Food and Nutrition Sciences
Vol.4 No.4(2013), Article ID:29745,7 pages DOI:10.4236/fns.2013.44051
Predictive Factors of Hyponatremia in Under-Five Severely Malnourished Children with Pneumonia Admitted to a Large Urban Hospital in Dhaka, Bangladesh: A Nested Case-Control Design
![]()
1Johns Hopkins Bloomberg School of Public Health, Baltimore, USA; 2Centre for Nutrition & Food Security (CNFS), International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh; 3Dhaka Shishu Hospital, Dhaka, Bangladesh.
Email: *chisti@icddrb.org
Copyright © 2013 Cheryl Kay Zogg et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 11th, 2013; revised March 11th, 2013; accepted March 18th, 2013
Keywords: Children; Diarrhea; Hyponatremia; Malnutrition; Pneumonia
ABSTRACT
Background: Hyponatremia is the most common electrolyte imbalance encountered in the management of diarrheal children. Common ramifications include cerebral edema and fatal outcomes. However, pediatric data remain lacking, particularly in developmental contexts where resources are limited and associated conditions like malnutrition and pneumonia are common. Aim: This study aimed to evaluate predicting factors associated with hyponatremia in children under five years of age with severe acute malnutrition (SAM) in Bangladesh. Methods: Using a nested case-control design, we compared clinical and laboratory characteristics of children with (n = 61) and without hyponatremia (n = 183) taken from a parent population of all children under five with SAM and clinical or radiological pneumonia admitted to Dhaka Hospital of icddr,b between April 2011 and June 2012 (n = 407). Results: Logistic regression analysis adjusting for potential confounders such as lack of breast feeding, duration of vomiting (days), and severe wasting revealed that older age (OR 1.05, 95%CI 1.02 - 1.07, p = 0.001) (5% increase in the relative odds of hyponatremia for each additional month of age), presence of diarrhea (OR 2.43, 95%CI 1.0 - 6.0, p = 0.05), and difficulty breathing (OR 1.52, 95%CI 1.0 - 2.05, p = 0.05) were significantly associated with hyponatremia. Conclusion: Our data suggest that older age, presence of diarrhea, and difficulty breathing in under-five children with SAM is independent predictors of hyponatremia. These findings underscore the importance of detecting simple clinical predictors early in order to facilitate appropriate management and to prevent potential ramifications of hyponatremia in SAM children, especially in resource-poor settings.
1. Introduction
Hyponatremia is the most common electrolyte imbalance encountered in children [1] and in the broader scope of clinical practice. Mild to moderate forms (<135 mmol/L) occur in approximately 25% of hospitalized children while more severe forms (<130 mmol/L) are found in 1% - 4% [2-10].
The disorder is associated with worsened outcomes in patients, is a major comorbidity factor for a variety of diseases, and is a known causative factor in the development of seizures and hyponatremic encephalopathy [1,11- 14]. Nevertheless, pediatric data remain lacking [1]
—a reality that underscores the importance of improving understanding of predictive factors involved in order to enable earlier and more complete detection of hyponatremia in children. Such issues become all the more important when considered in a developmental context where resources are inherently limited and associated conditions like malnutrition and pneumonia are common [2,5, 11,15-17].
Malnutrition remains a major public health concern—a fact reflected in the fourth Millennium Development Goal which calls for a two-thirds reduction in childhood mortality by the year 2015 [18]. It has been identified as an underlying factor in over 53% of the 10 - 11 million children under 5 years of age who die from preventable causes each year [19-22]. The condition impacts 13 million children worldwide and is responsible for more than 1.5 million child deaths each year [23].
When compounded with the influence of pneumonia, the leading cause of death in children of this age [24,25], a disturbing picture begins to emerge. High case-fatality rates (CFR) reported for both comorbidities in hyponatremic children necessitate the need for enhanced detection [2,5,11,15], yet research remains limited.
Twenty years ago, Samadi et al. [15] looked at the consequences of hyponatremia in children in Bangladesh and reported a CFR for children with acute diarrhea of >10%. Malnutrition, in particular, was found to directly relate to the incidence of hyponatremia, while case-fatality was related solely to serum sodium concentrations [15]. Since that time, no further studies have been published for Bangladesh and none have looked at the associated factors of hyponatremia in children with pneumonia and SAM, despite the high prevalence and reported CFRs.
Ultimately, despite reporting of hyponatremia since the mid-twentieth century, this highly prevalent disorder remains poorly understood [26]. Its association with numerous underlying disease states and its multiple etiologies with differing pathophysiologic mechanisms [26,27] make an understanding of hyponatremia difficult at best, yet by looking at the associated clinical and laboratory factors we can begin to identify children expected to be at highest risk.
