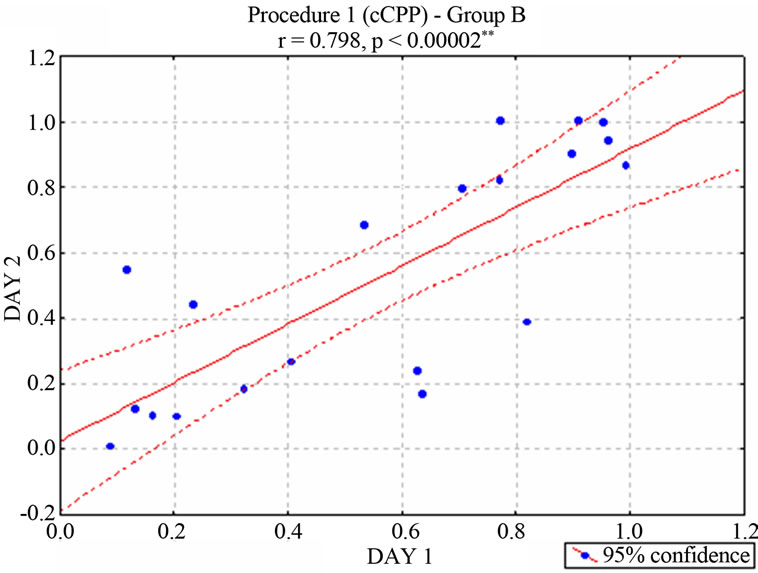
Figure 3. Correlation between the two ongoing stimulationinduced Place Preference trials–percentage of preference-in rats of the Group B. (According with the behavioral criterion defined in Procedure 1, 9 animals were considered positive, 6 negative and 5 neutral. Subjects of this neutral group were no longer stimulated).
lated animals in Group A were positive, 3 were negative, and 6 neutral, while 9 of the stimulated animals in Group B were positive, 6 were negative, and 5 neutral. The negative, neutral, positive, and intact animals were considered as independent groups.
3.2. Conditioned Taste Discrimination with Simultaneous Flavor and Place Cues (Procedure 2A)
Figure 4 depicts the percentage preferences for the taste stimulus associated with LPBe stimulation. The Group x Test ANOVA showed a significant effect of Group factor [F3,24 = 3.2539, p < 0.0392*].
Planned Comparisons between pairs of groups revealed that the overall results of the POSITIVE group significantly differed from those of the NEGATIVE group [F1,24 = 4.4858, p < 0.0447*] and the IMPLANTED control group [F1,24 = 5.4837, p < 0.0278*]. In fact, the positive group showed an increased preference for the taste stimulated in Test II [F(1,24) = 6.8991, p < 0.0147*], which was not observed in the other groups [ImplantedControl: F(1,24) = 0.1993, p < 0.6592; Negative: F(1,24) = 0.0437, p < 0.8360; Intact-Control: F(1,24) = 0.9268, p < 0.3452] .
Although no difference in overall liquid intake was found between the POSITIVE and INTACT control groups [F1,24 = 0.0014, p < 0.9705], they showed opposite tendencies towards the “preferred” taste stimulus (associated with LPBe stimulation) [Positive group vs. Intact control group in Test 1 vs. Test 2: F1,24 = 5.9385, p < 0.0226*].
Initially, no differences were found between the posi-
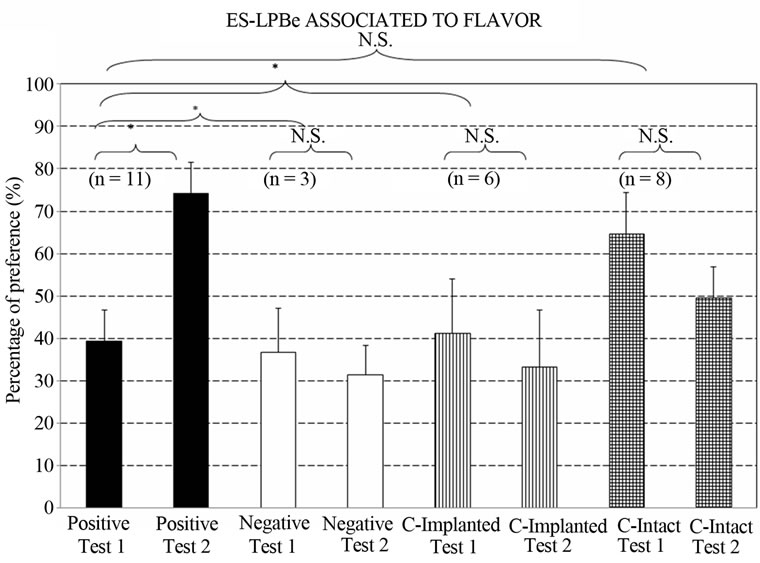
Figure 4. Percentage preference for the flavor associated with electrical stimulation of the LPBe in Tests I and II of flavor discrimination. (Groups: Positive; Negative; ControlImplanted; Control-Intact. Test I: after 4 conditioning trials and Test II: after 8 conditioning trials. [*] = p < 0.05; N.S. = not significant) (n = animals per group).
