Food and Nutrition Sciences
Vol. 3 No. 1 (2012) , Article ID: 17077 , 6 pages DOI:10.4236/fns.2012.31006
Comparison of Different Washing and Disinfection Protocols Used by Food Services in Southern Brazil for Lettuce (Lactuca sativa)
![]()
Programa de Pós-Graduação em Microbiologia Agrícola e do Ambiente, Universidade Federal do Rio Grande do Sul (PPGMAA/ UFRGS), Porto Alegre, Brazil.
Email: ana.beatriz@ufrgs.br
Received September 9th, 2011; revised October 19th, 2011; accepted October 26th, 2011
Keywords: Lettuce; Disinfection; Food Services
ABSTRACT
Different protocols have been used for washing and disinfecting leafy vegetables in Brazilian food services, however its effect on microbial reduction was barely studied. The aim of this study was to evaluate the effectiveness of lettuce washing and disinfecting protocols used by food services. The treatments evaluated were: washing in potable tap water (T1); washing and dipping in potable tap water (T2); washing with potable tap water and dipping in a solution of sodium hypochlorite (200 ppm of free chlorine) for 15 min (T3); and 30 min (T4); washing with potable tap water and dipping in 2% vinegar solution (T5); and 20% vinegar solution (T6). After the treatments, the samples were microbiologically evaluated to measure bacterial reductions. The microbial counts showed that the mean total mesophile reductions were: 0.64 log for T1, 0.75 log for T2, 2.06 log for T3, 2.46 log for T4, 1.68 log for T5 and 1.72 log for T6, and the mean total coliform reductions were: 1.11 log for T1, 1.09 log for T2, 2.29 log for T3, 2.35 log for T4, 1.6 log for T5 and 2.09 log for T6. Based on these results it can be concluded that protocols which used 200 ppm sodium hypochlorite for 15 to 30 min (T3 e T4), were the most effective, followed by treatment with 20% vinegar solution (T6). The latter method, however, turned the lettuce dark during the distribution period.
1. Introduction
The consumption of vegetables has been rising over the last few years due to a demand for healthier diets. Of the vegetables, lettuce (Lactuca sativa) is the most popular leaf vegetable among Brazilians [1,2]. On the other hand, vegetables can be potential vehicles of pathogenic microorganisms, contributing to the increasing number of foodborne diseases [2-4].
In the traditional growing method, lettuces stay in contact with the soil throughout their development. The humidity associated with organic fertilizers and water irrigation can provide ideal conditions for the contamination of these food products [4-6]. Furthermore, mishandling of the vegetables during processing can also turn these kinds of foods into vehicles of pathogenic bacteria [2,6].
Even though foodborne diseases are mostly caused by the consumption of animal food products, several outbreaks were related to vegetable and fruit consumption, many of them due to Salmonella Enteritidis, Campylobacter jejuni and Shigella sonnei after lettuce consumption [7-9]. Recently, in Germany (May 2011), thousands of infections occurred resulting in 877 cases of haemolytic uremic syndrome (HUS) with 32 deaths and 3,043 cases of enterohaemorrhagic Escherichia coli (EHEC) with 16 deaths [10]. The cause of this outbreak is not yet known, German officials have warned the public to avoid eating raw cucumbers, tomatoes and lettuce until further notice or until solid epidemiological or microbiological evidence regarding the source of the outbreak strain becomes available [11].
Leafy vegetables process generally involves the following steps: cleaning, trimming, coring, slicing, shredding, washing, disinfection, centrifugal drying and packaging. Washing and disinfection have been considered the only operations aimed actively reducing microbial populations on fresh cut vegetables [12] and for this reason they are important stages in food safety for raw vegetable consumption [6,13,14]. Different protocols have been used for washing and disinfection of leafy vegetables, and they comprise several washing times, kind of sanitizers and sanitizer concentrations, justifying its comparison. Chlorine and its various forms are the most commonly used disinfectants in food processing [9, 15]. The Food and Drug Administration (FDA) allows the use of sodium hypochlorite as a disinfectant agent for surfaces that come into contact with food in concentrations ranging from 50 to 200 ppm. In Brazil, this disinfectant is recommended by legislation at a concentration of 100 to 250 ppm/15 min for the disinfection cleaning cloths used in food preparation and the disinfection of vegetables [15].
Food services, mainly restaurants, have become very important in the modern lifestyle because an increasing number of people eat their meals outside of their households. The great variety of establishments, the differences in the number of meals served and the facilities and know-how of those involved in the preparation and handling of food must also be considered. Variations in these aspects might imply a lack of standardization of several food preparation procedures, including the disinfection of vegetables. Taking these considerations into account, the aim of the present study was to compare the different lettuce washing and disinfection protocols used by food services in Porto Alegre/Rio Grande do Sul, Southern Brazil.
2. Materials and Methods
The study was carried out in two stages. In the first stage, an observation study of buffet-style food services in Porto Alegre city took place. In the second stage, the washing and disinfection protocols used by the establishments visited were tested to determine their mesophilic aerobic bacteria and coliform reduction rates on lettuces.
