Advances in Chemical Engineering and Science
Vol. 2 No. 1 (2012) , Article ID: 16705 , 5 pages DOI:10.4236/aces.2012.21006
Synthesis of Capsaicin Oligosaccharides and Their Anti-Allergic Activity
——Synthesis of Capsaicin Oligosaccharides as Anti-Allergic Food-Additives
1Department of Chemistry, Faculty of Medicine, Oita University, Hasama-Machi, Japan
2Department of Pharmacology, Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Yamashiro-Cho, Japan
Email: *shimoda@med.oita-u.ac.jp
Received August 9, 2011; revised September 16, 2011; accepted September 27, 2011
Keywords: Glycosylation; Capsaicin; Lactobacillus delbrueckii; CGTase; Anti-Allergic Activity
ABSTRACT
The production of β-maltooligosaccharides of capsaicin was investigated using Lactobacillus delbrueckii and cyclodextrin glucanotransferase (CGTase) as biocatalysts. The cells of L. delbrueckii glucosylated capsaicin to give its β-glucoside. The β-glucoside of capsaicin was converted into the corresponding β-maltoside and β-maltotrioside by CGTase. On the other hand, β-melibioside and β-isomaltoside of capsaicin, which were two new compounds, were synthesized by chemical glycosylation. The β-glucoside, β-maltoside, β-melibioside, and β-isomaltoside of capsaicin showed inhibitory effects on IgE antibody production.
1. Introduction
Plants of the Capsicum species have been important sources of food, spices, and medicines for centuries worldwide. Capsaicin, N-[(4-hydroxy-3-methoxyphenyl)methyl]- 8-methyl-(E)-6-nonenamide, is the most pungent princeple among naturally occurring capsaicinoids. It has also been reported to decrease adipose tissue weight and serum triacylglycerol content in rats by enhancing energy metabolism [1]. Capsaicin has shown a wide range of pharmacological properties, such as analgesic, antigenotoxic, antimutagenic, and anticarcinogenic effects, and has been used to treat various peripheral painful conditions, including rheumatoid arthritis and diabetic neuropathy [2-5]. However, capsaicinoids possess extensive neurological toxicity, and direct irritant effects on skin and mucous membrane [6]. Furthermore, capsaicinoids are scarcely soluble in water and poorly absorbed after oral administration. These disadvantages prevent capsaicinoids from being used as food additives and medicines.
Glycosylation allows the conversion of water-insoluble and unstable organic compounds into the corresponding water-soluble and stable ones to improve their bioavailability and pharmacological properties [7-16]. In addition, glycosides of functional food-ingredients have been, recently, reported to show anti-allergic activity [17-19]. This paper describes the production of capsaicin maltooligosaccharides, and capsaicin β-melibioside and β-isomaltoside, which were two new compounds, by biocatalytic or chemical glycosylation. We also report the antiallergic activity, i.e., inhibitory effects on IgE antibody formation, of the capsaicin oligosaccharides.
2. Experimental
2.1. General
Capsaicin was purchased from Aldrich Chemical Co. The 1H and 13C nuclear magnetic resonance (NMR), H-H correlation spectroscopy (COSY), C-H COSY, and heteronuclear multiple-bond correlation (HMBC) spectra were recorded in CD3OD using a Varian XL-400 spectrometer (Varian Inc.). The chemical shifts were expressed in δ (ppm) referring to tetramethylsilane. The fast atom bombardment mass spectrometry (FABMS) spectra were measured using a JEOL MStation JMS-700 spectrometer (JEOL Ltd.). High performance liquid chromatography (HPLC) was carried out on a YMC-Pack R & D ODS column (150 mm × 30 mm) at 25˚C [solvent: methanol-water (9:11, v/v); detection: UV (280 nm); flow rate: 1.0 ml/min].
2.2. Synthesis of Capsaicin β-Maltoside and β-Maltotrioside
Culture medium used for growth of L. delbrueckii subsp. bulgaricus (Okayama University of Science) had the following composition (in grams per liter): 20 g of lactose, 5 g of yeast nitrogen base, 20 g of bacto casitone, 1 g of sorbitan monooleate, 2 g of ammonium citrate, 5 g of sodium acetate, 2 g of K2HPO4, 0.05 g of MnSO4, 0.1 g of MgSO4. The cells were grown in the culture medium with continuous shaking on a rotary shaker (120 rpm) at 30˚C.