tive and negative group (Test I: F1,24 = 0.0216, p < 0.8842), but their behaviors diverged with a larger number of association trials “flavor ↔ LPBe electrical stimulation” (Test II: F1,24 = 6.135, p < 0.0206*).
Implanted and intact control groups differed in liquid intake [F1,24 = 4.9851, p < 0.0351*], whereas no differences were observed in their performance in either test [F1,24 = 0.0850, p. < 0.7722].
3.3. Conditioned Placement Discrimination with Simultaneous Flavor and Place Cues (Procedure 2B)
Figure 5 depicts the percentage preferences for the flavored-stimulus consumed in the “stimulated” place. The Group x Test ANOVA showed a significant effect of the interaction [F3,24 = 3.27, p < 0.0386*].
Group by group planned comparisons only revealed differences between test I and II in the POSITIVE group [F1,24 = 7.2228, p < 0.0128*], with an increased intake of the flavored liquid presented in the “stimulated place”.
Additionally, comparison of the positive group with the two control groups considered together revealed a greater tendency by the positive group to consume the taste stimulus presented in the stimulated place (Test I vs. Test II: F1,24 = 5.3725, p < 0.0293*).
Finally, no significant difference in total intake was found between the positive and negative groups [F1,24 = 0.0030 p < 0.9566], but their intakes diverged as a consequence of learning (Test I vs. Test II: F1,24 = 4.7965, p < 0.0384*).
4. Discussion
Ongoing electrical stimulation of the LPBe generates concurrent place preferences ([20-23], the first part of this study). An additional and novel finding of this study is
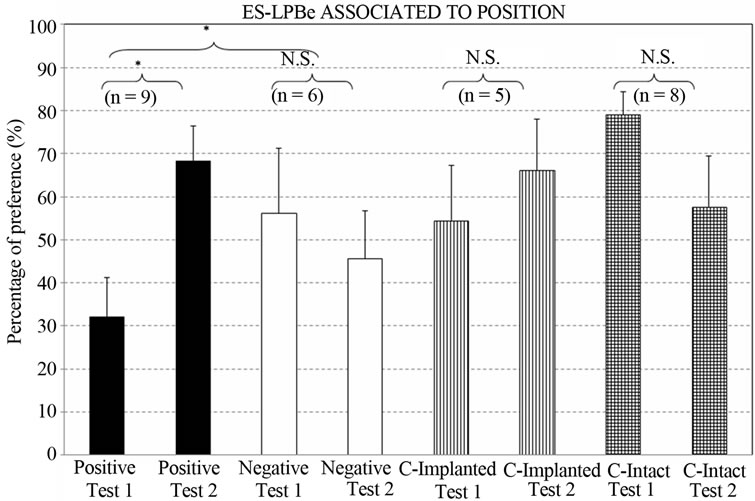
Figure 5. Percentage preference for the taste stimulus associated with the stimulated place. (Groups: Positive; Negative; Control-Implanted; Control-Intact. Test I: after 4 conditioning trials; Test II: after 8 conditioning trials. [*] = p < 0.05; N.S. = not significant) (n = animals per group).
that electrical stimulation of this pontine subnucleus immediately after exposure to the stimulus can also induce standard conditioned place preferences and specific flavor preferences; this result was not obtained in neurologically intact (non-stimulated) control rats. However, we also observed a subgroup of “negative” animals, and a third subgroup of “indifferent” animals, which showed no consistent preference or rejection behavior during the ongoing electrical stimulation procedure. This “indifferent” group was used as an “implanted non-stimulated control group” in subsequent conditioning procedures.