Building on the work of Samadi et al. [15], this study aimed to evaluate predicting factors associated with hyponatremia in children with SAM and pneumonia in Bangladesh.
2. Methods
2.1. Study Design and Population
The study was conducted as a nested case-control study within an ongoing hospital-based cohort of all children under 5 years of age presenting with severe acute malnutrition [defined by the WHO [28] as a very low weightfor-height ratio (below −3 z scores of the median WHO growth standards), by visible severe wasting, or by the presence of nutritional edema] and clinical or radiological pneumonia admitted to Dhaka Hospital of icddr,b over a 14 month period from April 2011 to June 2012. Dhaka Hospital of icddr,b is a specialized facility that treats more than 120,000 patients each year, particularly those with diarrheal and infectious disease. It primarily serves patients from poor socio-economic conditions in urban and peri-urban Dhaka, Bangladesh.
As per the parent study, inclusion was subject to obtaining written informed consent from appropriate parents/care-givers, and activities were approved by the Research Review and Ethical Review committees of icddr,b. Children were excluded from enrollment if their parents/care-givers did not provide consent.
Cases were selected based on a review of collected data to identify all previously enrolled subjects with hyponatremia (defined as a serum sodium level <130 mmol/L based on work by Chisti et al.) [29]. Sixty-one cases were identified. Controls were then randomly chosen from among remaining normonatremic study participants using the randomization feature of STATA (version 12.1; StataCorp LP, College Station, Texas) to give a ratio of three cases to one control. The unmatched casecontrol ratio was used to increase the statistical power of the analysis. This resulted in a total of 183 controls and a combined study population of 244 children under 5 years of age.
2.2. Data Collection Procedure
A review of previously collected data on enrolled study participants was used to ascertain information on socioeconomic and demographic characteristics, housing and environmental conditions, feeding practices of infants and children (0 - 24 months old), prior antibiotic use, possible tuberculosis contact, and use of medications at home. Original data collection for the parent study was completed using a study-specific standardized data collection form that was developed, pre-tested, and finalized by members of the current trial team. Information on clinically relevant (age, sex, rectal temperature, degree of dehydration (if any, usually in children presenting with diarrhea), nutritional status, peripheral perfusion status, features of sepsis and severe sepsis, arterial oxygen saturation, and outcome) and laboratory (blood glucose, complete blood count, serum electrolytes, lactate, and C-reactive protein) characteristics was further determined.
2.3. Statistical Analyses
Relevant data were entered into a personal computer database accessible only by the investigative team using SPSS (version 17.0; SPSS Inc., Chicago, Illinois) and were analyzed using STATA and SPSS. Differences in proportions and means were first compared via simple logistic regression on hyponatremic status in STATA to determine possible predictive factors. Fischer’s exact tests were employed as applicable. An alpha of 0.05 was considered the threshold for statistical significance (p < 0.05), and strength of association was determined by estimating odds ratios and corresponding 95%CIs.
We analyzed the following clinical and laboratory parameters in a univariate model: gender, age, SES, breast feeding, illness, fever, diarrhea, dehydration, vomiting, poor feeding practices, difficulty breathing, edema, convulsions, WHZ, WAZ, mid-upper arm circumference, respiratory rate, blood pressure, irritability, lethargy, hypoxemia, sepsis, death, hypoglycemia, total WBC count, bands, potassium, TCO2, creatinine, calcium, and magnesium.
Multiple logistic regression analyses were then preformed to identify characteristics that were associated independently with hyponatremia after adjusting for other singly significant variables. All variables statistically significantly associated with hyponatremia in children with severe acute malnutrition and pneumonia were in included in the model as independent variables. Hyponatremia was defined as the dependent variable.
3. Results
Between April 2011 and June 2012, a total of 407 children under the age of 5 years with SAM and pneumonia were enrolled in the parent study. Of these, 61 children (15%) were found to have serum sodium levels <130 mm/L and were considered hyponatremic (cases). From the remaining 346 patients (85%), we randomly selected 183 controls.
The median age of cases and controls were 14.7 (0.8 - 60.0) and 9.0 (0.7 - 56.0) months respectively, and the difference was significant (Table 1). Among the cases, 40 children (65.6%) were male as compared to 103 children (56.3%) among the controls (Table 1). Univariate analysis of clinical characteristics suggested that children with hyponatremia were statistically significantly more likely to be older, to have been continuously breast fed during the neonatal period, to have a longer duration of vomiting, to have difficulty breathing, and to be more severely wasted than normonatremic controls (Table 1). The presence of diarrhea on admission was found to be statistically significant with a p-value of 0.05, and in contrast to the findings from 1983 [15], hyponatremic children were not found to be significantly more likely to die (Table 1). Other considered clinical characteristics were comparable between the groups and are included in Table 1. Univariate analysis of laboratory characteristics, included in Table 2, were statistically insignificant.