The cultures of L. delbrueckii were grown in 500 ml conical flasks containing 200 ml of culture medium at 30˚C. Prior to use for the experiments, the cells were harvested by centrifugation at 1000 g for 15 min. The β- glucosides were prepared as follows. A total of 2 mmol of substrate (0.2 mmol/flask) was added to ten 300-ml conical flasks containing 5 g of L. delbrueckii cells and 1 g of glucose in 100 ml of freshly prepared culture medium. The mixture was incubated with continuous shaking on a rotary shaker (120 rpm) for 5 days at 30˚C. The reaction mixture was centrifuged at 8000 g for 15 min to remove the cells and the supernatant was extracted with n-butanol. The n-butanol fraction was purified by preparative HPLC on YMC-Pack R & D ODS column to give β-glucoside product.
To a solution containing 0.1 mmol of β-glucoside of capsaicin and 5 g of starch in 25 mM of sodium phosphate buffer (pH 7.0) was added 100 U of CGTase. The reaction mixture was stirred at 40˚C for 24 h, and then the mixture was centrifuged at 3000 g for 10 min. The supernatant was subjected on to a Sephadex G-25 column equilibrated with water to remove CGTase. The fractions containing glycosides were purified by preparative HPLC on YMC-Pack R & D ODS column to give β- maltooligoside products.
2.3. Synthesis of Capsaicin β-Melibioside and β-Isomaltoside
A typical procedure to synthesize β-melibioside products is as follows. Hepta-O-acetyl-α-D-melibiosyl fluoride was prepared from octaacetylated maltoside and hydrogen fluoride by modification of Noyori’s method. Under a nitrogen atmosphere, BF3·OEt2 was added to a mixture of hepta-O-acetyl-α-D-melibiosyl fluoride (1.5 mmol), starting material (capsaicin, 1 mmol), and 1,1,3,3-tetramethylguanidine (3 mmol) in acetonitrile (20 mL) at room temperature. After the mixture had been stirred for 2 h, saturated aqueous sodium hydrogen carbonate was added. Organic materials were extracted twice with ethyl acetate, washed with saturated aqueous potassium hydrogen surfonate and brine, dried, and concentrated. The residue was chromatographed on silica gel to give capsaicin hepta-O-acetyl-α-D-melibioside. Deacetylation of capsaicin hepta-O-acetyl-α-D-melibioside with 3% potassium carbonate in methanol afforded capsaicin β-melibioside. The other glycoside, i.e., β-isomaltoside of capsaicin, was prepared by the similar procedure.
Spectral data of new compounds are as follows.
Capsaicin β-melibioside: FABMS: m/z 652 [M + Na]+; 1H NMR (400 MHz, CD3OD): δ 0.95 (6H, d, J = 6.8 Hz, H-16, 17), 1.38 (2H, q, J = 7.6 Hz, H-11), 1.62 (2H, q, J = 7.6 Hz, H-10), 2.00 (2H, m, H-12), 2.20 (3H, m, H-9, 15), 3.22 - 3.82 (12H, m, H-2', 2", 3', 3", 4', 4", 5', 5", 6', 6"), 3.83 (3H, s, OCH3), 4.28 (2H, s, H-7), 4.41 (1H, d, J = 8.0 Hz, H-1"), 4.75 (1H, d, J = 3.2 Hz, H-1'), 5.30 - 5.41 (2H, m, H-13, 14), 6.80 (1H, dd, J = 8.0, 1.6 Hz, H-6), 6.92 (1H, d, J = 1.6 Hz, H-2), 7.08 (1H, d, J = 8.0 Hz, H-5); 13C NMR (100 MHz, CD3OD): 23.2 (C-16, C-17), 26.5 (C-10), 30.3 (C-11), 32.0 (C-15), 33.3 (C-12), 36.9 (C-9), 43.5 (C-7), 56.5 (OCH3), 62.7 (C-6"), 68.4 (C-6'), 70.4 (C-4"), 71.0 (C-4'), 71.5 (C-2"), 72.2 (C-3"), 75.8 (C-2'), 76.4 (C-3', C-5"), 77.8 (C-5'), 100.5 (C-1'), 105.8 (C-1"), 113.3 (C-2), 118.2 (C-5), 121.2 (C-6), 127.7 (C-13), 135.1 (C-1), 139.0 (C-14), 147.0 (C-4), 150.5 (C-3), 176.0 (C-8). Calcd. for C30H47NO13: C, 55.25; H, 7.26; N, 2.15. Found: C, 55.12; H, 7.20; N, 2.10.