It has repeatedly been shown that a single brain nucleus may be the substrate for both appetitive and aversive motivational processes [42-44]. With respect to the electrical brain stimulation, the stainless steel 00 electrodes used in our study can activate cell bodies, initial axon segments and Ranvier nodules within a small spherical field of electrical influence [45-47]. This may allow dissociation among different functional systems that are anatomically very close to the electrode tip [46], and it would only require a modification of current parameters to active some or other systems. Specifically, electrical stimulation of the LPBe may simultaneously activate opposite behavioral processes, as observed with the stimulation of other brain areas (e.g., eating, drinking, selfstimulation, aversion, etc.) after LH stimulation [47,48] or pain and analgesia after PAG stimulation [49,50]. This would explain the existence of the three behaviorallydifferentiated groups in our study.
In the first part of this study, in which a concurrent stimulation-induced place preference test was conducted, we replicated the results of previous studies, in which animals show preferences (or aversions) for the compartment associated with simultaneous electrical stimulation of the LPBe in a rectangular maze where they can move around freely [20-22]. These data are compatible with studies that include the LPBe among the brain areas involved in the processing of various foods and/or reinforcing substances, e.g., lactose, sucrose, glucose, polycose, saccharine···, [12-14,51]. Likewise, it has been observed that the administration of drugs such as fenfluramine [15,16], amphetamines [18] or opiates [19] may induce C-fos immunoreactivity in different brain regions, and among them, the LPBe.
Our findings are in agreement with published data on conditioned place preferences with liquids or food administered to animals confined within a specific compartment of a T-Maze [24,52,53]. In addition, Bechara et al. (1993) [31] showed that lesions of the dorsolateral end of the parabrachial area, supposedly including the LPBe, blocked aversive place conditioning induced by peripheral morphine.
In the present investigation, we verified that animals can associate a place (the site of taste stimulus presentation) with subsequent electrical stimulation of the LPBe, regardless of the taste stimulus and despite the potential interference of its presence. We observed the contrary effect in the small group of “negative” animals in which electrical stimulation of the LPBe produced aversive effects, who learned a preference for the place not associated with stimulation of the LPBe [20-22]. We also show that the reinforcing electrical activation can sustain a standard conditioned place preference, i.e., a discriminative learning in a choice test between two simultaneously presented stimuli in the absence of stimulation.
By using a similar procedure, animals underwent a specific conditioned taste discrimination task in which they were simultaneously offered 2 flavors in 2 different positions (left/right) that were varied throughout the learning (Procedure 2A). Under these circumstances, animals develop specific preferences for the flavor stimuli associated with electrical stimulation of the LPBe, regardless of the place of their presentation, allowing dissociation between the two stimulus indexes (place/ flavor). It was previously reported that this stimulation generated preferences for associated taste stimuli, but the flavors were presented in fixed positions [20,23]. Likewise, LPBe lesions were previously found to interrupt the association between a flavor and the intragastric infusion of rewarding foods [11] and between a flavor and the administration of aversive products [9], but the flavors were always presented in fixed positions in these studies. In other words, unlike in the present study, it was not possible to dissociate between taste stimuli and other space, proprioceptive, or place indexes presented in the learning setting.
The global consumption of taste-olfactory stimuli by our non-stimulated control animals did not differ from that of the positive or negative groups, but they showed no consistent preference or rejection behaviors towards a specific place or flavor at any stage of the learning.
The procedure used in the present study implies temporal contiguity between the stimuli (taste/place and brain stimulation) and also means that the animals benefit from an increase in the number of trials ([20-23], present study). These results are similar to those obtained in certain stressful situations (acute or chronic stress, emotionally arousing material, and even drug addiction), which induce implicit learning modalities (learned implicit behaviors) that promote stereotyped behaviors and may involve the participation of certain visceral pathways [54-56]. In fact, the LPBe constitutes one of the main central relays in the processing of visceral cues [1,4-6], and specific lesions of this visceral brain subnucleus impair implicit but not explicit taste aversive learning [9-11]. Implicit learning requires time contiguity between stimuli, benefits from repetition, and the acquisition and retention process is rigid [9-11,57].
Nonetheless, some learning modalities evidence a biological sensory constraint that favors the establishment of selective stimulus associations, e.g. tastes with gastrointestinal malaise or light/noise with shock [26,27,57]. Moreover, rewarding substances (e.g., morphine) can simultaneously produce conditioned taste aversion and conditioned place preference at similar dosages [31], preferentially associating their aversive properties with flavors and their rewarding effects with environmental cues present at the time of administration. Parker et al. suggested that the avoidance reactions generated by taste stimuli associated with drugs of abuse (e.g., morphine) differ from the disgust produced by tastes associated with some noxious products such as lithium chloride [58-60].