Subsequent multiple logistic regression-accounting for the influence of the other identified variables—showed that children with hyponatremia were statistically significantly more likely than normonatremic controls to be older, to present with diarrhea on admission, and to present with difficulty breathing on admission (Table 3).
4. Discussion
We observed significantly greater age in SAM children with hyponatremia and pneumonia as compared to those without hyponatremia. Findings of increased hyponatremic burden among children expected to be healthier due to more advanced physiologic and immune development associated with increased age are of interest for their peculiarity and importantly suggest a conditional manifesttation that we may not fully understand.
It has long been known that severely malnourished children present with different pathophysiological mechanisms than normally or well-nourished children as a result of their bodies’ inability to carry out the routine operations of normal pathways. These reductive adaptations described in the work of Collins et al. [23] may help explain the findings of the present study, suggesting that as the severity of nutritional insult increases, reducetive adaptations progressively limit the body’s ability to respond to stresses such as infection.
Differences in difficulty breathing on admission can be more easily understood. Assuming a clear (or protected) airway and that the observation is not due to some form of obvious obstruction, respiratory findings in children with pneumonia are common [30]. Its pathogenic presence can lead to the development of acute respiratory distress syndrome wherein alveolar injury begets diffuse alveolar damage [31-34]. These children are also likely to be acidotic (low pH) due to under perfusion of the tissues and lactic acid build-up that a failing renal system may be unable to clear. The presence of increased acid load triggers the renal-pulmonary compensatory response, characteristic of metabolic acidosis within this population [35].
Diarrhea on admission has been well characterized [15], yet no further studies have been published for Bangladesh. Limited research within the region reports highly mixed results. Work by Memon et al. [16] found a statistically significant difference in the prevalence of hyponatremia (p < 0.001) between malnourished children under five in Hyderabad, India with and without diarrhea while work by Mishra et al. [17] found that serum sodium level was not significantly associated with nutriational state (p = 0.054) among malnourished children under five with diarrhea in Kathmandu, Nepal. In general terms, it is thought that anything that decreases energy levels at the gut endothelium (such as the results of pneumonia, malnutrition, and hyponatremia acting within the body) can result in a loss of tight junction integrity and consequent gut fluid losses.
Twenty years ago, Samadi et al. [15] looked at the consequences of hyponatremia in children in Bangladesh and reported a CFR for children with acute diarrhea of greater than ten percent. The present study failed to corroborate this result, suggesting that in 2012, hyponatremic children in Bangladesh with pneumonia and malnu-
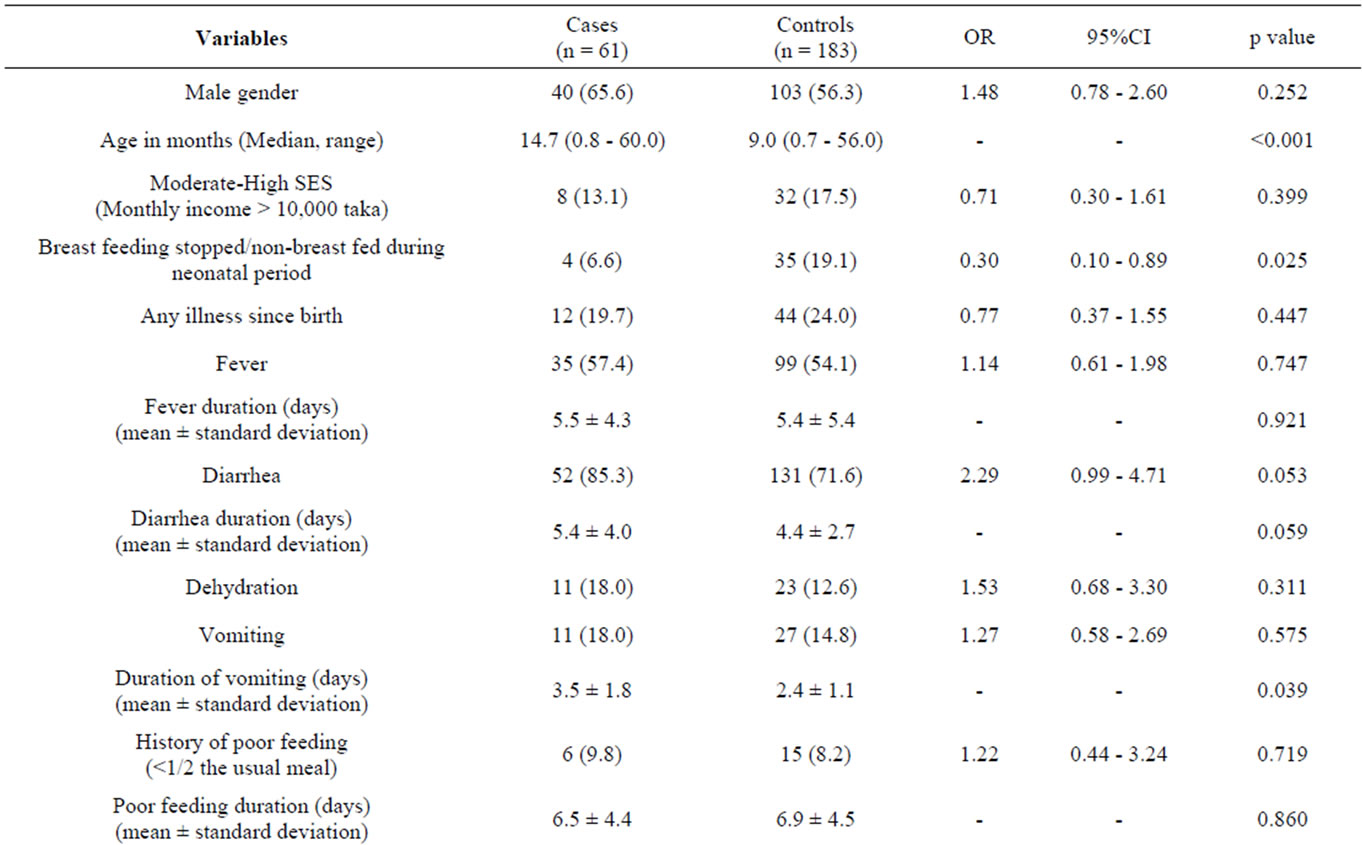
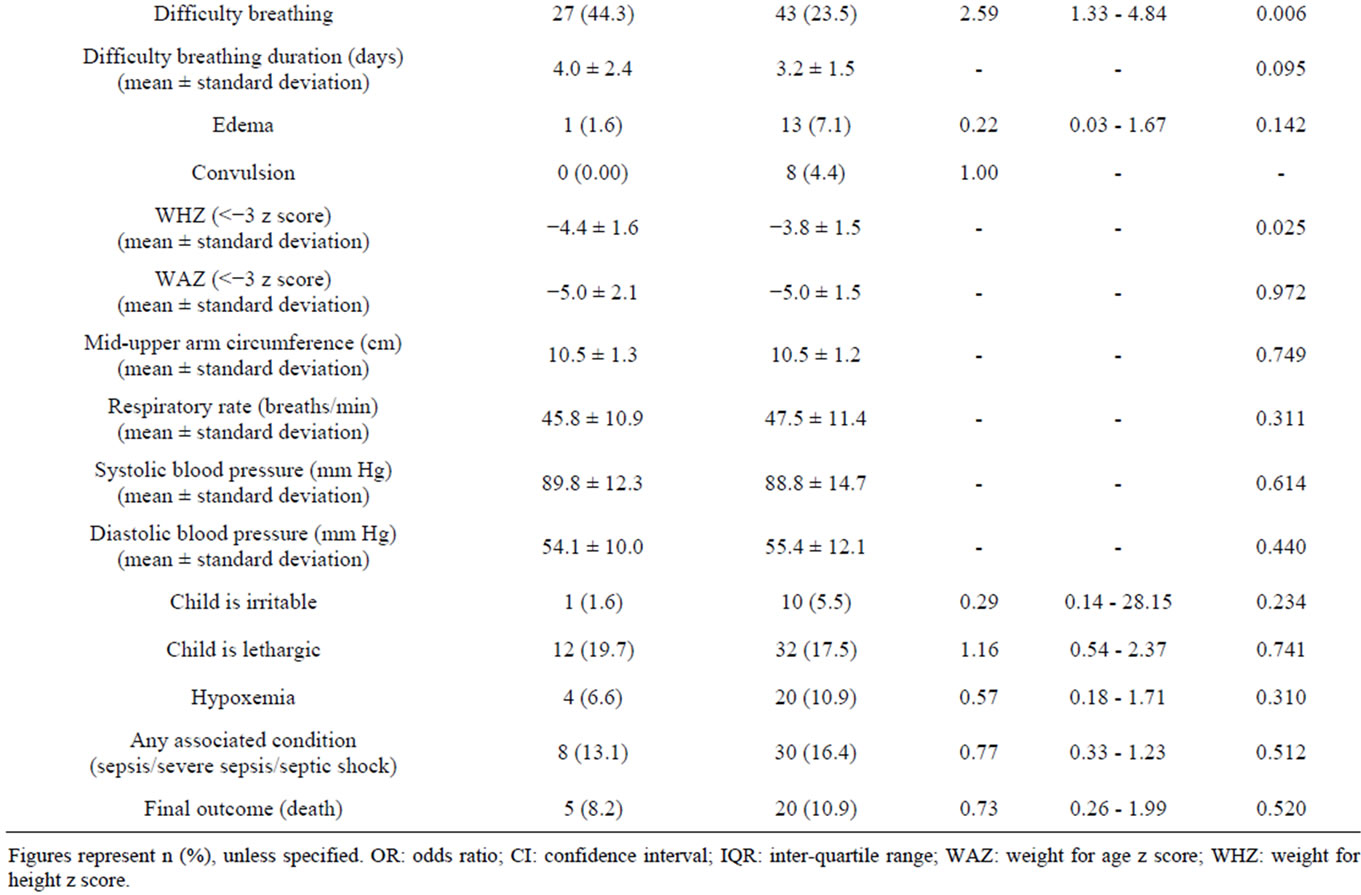
Table 1. Clinical characteristics of under-five children with hyponatremia (cases) and without hyponatremia (controls).