Capsaicin β-isomaltoside: FABMS: m/z 652 [M + Na]+; 1H NMR (400 MHz, CD3OD): δ 0.95 (6H, d, J = 6.8 Hz, H-16, 17), 1.38 (2H, q, J = 7.6 Hz, H-11), 1.60 (2H, q, J = 7.6 Hz, H-10), 1.99 (2H, m, H-12), 2.22 (3H, m, H-9, 15), 3.27 - 3.88 (12H, m, H-2', 2", 3', 3", 4', 4", 5', 5", 6', 6"), 3.83 (3H, s, OCH3), 4.28 (2H, s, H-7), 4.49 (1H, d, J = 7.6 Hz, H-1"), 4.82 (1H, d, J = 2.4 Hz, H-1'), 5.29 - 5.41 (2H, m, H-13, 14), 6.80 (1H, d, J = 8.0, 1.6 Hz, H-6), 6.92 (1H, d, J = 1.6 Hz, H-2), 7.09 (1H, d, J = 8.0 Hz, H-5); 13C NMR (100 MHz, CD3OD): 23.2 (C-16, C-17), 26.5 (C-10), 30.3 (C-11), 32.2 (C-15), 33.3 (C-12), 36.9 (C-9), 43.5 (C-7), 56.5 (OCH3), 62.7 (C-6"), 68.5 (C-6'), 71.5 (C-4'), 72.0 (C-4"), 72.8 (C-5"), 73.4 (C-2"), 73.7 (C-3"), 75.2 (C-2'), 76.4 (C-3'), 78.0 (C-5'), 100.4 (C-1'), 106.0 (C-1") 113.3 (C-2), 118.0 (C-5), 121.2 (C-6), 127.7 (C-13), 135.1 (C-1), 139.0 (C-14), 147.0 (C-4), 150.5 (C-3), 176.0 (C-8). Calcd. for C30H47NO13: C, 55.25; H, 7.26; N, 2.15. Found: C, 55.20; H, 7.04; N, 2.09.
2.4. Suppressive Action on IgE Antibody Formation
The inhibitory action of β-glycosides of capsaicin on IgE antibody formation was examined as follows. Glutenin was used as the antigen (1 mg/rat), and Al(OH)3 and pertussis vaccine were used as the adjuvants (20 mg and 0.6 ml/rat, respectively). Sensitization was made by injection of a mixture (0.6 ml) of the antigen and the adjuvant into the paws of each rat (male, ca. 200 g). Paw edema was measured 24 h after injection and the treated rats were divided in groups with an equal average swelling volume. Each sample was dissolved in physiological saline containing 10% Nikkol and the solution was injected daily into the rat for 11 d starting on the day of grouping. Hydrocortisone was used as the positive control. The amount of IgE was measured by the passive cutaneous anaphylaxis method on the 15th day [20]. The results were expressed as average of plasma IgE level of 7 rats administered a total of 10 mg/kg of each test compound.
3. Results and Discussions
3.1. Biocatalytic Synthesis of Capsaicin β-Maltoside and β-Maltotrioside
The biotransformation of capsaicin (1) was investigated using the cultured cells of L. delbrueckii. Cells of L. delbrueckii were incubated with capsaicin (1) in the presence of glucose for 5 days. Product 2 was isolated from n-butanol extracts of cell cultures by preparative HPLC (7% yield). The chemical structure of 2 was determined by spectroscopic methods such as HRFABMS, 1H and 13C NMR as capsaicin β-D-glucoside (2). Next, the glucoside product 2 was further glycosylated by cyclodextrin glucanotransferase (CGTase) to give two compounds 3 and 4 in 41 and 35% yield. The structures of products 3 and 4 were identified as capsaicin β-maltoside and cap-saicin β-maltotrioside by HRFABMS, 1H and 13C NMR, H-H COSY, C-H COSY, and HMBC spectra. The synthetic route of capsaicin maltooligosides is shown in Figure 1.