In the present study, no difference in terms of specificity was observed between the learned taste and place tasks. Given that the reinforcing effect of ongoing electrical stimulation of the LPBe can be blocked by naloxone administration, an opiate mechanism might be involved [20,22]. In this regard, it has been proposed that an opiate system may participate in positive and negative motivational processes through differential action on µ and κ receptors in this parabrachial area [35,36,61].
The effect of natural substances such as food [34,36] and of certain drugs [13,14,18,62,63] may be exerted via the LPBe. These reinforcing effects may modify the hedonic component and/or motivational value of stimuli present in a given setting, acting as incentives that guide behavior regardless of their nature [64,65]. Thus, preferences have been induced for places where animals find liquid, food, or sexual reward [24,52,53,65,66]. Conversely, administration of the opiate antagonist naloxone has inhibited the hedonic effect under different experimental conditions, attenuating the startle response in the presence of appetizing foods (sweetened condensed milk) and reducing the voluntary consumption or CPP associated with their intake [38]. More specifically, lesions of the whole lateral parabrachial area or of the LPBe alone abolished the rewarding effects of appetizing foods [67], intragastric nutrients [11], and morphine [31], and these rewarding effects were also inhibited by pharmacological blockade with naloxone [20,22,35].
In conclusion, this study demonstrates that animals can develop standard conditioned preferences/aversions towards specific flavors or place stimuli associated with subsequent electrical stimulation of the LPBe. The reinforcing effect of LPBe stimulation does not appear to be determined by biological sensory constraints towards a given type of stimuli, as is the case in taste aversion or exteroceptive learning. It appears that, in our procedure, this general rewarding effect may initially be conditioned to any type of cue, regardless of the stimulus modality.
5. Acknowledgements
This research was supported in part by the University of Granada and Spanish Ministry of Education and Culture (National R + D Plan PB98-1284; BSO2003-06627 and PSI2010-17400).
The authors are grateful to Richard Davies for assistance with the English version of this paper. Part of this study was submitted by the first author for her PhD degree in Psychology (Psychobiology) at the University of Granada. Part of this study was presented in abstract form at the XIII Congress of the Spanish Society of Neuroscience in Salamanca, Spain.
REFERENCES
- C. E. Fulwiler and C. B. Saper, “Subnuclear Organization of the Efferent Connections of the Parabrachial Nucleus in the Rat,” Brain Research, Vol. 319, No. 3, 1984, pp. 229-259.
- C. B. Halsell and S. P. Travers, “Anterior and Posterior Oral Cavity Responsive Neurons Are Differentially Distributed among Parabrachial Subnuclei in Rat,” Journal of Neurophysiology, Vol. 78, No. 2, 1997, pp. 920-938.
- S. Papas and A. V. Ferguson, “Electrophysiological Characterization of Reciprocal Connections between the Parabrachial Nucleus and the Area Postrema in the Rat,” Brain Research Bulletin, Vol. 24, No. 4, 1990, pp. 577- 582. doi:10.1016/0361-9230(90)90162-S
- S. De Lacalle and C. R. Saper, “Calcitonin Gene-Related Peptide-Like Immunoreactivity Marks Putative Visceral Sensory Pathways in Human Brain,” Neuroscience, Vol. 100, No. 1, 2000, pp. 115-130. doi:10.1016/S0306-4522(00)00245-1
- H. Karimnamazi, S. P. Travers and J. B Travers, “Oral and Gastric Input to the Parabrachial Nucleus of the Rat,” Brain Research, Vol. 957, No. 2, 2002, pp. 193-206. doi:10.1016/S0006-8993(02)03438-8
- I. E. De Araujo, “Gustatory and Homeostatic Functions of the Rodent Parabrachial Nucleus,” Annals of the New York Academy of Sciences, Vol. 1170, 2009, pp. 383-391. doi:10.1111/j.1749-6632.2009.03923.x
- T. Yamamoto, M. Takemura, T. Inui, K. Torii, N. Maeda, M. Ohmoto, I. Matsumoto and K. Abe, “Functional Organization of the Rodent Parabrachial Nucleus,” Annals of the New York Academy of Sciences, Vol. 1170, 2009, pp. 378-382. doi:10.1111/j.1749-6632.2009.03883.x
- K. Hashimoto, K. Obata and H. Ogawa, “Characterization of parabrachial subnuclei in mice with regard to salt tastants: posible independence of taste relay from visceral processing,” Chemical Senses, Vol. 34, 2009, pp. 253-267. doi:10.1093/chemse/bjn085
- C. Mediavilla, F. Molina and A. Puerto, “The Role of the Lateral Parabrachial Nuclei in Concurrent and Sequential Taste Aversion Learning in Rats,” Experimental Brain Research, Vol. 134, No. 4, 2000, pp. 497-505. doi:10.1007/s002210000497
- C. Mediavilla, F. Molina and A. Puerto, “Effects of a Flavor-Placement Reversal Test after Different Modalities of Taste Aversion Learning,” Neurobiology of Learning and Memory, Vol. 76, No. 2, 2001, pp. 209-224. doi:10.1006/nlme.2000.3990
- M. A. Zafra, M. J. Simon, F. Molina and A. Puerto, “The Role of the External Lateral Parabrachial Subnucleus in Flavor Preferences Induced by Pre-Digested Food Administered Intragastrically,” Brain Research, Vol. 950, No. 1-2, 2002, pp. 155-164. doi:10.1016/S0006-8993(02)03032-9
- L. Wang, S. Cardin, V. Martinez, I. Tache and C. K. Lloyd, “Duodenal Loading with Glucose Induces Fos Expression in Rat Brain: Selective Blockade by Devazepide,” American Journal of Physiology, Vol. 277, No. 3, 1999, pp. R667-R674.