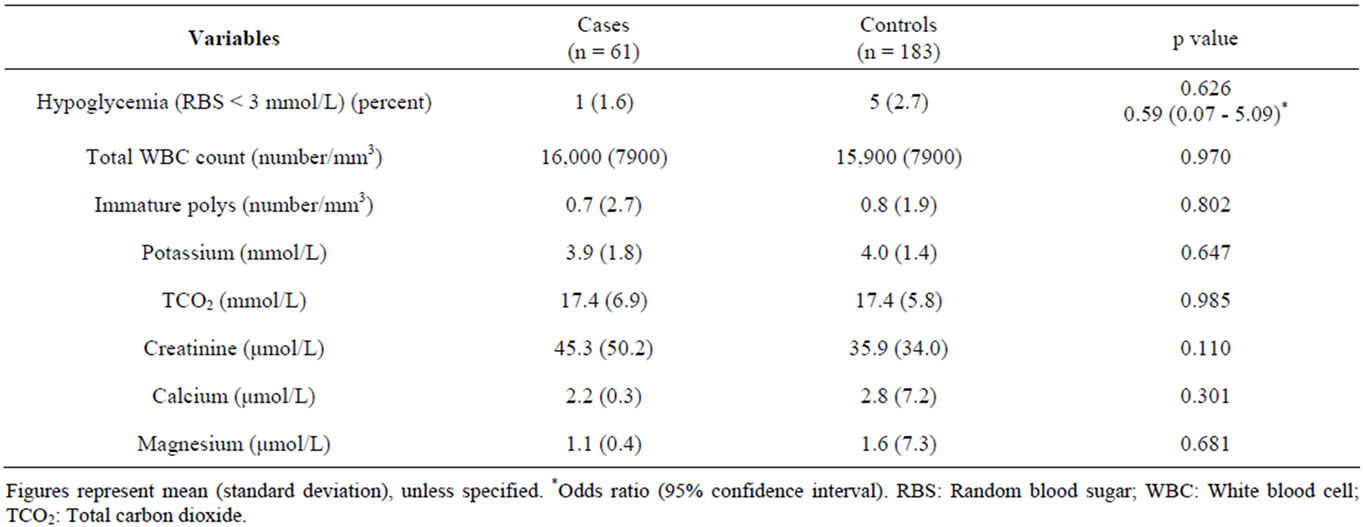
Table 2. Laboratory characteristics of under-five children with hyponatremia (cases) and without hyponatremia (controls).
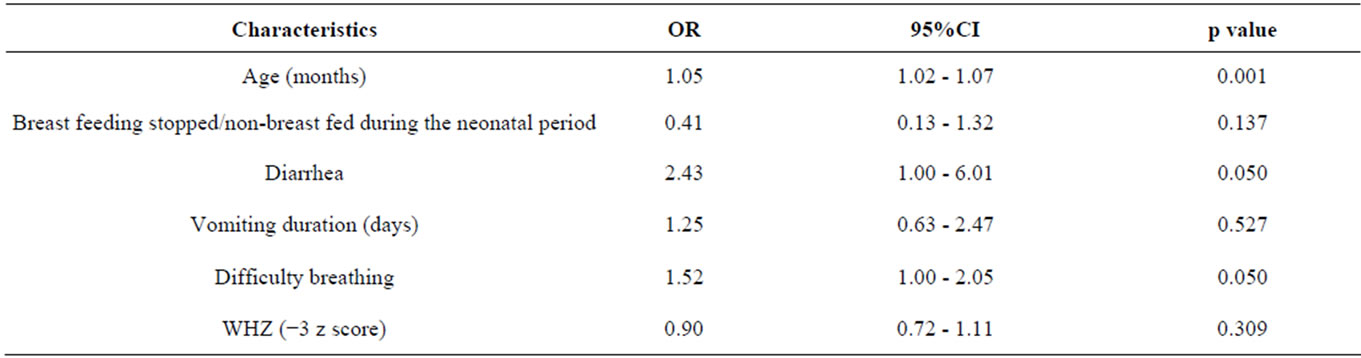
Table 3. Results of multiple logistic regression analysis to explore the independent factors associated with hyponatremia in under-five children with pneumonia and SAM.