3.2. Chemical Synthesis of Capsaicin β-Melibioside and β-Isomaltoside
Capsaicin glycosides, including β-melibioside 5 and β- isomaltoside 6, were prepared by chemical syntheses in the presence of 1,1,3,3-tetramethylguanizine. Hepta-Oacetyl-α-D-melibiosyl fluoride was prepared from octaacetylated melibioside and hydrogen fluoride by modification of Noyori’s method in 85% yield. Under a nitrogen atmosphere, BF3·OEt2 was added to a mixture of hepta-O-acetyl-α-D-melibiosyl fluoride, capsaicin, and 1,1,3,3-tetramethylguanidine in acetonitrile at room temperature. Organic materials were extracted twice with ethyl acetate, washed with saturated aqueous potassium hydrogen surfonate and brine, dried, concentrated, and chromatographed on silica gel to give capsaicin heptaO-acetyl-α-D-melibioside in 50% yield. Deacetylation of capsaicin hepta-O-acetyl-α-D-melibioside with 3% potassium carbonate in methanol afforded capsaicin β-melibioside 5 (total yield, 43%). The synthetic route of capsaicin β-melibioside is shown in Figure 2. Capsaicin β- isomaltoside 6 was prepared by the similar procedure (total yield, 51%).
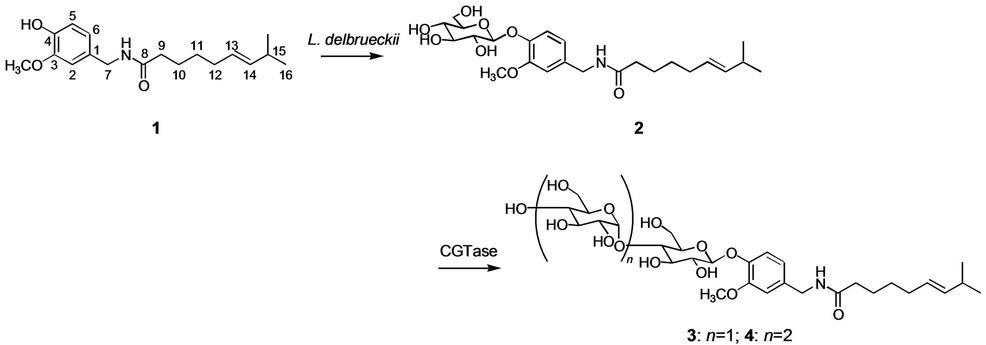
Figure 1. Synthesis of capsaicin maltooligosides by L. delbrueckii and CGTase.
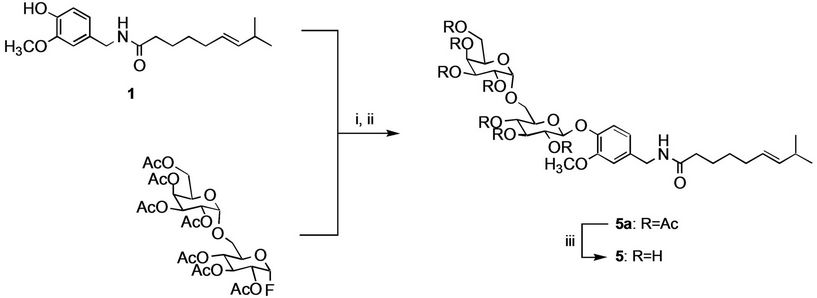
Figure 2. Synthesis of capsaicin β-melibioside. Reagents and conditions: (i) BF3·OEt2, 1,1,3,3-tetramethylguanidine; (ii) aq. sodium hydrogen carbonate; (iii) 3% potassium carbonate/methanol.
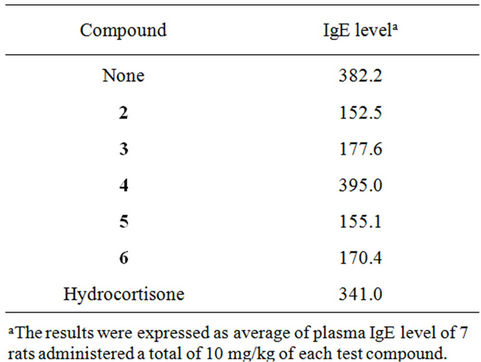
Table 1. Suppressive action of capsaicin β-glycosides 2 - 6 on IgE antibody formation.
The FABMS spectrum of 5 showed a peak at m/z 652 [M+Na]+ suggesting a molecular formula of C30H47NO13. The 1H and 13C NMR data of the sugar moiety of 5 agreed with those of β-melibiose. Each signal in the NMR spectra of 5 was assigned by H-H COSY, C-H COSY, and HMBC analyses. The HMBC spectrum of 5 showed correlations between the proton signal at δ 4.75 (H-1') and the carbon signal at δ 147.0 (C-4), and between the proton signal at δ 4.41 (H-1”) and the carbon signal at δ 68.4 (C-6'), which confirmed that the inner β-D-glucopyranosyl residue was attached to the 4-hydroxyl group of capsaicin and that the second β-D-galactopyranosyl residue and the inner β-D-glucopyranosyl residue were 1,6-linked. Therefore, the structure of 5 was determined to be capsaicin β-melibioside. Compound 5 has not been identified previously.