- T. Yamamoto and K. Sawa, “C-Fos-Like Immunoreactivity in the Brainstem Following Gastric Loads of Various Chemical Solutions in Rats,” Brain Research, Vol. 866, 2000, pp. 135-143. doi:10.1016/S0006-8993(00)02241-1
- T. Yamamoto and K. Sawa, “Comparison of c-Fos-Like Immunoreactivity in the Brainstem Following Intraoral and Intragastric Infusions of Chemical Solutions in Rats,” Brain Research, Vol. 866, No. 1-2, 2000, pp. 144-151. doi:10.1016/S0006-8993(00)02242-3
- B. H. Li and N. E. Rowland, “Effects of Vagotomy on Cholecystokininand Dexfenfluramine-Induced Fos-Like Immunoreactivity in the Rat Brain,” Brain Research Bulletin, Vol. 37, No. 6, 1995, pp. 589-593. doi:10.1016/0361-9230(95)00045-G
- R. Trifunovic and S. Reilly, “Medial versus Lateral Parabrachial Nucleus Lesions in the Rat: Effects Cholecystokininand D-Fenfluramine-Induced Anorexia,” Brain Research, Vol. 894, No. 2, 2001, pp. 288-296. doi:10.1016/S0006-8993(01)02037-6
- C. F. Elias, F. Kelly, C. E. Lee, R. S. Ahima, D. J. Drucker, C. B. Saper and J. K. Elmquist, “Chemical Characterization of Leptin-Activated Neurons in the Rat Brain,” Journal of Comparative Neurology, Vol. 423, No. 2, 2000, pp. 261-281. doi:10.1002/1096-9861(20000724)423:2<261::AID-CNE6>3.0.CO;2-6
- N. Sakai and T. Yamamoto, “Conditioned Taste Aversion and c-Fos Expression in the Rat Brainstem after Administration of Various USs,” Neuroreport, Vol. 8, No. 9-10, 1997, pp. 2215-2220. doi:10.1097/00001756-199707070-00025
- N. L. Chamberlin, A. Mansour, S. J. Watson and C. B. Saper, “Localization of Mu-Opioid Receptors on Amygdaloid Projection Neurons in the Parabrachial Nucleus of the Rat,” Brain Research, Vol. 827, No. 1-2, 1999, pp. 198-204. doi:10.1016/S0006-8993(99)01168-3
- M. J. Simon, R. García, M. A. Zafra, F. Molina and A. Puerto, “Learned Preferences Induced by Electrical Stimulation of a Food-Related Area of the Parabrachial Complex: Effects of Naloxone,” Neurobiology of Learning & Memory, Vol. 87, No. 3, 2007, pp. 332-342. doi:10.1016/j.nlm.2006.09.009
- M. J. Simon, F. Molina and A. Puerto, “Conditioned Place Preference But Not Rewarding Self-Stimulation after Electrical Activation of the External Lateral Parabrachial Nucleus,” Behavioral Brain Research, Vol. 205, No. 2, 2009, pp. 443-449. doi:10.1016/j.bbr.2009.07.028
- M. J. Simon, R. Garcia and A. Puerto, “Concurrent Stimulation-Induced Place Preference in Lateral Hypothalamus and Parabrachial Complex: Differential Effects of Naloxone,” Behavioral Brain Research, Vol. 225, No. 1, 2011, pp. 311-316. doi:10.1016/j.bbr.2011.07.029
- M. J. Simon, M. A. Zafra, F. Molina and A. Puerto, “Consistent Rewarding or Aversive Effects of the Electrical Stimulation of the Lateral Parabrachial Complex,” Behavioral Brain Research, Vol. 190, No. 1, 2008, pp. 67-73. doi:10.1016/j.bbr.2008.02.036
- T. Spiteri, G. Le Pape and A. Agmo, “What Is Learned during Place Preference Conditioning? A Comparison of Foodand Morphine-Induced Reward,” Psychobiology, Vol. 28, No. 3, 2000, pp. 367-382.