trition receiving treatment at the same hospital were not significantly more likely to die. The original results of Samadi et al. [15] indicate that the observed effect was related solely to serum sodium concentrations, largely negating the potential impact of different patient groups (diarrhea vs. malnutrition and pneumonia). While this remains an important consideration and a possible limitation of either study, a more likely explanation can be found in the work of Ahmed et al. [36] which describes the development of a “standardized protocol for acutephase treatment of children with severe malnutrition and diarrhea, with the aim of reducing mortality” that has been in effect at icddr,b for more than 10 years. Their reported relative 48% reduction in mortality appears to be corroborated by the present study’s findings of a lack of statistical significance [36].
Another possible limitation comes in a lack of correction for multiple comparisons within our study. Given the stated objective of identifying predicting factors associated with hyponatremia in children with SAM and pneumonia in Bangladesh in order to enhance critical and early detection, we felt that it was equally (if not more) important to protect against Type II errors (overlooking a factor that has predictive value) as it was to ensure statistical significance of the ones we did identify (preventing a Type I error). Employing statistical measures such as Bonferroni corrections offer an effective means of reducing the probability of making a Type I error but at the expense of increasing the probability of making a Type II.
In conclusion, hyponatremia in under-five children with severe acute malnutrition and radiological or clinical pneumonia is associated with increased age and presence of diarrhea and difficulty breathing on admission relative to comparable normonatremic controls. It was found not to reflect a statistically significant difference in the likelihood of death between the two groups— a change from findings in the 1980s which reported a CFR of >10%. Such a change is likely attributable to the successful implementation of protocolled management of diarrhea and malnutrition designed to reduce mortality over the last 10 years at icddr,b. These findings underscore the importance of early detection to facilitate appropriate care and its potential to mitigate the myriad of devastating consequences associated with symptomatic and advanced disease.
REFERENCES
- M. L. Moritz and J. C. Ayus, “New Aspects in the Pathogenesis, Prevention, and Treatment of Hyponatremic Encephalopathy in Children,” Pediatric Nephrology, Vol. 25, No. 7, 2010, pp. 1225-1238. doi:10.1007/s00467-009-1323-6
- A. Sakellaropoulou, M. Hatzistilinou, M. Eboriadou and F. Athanasiadou-Piperopoulou, “Hyponatremia in Cases of Children with Pneumonia,” Achieves of Medical Science, Vol. 6, No. 4, 2010, pp. 578-583. doi:10.5114/aoms.2010.14471
- R. J. Anderson, H. M. Chung, R. Kluge and R. W. Schrier, “Hyponatremia: A Prospective Analysis of Its Epidemiology and the Pathogenic Role of Vasopressin,” Annals of Internal Medicine, Vol. 102, No. 2, 1985, pp. 164-168.
- H. Hasegawa, S. Okubo, Y. Ikezumi, K. Uchiyama, T. Hirokawa, H. Hirano and M. Uchiyama, “Hyponatremia Due to an Excess of Arginine Vasopressin Is Common in Children with Febrile Disease,” Pediatric Nephrology, Vol. 24, No. 3, 2009, pp. 507-511. doi:10.1007/s00467-008-1053-1
- M. Don, G. Valerio, M. Korppi and Canciani M, “Hyponatremia in Pediatric Community-Acquired Pneumonia,” Pediatric Nephrology, Vol. 23, No. 12, 2008, pp. 2247- 2253. doi:10.1007/s00467-008-0910-2
- E. J. Hoorn, D. Geary, M. Robb, M. L. Halperin and D. Bohn, “Acute Hyponatremia Related to Intravenous Fluid Administration in Hospitalized Children: An Observational Study,” Pediatrics, Vol. 113, No. 5, 2004, pp. 1279- 1284. doi:10.1542/peds.113.5.1279
- K. Armon, A. Riordan, S. Playfor, G. Millman and A. Khader, “Hyponatraemia and Hypokalemia during Intravenous Fluid Administration,” Archives of Disease in Childhood, Vol. 93, 2008, pp. 285-287. doi:10.1136/adc.2006.093823
- A. Wattad, M. L. Chiang and L. L. Hill, “Hyponatremia in Hospitalized Children,” Clinical Pediatrics, Vol. 31, No. 3, 1992, pp. 153-157. doi:10.1177/000992289203100305
- M. L. Moritz and J. C. Ayus, “Disorders of Water Metabolism in Children: Hyponatremia and Hypernatremia,” Pediatrics in Review, Vol. 23, No. 11, 2002, pp. 371-380.