Product 6 was assigned a Mr of 652 [M + Na]+ in the FABMS spectrum, which suggested a molecular formula of C30H47NO13. In the 1H and 13C NMR spectra of 6, the coupling pattern of the sugar proton signals and the chemical shifts of the sugar carbon signals indicated that the sugar component in 6 was β-isomaltose. Correlations were observed in the HMBC spectrum between the proton signal at δ 4.82 (H-1’) and the carbon signal at δ 147.0 (C-4), and between the proton signal at δ 4.49 (H-1") and the carbon signal at δ 68.5 (C-6'). These results confirmed that the inner β-D-glucopyranosyl residue was attached to the phenolic hydroxyl group of capsaicin and that the second β-D-glucopyranosyl residue and the inner β-D-glucopyranosyl residue were 1,6-linked. Thus, compound 6 was identified as capsaicin β-isomaltoside, which is a new compound.
3.3. Anti-Allergic Activity of Capsaicin β-Glycosides
The effects of capsaicin β-glycosides 2-6 on IgE antibody formation were investigated by in vivo bioassay using glutenin as an antigen [20]. The average of rat plasma IgE level after treatment of glutenin with or without test compounds was examined. As shown in Table 1, capsaicin β-D-glucoside (2) and capsaicin β-maltoside (3) showed strong inhibitory action on IgE antibody generation. On the other hand, capsaicin β-maltotrioside (4) did not exhibit the inhibitory action on IgE antibody formation. Capsaicin β-melibioside (5) and capsaicin β-isomaltoside (6) also showed inhibitory action on IgE antibody formation.
4. Conclusions
The results of this experiment demonstrate that cultured cells of L. delbrueckii converted capsaicin into its glucoside and that CGTase glycosylate capsaicin glucoside to capsaicin β-maltoside and capsaicin β-maltotrioside. Also, capsaicin β-melibioside 5 and capsaicin β-isomaltoside 6 were successfully prepared by chemical syntheses in the presence of 1,1,3,3-tetramethylguanizine. Capsaicin β-glucoside, capsaicin β-maltoside, capsaicin β-melibioside, and capsaicin β-isomaltoside showed strong inhibitory action on IgE antibody generation. On the other hand, capsaicin β-maltotrioside did not exhibit antiallergic activity. It should be emphasized that capsaicin monoand disaccharides would be useful functional food-ingredients which show anti-allergic activity toward glutenin. Further studies on the physiological activities of capsaicin glycosides are now in progress.
REFERENCES
- T. Kawada, K. Hagihara and K. Iwai, “Effects of Capsaicin on Lipid Metabolism in Rats Fed a High Fat Diet,” Journal of Nutrition, Vol. 116, No. 7, 1985, pp. 1272- 1278.
- Y. J. Surh and S. S. Lee, “Capsaicin, a Double Edged Sword: Toxicity, Metabolism, and Chemopreventative Potential (Review),” Life Sciences, Vol. 56, No. 22, 1995, pp. 1845-1855. doi:10.1016/0024-3205(95)00159-4
- K. K. Park, K. S. Chun, J. I. Yook and Y. J. Surh, “Lack of Tumor Promoting Activity of Capsaicin, a Principal Pungent Ingredient of Red Peppers, in Mouse Skin Carcinoens,” Anticancer Research, Vol. 18, No. 6A, 1998, pp. 4201-4205.
- Y. J. Surh, E. Lee and J. M. Lee, “Chemopreventive Properties of Some Pungent Ingredients Present in Red Pepper and Ginger,” Mutation Research, Vol. 402, No. 1, 1998, pp. 259-267. doi:10.1016/S0027-5107(97)00305-9
- M. H. Ward and L. Lopez-Carrillo, “Dietary Factors and the Risk of Gastric Cancer in Mexico City,” American Journal of Epidemiology, Vol. 149, No. 10, 1999, pp. 925-932.