- L. A. Parker, “Taste Avoidance and Taste Aversion: Evidence for Two Different Processes,” Learning and Behavior, Vol. 31, No. 2, 2003, pp. 165-172. doi:10.3758/BF03195979
- J. Garcia, W. G. Hankins and K. W. Rusiniak, “Behavioral Regulation of the Milieu Interne in Man and Rat,” Science, Vol. 185, 1974, pp. 824-831. doi:10.1126/science.185.4154.824
- M. Domjan, “Pavlovian Conditioning: A Functional Perspective,” Annual Review of Psychology, Vol. 56, 2005, pp. 179-206. doi:10.1146/annurev.psych.55.090902.141409
- A. Bechara and D. Van der Kooy, “The Tegmental Pedunculopontine Nucleus: A Brain-Stem Output of the Limbic System Critical for the Conditioned Place Preferences Produced by Morphine and Amphetamine,” The Journal of Neuroscience, Vol. 9, No. 10, 1989, pp. 3400- 3409.
- A. Bechara and D. Van der Kooy, “A Single Brain Stem Substrate Mediates the Motivational Effects of Both Opiates and Food in Nondeprived Rats But Not in Deprived rats,” Behavioral Neuroscience, Vol. 106, No. 2, 1992, pp. 351-363. doi:10.1037/0735-7044.106.2.351
- A. Bechara and D. Van der Kooy, “Lesions of the Tegmental Pedunculopontine Nucleus: Effects on the Locomotor Activity Induced by Morphine and Amphetamines,” Pharmacology, Biochemistry & Behavior, Vol. 42, 1992, pp. 9-18. doi:10.1016/0091-3057(92)90438-L
- A. Bechara, G. M. Martin, A. Pridgar and D. Van der Kooy, “The Parabrachial Nucleus: A Brain Stem Substrate Critical for Mediating the Aversive Motivational Effects of Morphine,” Behavioral Neuroscience, Vol. 107, No. 1, 1993, pp. 147-160. doi:10.1037/0735-7044.107.1.147
- T. V. Jaeger and D. Van der Kooy, “Morphine Acts in the Parabrachial Nucleus, a Pontine Viscerosensory Relay, to Produce Discriminative Stimulus Effects,” Psychopharmacology, Vol. 110, No. 1-2, 1993, pp. 76-84. doi:10.1007/BF02246953
- T. V. Jaeger and D. Van der Kooy, “Separate Neural Substrates Mediate the Motivating and Discriminative Properties of Morphine,” Behavioral Neuroscience, Vol. 110, No. 1, 1996, pp. 181-201. doi:10.1037/0735-7044.110.1.181
- K. D. Carr, D. O. Aleman, T. H. Bak and E. J. Simon, “Effects of Parabrachial Opioid Antagonism on Stimulation-Induced Feeding,” Brain Research, Vol. 545, No. 1-2, 1991, pp. 283-286. doi:10.1016/0006-8993(91)91298-F
- S. Moufid-Bellancourt, R. Razafimanalina and L. Velley, “Interaction between μ and κ Receptors Located in the Parabrachial Area in the Opioid Control of Preference Threshold for Saccharine: Modulatory Role of Lateral Hypothalamic Neurons,” Behavioral Pharmacology, Vol. 7, No. 8, 1996, pp. 798-809.
- T. D. Wolinsky, K. D. Carr, J. M. Hiller and E. J. Simon, “Chronic Food Restriction Alters μ and κ Opioid Receptor Binding in the Parabrachial Nucleus of the Rat: A Quantitative Autoradiographic Study,” Brain Research, Vol. 706, No. 2, 1996, pp. 333-336. doi:10.1016/0006-8993(95)01337-7
- J. D. Wilson, D. M. Nicklous, V. J. Aloyo and K. J. Simansky, “Peptides that Regulate Food Intake. An Orexigenic Role for μ-Opioid Receptors in the Lateral Parabrachial Nucleus,” American Journal of Physiology, Vol. 285, No. 5, 2003, pp. R1055-R1065.