- F. Laczi, “Etiology, Diagnostics and Therapy of Hyponatremias,” Orvosi Hetilap, Vol. 149, No. 29, 2008, pp. 1347-1354. doi:10.1556/OH.2008.28409
- M. D. Zilberberg, A. Exuzides, J. Spalding, A. Foreman, A. G. Jones, C. Colby and A. F. Shorr, “Epidemiology, Clinical and Economic Outcomes of Admission Hyponatremia among Hospitalized Patients,” Current Medical Research & Opinion, Vol. 24, No. 6, 2008, pp. 1601- 1608. doi:10.1185/03007990802081675
- F. Cetinkaya, E. Sennaroglu and S. Comu, “Etiologies of Seizures in Young Children Admitted to an Inner City Hospital in a Developing Country,” Pediatric Emergency Care, Vol. 24, No. 11, 2008, pp. 761-763. doi:10.1097/PEC.0b013e31818c2652
- H. Caksen, D. Odabas, S. Sar, V. Celebi, S. Arslan, M. Kuru and M. Abduhandan, “Hyponatremic Dehydration: An Analysis of 78 Cases,” International Urology & Nephrology, Vol. 33, No. 3, 2001, pp. 445-448. doi:10.1023/A:1019563222488
- H. C. Farrar, V. T. Chande, D. F. Fitzpatrick and S. J. Shema, “Hyponatremia as the Cause of Seizures in Infants: A Retrospective Analysis of Incidence, Severity, and Clinical Predictors,” Annals of Emergency Medicine, Vol. 26, No. 1, 1995, pp. 42-48. doi:10.1016/S0196-0644(95)70236-9
- A. R. Samadi, M. A. Wahed, M. R. Islam and S. M. Ahmed, “Consequences of Hyponatraemia and Hypernatraemia in Children with Acute Diarrhoea in Bangladesh,” British Medical Journal, Vol. 286, No. 6366, 1983, pp. 671-673. doi:10.1136/bmj.286.6366.671
- Y. Memon, R. Majeed, M. H. Ghani and S. Shaikh, “Serum Electrolytes Changes in Malnourished Children with Diarrhoea,” Pakistan Journal of Medical Sciences, Vol. 23, No. 5, 2007, pp. 760-764.
- S. K. Mishra, S. P. Bastola and B. Jha, “Biochemical Nutritional Indicators in Children with Protein Energy Malnutrition Attending Kanti Children Hospital, Kathmandu, Nepal,” Kathmandu University Medical Journal, Vol. 7, No. 2, 2009, pp.129-134.
- World Health Organization, “MDG 4: Reduce Child Mortality,” 2012. http://www.who.int/topics/millennium_development_goals/child_mortality/en/index.html
- J. Bryce, C. Boschi-Pinto, K. Shibuya, R. E. Black and WHO Child Health Epidemiology Reference Group, “WHO Estimates of the Causes of Death in Children,” The Lancet, Vol. 365, No. 9465, 2005, pp. 1147-1152. doi:10.1016/S0140-6736(05)71877-8
- L. E. Caulfield, M. de Onis and R. E. Black, “Undernutrition as an Underlying Cause of Child Deaths Associated with Diarrhea, Pneumonia, Malaria, and Measles,” The American Journal of Clinical Nutrition, Vol. 80, No. 1, 2002, pp. 193-198.
- A. I. Rice, I. Sacco, A. Hyder and R. E. Black, “Malnutrition as an Underlying Cause of Childhood Deaths Associated with Infectious Diseases in Developing Countries,” Bulletin of the World Health Organization, Vol. 78, No. 10, 2000, pp. 1207-1221.
- D. I. Pelletier and E. A. Frongillo, “Changes in Child Survival are Strongly Associated with Changes in Malnutrition in Developing Countries,” The Journal of Nutrition, Vol. 133, No. 1, 2003, pp. 107-119.
- S. Collins, N. Dent, P. Binns, P. Bahwere, K. Sadler and A. Hallam, “Management of Severe Acute Malnutrition in Children,” The Lancet, Vol. 368, No. 9551, 2006, pp. 1992-2000. doi:10.1016/S0140-6736(06)69443-9
- R. Hajjeh and C. G. Whitney, “Call to Action on World Pneumonia Day,” Emerging Infectious Diseases, Vol. 18, No. 11, 2012, pp. 1896-1897.