- T. Watanabe, T. Kawada, M. Yamamoto and K. Iwai, “Capsaicin, a Pungent Principle of Hot Red Pepper, Evokes Catecholamine Secretion from the Adrenal Medulla of Anesthetized Rats,” Biochemical and Biophysical Research Communications, Vol. 142, No. 1, 1987, pp. 259-264. doi:10.1016/0006-291X(87)90479-7
- E. Lewinson, E. Berman, Y. Mazur and J. Gressel, “Glucosylation of Exogenous Flavanones by Grapefruit (Citrus paradisi) Cell Cultures,” Phytochemistry, Vol. 25, 1986, pp. 2531-2535. doi:10.1016/S0031-9422(00)84502-1
- M. Tabata, Y. Umetani, M. Ooya and S. Tanaka, “Glucosylation of Phenolic Compounds by Plant Cell Cultures,” Phytochemistry, Vol. 27, No. 3, 1988, pp. 809- 813. doi:10.1016/0031-9422(88)84097-4
- B. Upmeier, J. E. Thomzik and W. Barz, “Nicotinic Acid- N-Glucoside in Heterotrophic Parsley Cell Suspension Cultures,” Phytochemistry, Vol. 27, No. 11, 1988, pp. 3489-3493. doi:10.1016/0031-9422(88)80754-4
- T. Furuya, M. Ushiyama, Y. Ashida and T. Yoshikawa, “Biotransformation of 2-Phenylpropionic Acid in Root Culture of Panax ginseng,” Phytochemistry, Vol. 28, No. 2, 1989, pp. 483-487. doi:10.1016/0031-9422(89)80036-6
- M. Ushiyama, T. Asada, T. Yoshikawa and T. Furuya, “Biotransformation of Aromatic Carboxylic Acids by Root Culture of Panax ginseng,” Phytochemistry, Vol. 28, No. 11, 1989, pp. 1859-1869. doi:10.1016/S0031-9422(00)97875-0
- T. Suga and T. Hirata, “Biotransformation of Exogenous Substrates by Plant Cell Cultures,” Phytochemistry, Vol. 29, No. 8, 1990, pp. 2393-2406. doi:10.1016/0031-9422(90)85155-9
- K. Ishihara, H. Hamada, T. Hirata and N. Nakajima, “Biotransformation Using Plant Cultured Cells,” Journal of Molecular Catalysis B: Enzymatic, Vol. 23, No. 2-6, 2003, pp. 145-170.
- K. Morohoshi, F. Shiraishi, Y. Oshima, T. Koda, N. Nakajima, J. S. Edmonds and M. Morita, “Synthesis and EsTrogenic Activity of Bisphenol a Monoand Di-Beta-DGlucopyranosides, Plant Metabolites of Bisphenol A,” Environmental Toxicology and Chemistry, Vol. 22, No. 10, 2003, pp. 2275-2279. doi:10.1897/02-464
- S. Kwon, K. Shimoda, H. Hamada, K. Ishihara, N. Masuoka and H. Hamada, “High Production of β-Thujaplicin Glycosides by Immobilized Plant Cells of Nicotiana tabacum,” Acta Biologica Hungarica, Vol. 59, No. 3, 2008, pp. 347-355. doi:10.1556/ABiol.59.2008.3.8
- H. Katsuragi, K. Shimoda, A. Ohiro and H. Hamada, “Glycosylation of Capsaicinoids with Panax ginseng Stimulated by Salicylic Acid,” Acta Biologica Hungarica, Vol. 61, No. 4, 2010, pp. 449-456. doi:10.1556/ABiol.61.2010.4.8
- K. Shimoda, H. Hamada, “Synthesis of β-Maltooligosaccharides of Glycitein and Daidzein and Their Anti-Oxidant and Anti-Allergic Activities,” Molecules, Vol. 15, No. 8, 2010, pp. 5153-5161. doi:10.3390/molecules15085153
- K. Shimoda and H. Hamada, “Production of Hesperetin Glycosides by Xanthomonas campestris and Cyclodextrin Glucanotransferase and Their Anti-Allergic Activities,” Nutrients, Vol. 2, No. 2, 2010, pp. 171-180. doi:10.3390/nu2020171
- K. Shimoda and H. Hamada, “Enzymatic Synthesis and Anti-Allergic Activities of Curcumin Oligosaccharides,” Biochemistry Insights, Vol. 2010, No. 3, 2010, pp. 1-5.
- A. Koda, T. Miura, N. Inagaki, O. Sakamoto, A. Arimura, H. Nagai and H. Mori, “A Method for Evaluating Anti- Allergic Drugs by Simultaneously Induced Passive Cutaneous Anaphylaxis and Mediator Cutaneous Reactions,” International Archives of Allergy and Applied Immunology, Vol. 92, No. 3, 1990, pp. 209-216. doi:10.1159/000235179
NOTES
*Corresponding author.