- M. Schneider, V. Heise and R. Spanagel, “Differential Involvement of the Opioid Receptor Antagonist Naloxone in Motivational and Hedonic Aspects of Reward,” Behavioral Brain Research, Vol. 208, No. 2, 2010, pp. 466- 472. doi:10.1016/j.bbr.2009.12.013
- L. Parker, A. Failor and K. Weidman, “Conditioned Preferences in the Rat with an Unnatural Need State: Morphine Withdrawal,” Journal of Comparative Physiological Psychology, Vol. 82, No. 2, 1973, pp. 294-300. doi:10.1037/h0033921
- G. Paxinos and C. Watson, “The Rat Brain in Stereotaxic Coordinates,” 4th Edition, Academic Press, San Diego, 2005.
- C. Mediavilla, F. Molina and A. Puerto, “Bilateral Lesions in the Cerebellar Interpositus-Dentate Region Impair Taste Aversion Learning in Rats,” Physiology & Behavior, Vol. 65, No. 1, 1998, pp. 25-33. doi:10.1016/S0031-9384(98)00083-3
- J. D. Salamone, “The Involvement of Nucleus Accumbens Dopamine in Appetitive and Aversive Motivation,” Behavioral Brain Research, Vol. 61, No. 2, 1994, pp. 117-133. doi:10.1016/0166-4328(94)90153-8
- D. Small, M. D. Gregory, Y. E. Mark, D. Gitelman, M. M. Mesulam and T. Parrish, “Dissociation of Neural Representation of Intensity and Affective Valuation in Human Gestation,” Neuron, Vol. 39, No. 4, 2001, pp. 701-711. doi:10.1016/S0896-6273(03)00467-7
- S. M. Reynolds and K. C. Berridge, “Positive and Negative Motivation in Nucleus Accumbens Shell: Bivalent Rostrocaudal Gradients for GABA-Elicitated Eating, Taste ‘Liking’/‘Disliking’ Reactions, Place Preference/ Avoidance, and Fear,” Journal of Neuroscience, Vol. 22, No. 16, 2002, pp. 7308-7320.
- J. B. Rank, “Which Elements Are Excited in Electrical Stimulation of Mammalian Central Nervous System: A Review,” Brain Research, Vol. 98, No. 3, 1975, pp. 417- 440. doi:10.1016/0006-8993(75)90364-9
- J. S. Yeomans, “Principles of Brain Stimulation,” Oxford University Press, New York, 1990.
- R. D. Hawkins, P. L. Roll, A. Puerto and J. S. Yeomans, “Refractory Periods of Neurons Mediating StimulationElicited Eating and Brain Stimulation Reward: Interval Scale Measurement and a Test of a Model of Neural Integration,” Behavioral Neuroscience, Vol. 97, No. 3, 1983, pp. 416-432. doi:10.1037/0735-7044.97.3.416
- A. Gratton and R. A Wise, “Brain Stimulation Reward in the Lateral Hypothalamic Medial Forebrain Bundle: Mapping of Bourdaries and Homogeneity,” Brain Research, Vol. 274, No. 1, 1983, pp. 25-30. doi:10.1016/0006-8993(83)90518-8
- D. J. Mayer, T. L. Wolfle, H. Akil, B. Carder and J. C. Liebeskind, “Analgesia from Electrical Stimulation in the Brainstem of the Rat,” Science, Vol. 174, No. 4016, 1971, pp. 1351-1354. doi:10.1126/science.174.4016.1351
- W. A. Prado and M. H Roberts, “An Assessment of the Antinociceptive and Aversive Effects of Stimulating Identified Sites in the Rat Brain,” Brain Research, Vol. 340, No. 2, 1985, pp. 219-228. doi:10.1016/0006-8993(85)90917-5
- T. Yamamoto, T. Shimura, N. Sakai and N. Ozaki, “Representation of Hedonics and Quality of Taste Stimuli in the Parabrachial Nucleus of the Rat,” Physiology & Behavior, Vol. 56, No. 6, 1994, pp. 1197-1202. doi:10.1016/0031-9384(94)90366-2
- C. Arnold and A. Agmo, “The Importance of the Stomach for Conditioned Place Preference Produced by Drinking Sucrose in Rats,” Psychobiology, Vol. 27, No. 4, 1999, pp. 541-546.