- L. Liu, H. L. Johnson, S. Cousens, J. Perin, S. Scott, J. E. Lawn, I. Rudan, H. Campbell, R. Cibulskis, M. Li, C. Mathers, R. E. Black and Child Health Epidemiology Reference Group of WHO and UNICEF, “Global, Regional, and National Causes of Child Mortality: An Updated Systematic Analysis for 2010 with Time Trends Since 2000,” The Lancet, Vol. 379, No. 9832, 2012, pp. 2151-2161. doi:10.1016/S0140-6736(12)60560-1
- J. G. Verbalis, S. R. Goldsmith, A. Greenberg, R. W. Schrier and R. H. Sterns, “Hyponatremia Treatment Guidelines 2007: Expert Panel Recommendations,” The American Journal of Medicine, Vol. 120, No. 11, 2007, pp. S1-S21. doi:10.1016/j.amjmed.2007.09.001
- B. J. Feldman, S. M. Rosenthal, G. A. Vargas, R. G. Fenwick, E. A. Huang, M. Matsuda-Abedini, R. H. Lustig, R. S. Mathias, A. A. Portale, W. L. Miller and S. E. Gitelman, “Nephrogenic Syndrome of Inappropriate Antidiuresis,” The New England Journal of Medicine, Vol. 352, 2005, pp. 1884-1890. doi:10.1056/NEJMoa042743
- World Health Organization and United Nations Children’s Fund, “WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children,” WHO Press, Geneva, 2009.
- M. J. Chisti, T. Ahmed, P. K. Bardhan and M. A. Salam, “Evaluation of Simple Laboratory Investigations to Predict Fatal Outcomes in Infants with Severe Malnutrition Presenting in an Urban Diarrhoea Treatment Centre in Bangladesh,” Topical Medicine & International Health, Vol. 15, No. 11, 2010, pp. 1322-1325. doi:10.1111/j.1365-3156.2010.02619.x
- C. G. Murphy, A. C. van de Pol, M. B. Harper and R. G. Bachur, “Clinical Predictors of Occult Pneumonia in the Febrile Child,” Academic Emergency Medicine, Vol. 14, No. 3, 2007, pp. 243-249. doi:10.1111/j.1553-2712.2007.tb01781.x
- C. A. Piantadosi and D. A. Schwartz, “The Acute Respiratory Distress Syndrome,” Annals of Internal Medicine, Vol. 141, No. 4, 2004, pp. 460-470.
- F. S. Calandrino Jr., D. J. Anderson, M. A. Mintun and D. P. Schuster, “Pulmonary Vascular Permeability during the Adult Respiratory Distress Syndrome: A Positron Emission Tomographic Study,” American Journal of Respiratory & Critical Care Medicine, Vol. 138, No. 2, 1988, pp. 421-428.
- L. B. Ware and M. A. Matthay, “Alveolar Fluid Clearance Is Impaired in the Majority of Patients with Acute Lung Injury and the Acute Respiratory Distress Syndrome,” American Journal of Respiratory & Critical Care Medicine, Vol. 163. No. 6, 2001, pp. 1376-1383.
- W. R. Baumann, R. C. Jung, M. Koss, C. T. Boylen, L. Navarro and O. P. Sharma, “Incidence and Mortality of Adult Respiratory Distress Syndrome: A Prospective Analysis from a Large Metropolitan Hospital,” Critical Care Medicine, Vol. 14, No. 1, 1986, pp. 1-4. doi:10.1097/00003246-198601000-00001
- M. J. Chisti, T. Ahmed, H. Ashraf, A. S. G. Faruque, P. K. Bardhan, S. K. Dey, S. Huq, S. K. Das and M. A. Salam, “Clinical Predictors and Outcome of Metabolic Acidosis in Under-Five Children Admitted to an Urban Hospital in Bangladesh with Diarrhea and Pneumonia,” PLoS One, Vol. 7, No. 6, 2012, Article ID: e39164. doi:10.1371/journal.pone.0039164
- T. Ahmed, M. Ali, M. M. Ullah, I. A. Choudhury, M. E. Haque, M. A. Salam, G. H. Rabbani, R. M. Suskind and G. J. Fuchs, “Mortality in Severely Malnourished Children with Diarrhoea and Use of a Standardized Management Protocol,” The Lancet, Vol. 353, No. 9168, 1999, pp. 1919-1922. doi:10.1016/S0140-6736(98)07499-6
NOTES
*Corresponding author and senior author.