- T. L. Stefurak and D. van der Kooy, “Saccharin’s Rewarding, Conditioned Reinforcing, and Memory-Improving Properties: Mediation by Isomorphic or Independent Processes?” Behavioral Neuroscience, Vol. 106, No. 1, 1992, pp. 125-139. doi:10.1037/0735-7044.106.1.125
- L. Schwabe, O. T. Wolf and M. S. Oitzl, “Memory Formation under Stress: Quantity and Quality,” Neuroscience & Biobehavioral Reviews, Vol. 34, No. 4, 2010, pp. 584- 591. doi:10.1016/j.neubiorev.2009.11.015
- L. Schwabe, O. T. Wolf and A. Dickinson, “Stress, Habits, and Drug Addiction: A Psychoneuroendocrinological Perspective,” Experimental and Clinical Psychopharmacology, Vol. 19, No. 1, 2011, pp. 53-63. doi:10.1037/a0022212
- L. Schwabe, M. Joëls, B. Roozendaal, O. T. Wolf and M. S. Oitzl, “Stress Effects on Memory: An Update and Integration,” Neuroscience & Biobehavioral Reviews, Vol. 36, No. 7, 2012, pp. 1740-1749. doi:10.1016/j.neubiorev.2011.07.002
- A. Chung, S. K. Barot, J. J. Kim and I. L. Bernstein, “Biologically Predisposed Learning and Selective Associations in Amygdalar Neurons,” Learning & Memory, Vol. 18, No. 6, 2011, pp. 371-374. doi:10.1101/lm.2053711
- L. A. Parker, “Rewarding Drugs Produce Taste Avoidance, but Not Taste Aversion,” Neuroscience & Biobehavioral Reviews, Vol. 19, No. 1, 1995, pp. 143-151. doi:10.1016/0149-7634(94)00028-Y
- L. A. Parker, J. A. Cyr, A. N. Santi and P. D. Burton, “The Aversive Properties of Acute Morphine Dependence Persist 48 h after a Single Exposure to Morphine. Evaluation by Taste and Place Conditioning,” Pharmacology, Biochemistry & Behavior, Vol. 72, No. 1-2, 2002, pp. 87-92. doi:10.1016/S0091-3057(01)00724-9
- [61] S. Peciña, K. C. Berridge and L. A. Parker, “Pimozide Does Not Shift Palatability: Separation of Anhedonia from Sensoriomotor Suppression by Taste Reactivity,” Pharmacology, Biochemistry & Behavior, Vol. 58, No. 3, 2002, pp. 801-811. doi:10.1016/S0091-3057(97)00044-0
- [62] R. Spanagel, A. Herz and T. S. Shippenberg, “Opposing Tonically Active Endogenous Opioid Systems Modulate the Mesolimbic Dopaminergic Pathway,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 89, No. 6, 1992, pp. 2046-2050. doi:10.1073/pnas.89.6.2046
- [63] H. B. Gutstein, J. L. Thome, J. L. Fine, S. J. Watson and H Akil, “Pattern of c-Fos mRNA Induction in Rat Brain by Acute Morphine,” Canadian Journal of Physiology and Pharmacology, Vol. 76, No. 3, 1998, pp. 294-303. doi:10.1139/y98-027
- [64] S. D. Grabus, J. R., Glowa and A. L. Riley, “Morphineand Cocaine-Induced c-Fos Levels in Lewis and Fischer Rat Strains,” Brain Research, Vol. 998, No. 1, 2004, pp. 20-28. doi:10.1016/j.brainres.2003.11.007
- [65] A. H. Söderpalm and K. C. Berridge, “The Hedonic Impact and Intake of Food Are Increased by Midazolam Microinjection in the Parabrachial Nucleus,” Brain Research, Vol. 877, No. 2, 2000, pp. 288-297. doi:10.1016/S0006-8993(00)02691-3
- [66] J. P. Schroeder and M. G. Packard, “Differential Effects of Intra-Amygdala Lidocaine Infusion on Memory Consolidation and Expression of a Food Conditioned Place Preference,” Psychobiology, Vol. 28, No. 4, 2000, pp. 486-491.
- [67] S. P. Garcia-Horsman, A. Agmo and R. G. Paredes, “Infusions of Naloxone into the Medial Preoptic Area, Ventromedial Nucleus of the Hypothalamus, and Amygdala Block Conditioned Place Preference Induced by Paced Mating Behavior,” Hormones & Behavior, Vol. 54, No. 5, 2008, pp. 709-716. doi:10.1016/j.yhbeh.2008.07.011
- [68] G. L. Edwards and R. C. Ritter, “Lateral Parabrachial Lesions Attenuate Ingestive Effects of Area Postrema Lesions,” American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, Vol. 256, No. 2, 1989, pp. R306-R312.
NOTES
*Corresponding author.