2.1. Characterization of the Food Services and Identification of Lettuce Washing and Disinfection Protocols
Seventy-two buffet-style restaurants in two districts of Porto Alegre city were visited between July and November 2004. The food services were randomly chosen from those registered with the Porto Alegre City Industry and Commerce Secretary. Of these, 10 food services in the Moinhos de Vento district, making up 28.5% of the 35 registered in the area, and 62 food services located downtown, making up 25.7% of the 241 registered in the area, were visited. The districts of Moinhos de Vento and downtown Porto Alegre were chosen to represent two distinct realities. The first is an area with establishments that generally serve meals to a section of the population with a higher purchasing power than in downtown Porto Alegre. Some data on the restaurants were collected in order to provide a brief set of characteristics of the environment where the protocols were carried out.
2.2. Lettuce Washing and Disinfection Protocols Used by the Restaurants
The main lettuce washing and disinfection protocols identified in the restaurants were tested on 10 replicates to determine their effectiveness at reducing the number of microorganisms on leaf lettuce. Leaf lettuce was chosen because it is the most popular type of lettuce among the food services that were visited. The samples were purchased between January and February 2005 from eight different retail outlets in Porto Alegre city; the samples had previously been packed in sterilized containers and were transported at room temperature to the Laboratório de Medicina Veterinária Preventiva at UFRGS. Five units of leaf lettuce were separately purchased and the leaves were mixed and then divided into 100 g samples to be submitted to the following treatments: (T0) lettuce without any treatment; (T1) washing with potable tap water; (T2) washing and dipping for 30 min in potable tap water; (T3) washing with potable tap water and dipping in a solution of sodium hypochlorite (Q. Boa®) with 200 ppm of free chlorine for 15 min; (T4) washing with potable tap water and dipping in s solution of sodium hypochlorite (Q. Boa®) with 200 ppm of free chlorine for 30 min; (T5) washing in potable tap water and dipping for 15 min in a solution of 2% vinegar (fermented acetic acid from white wine and alcohol—Agrin Prinz®); (T6) washing with tap potable water and dipping for 15 minutes in solution of vinegar 20% (fermented acetic acid from white wine and alcohol—Agrin Prinz®).
In the case of treatments T2 to T6, the lettuce leaves were rinsed for 30 s with potable tap water after treatment, before undergoing microbiological analyses.
Samples of 25 g of lettuce from each of the treatments were placed in 225 ml of saline solution (0.85% NaCl) and homogenized in a Stomacher blender twice for 30 s each. Aliquots of 1 ml were serially diluted in 9 ml of saline solution to be submitted to bacterial enumeration as described below.
2.3. Total Mesophilic Aerobic Bacterial Counts
A 1 ml sample from each dilution, relative to each treatment, was placed in sterile Petri dishes to which approximately 15 ml of Plate Count Agar (PCA, Merckâ, Darmstadt, Germany) was pour plated at 45˚C. The dishes were slightly agitated and then left at room temperature for agar solidification. Later, the dishes were incubated for 48 h at 37˚C. Dishes that presented 25 to 250 colonies were selected and counted. The colony count was determined according to Silva et al. [16] and expressed in CFU/g of lettuce. All counts were performed in duplicate.
2.4. Total Coliform and Thermotolerant Bacterial Count
From each dilution, relative to each treatment, 1 ml samples were placed in sterile Petri dishes to which approximately 15 ml of Violet Red Bile Agar (VRBA, Oxoid®, Basingstoke, UK) was pour plated at approximately 45˚C; the mixture was then homogenized. After agar solidification at room temperature, an over layer of 5 ml VRBA was added. Following this procedure, the dishes were incubated for 48 h at 37˚C. Dishes that presented 25 to 250 colonies were selected for counting. Counting was performed according to Silva et al. [15] and expressed in CFU/g of lettuce. All counts were performed in duplicate. Five typical coliform colonies were selected from the dishes and individually transferred to tubes containing EC broth (Merck®, Darmstadt, Germany), which were incubated in a hot water bath for 48 h at 44.5˚C. Tubes that showed growth and the presence of gas were confirmed for thermotolerant coliforms. The number of CFU/g was calculated in terms of the number of typical colonies, the inoculated dilution and the percentage of confirmed colonies [16].
2.5. Calculating the Coliform and Mesophilic Aerobic Bacteria Count Reductions
For each repetition that was performed for each treatment (T1 to T6), the following formula was applied, where n = 1 to 6; RCT = total coliform bacteria reduction; RM = total mesophilic aerobic bacteria reduction:
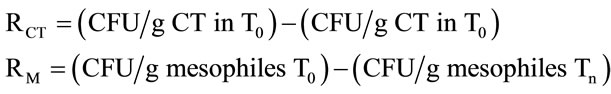
2.6. Statistical Analysis
The bacteria reduction obtained from each of the different protocols was analysed using Tukey’s multiple comparison test [17] We used a level of significance of p < 0.05 for each of the tests.
3. Results and Discussion
3.1. Characteristics of the Food Services Visited
The food services that were visited did not show a statistical difference in the number of meals they served, since 49% of the establishments served from 51 to 200 meals. There was significant statistical difference (p < 0.01) between the prices charged for meals in the two districts. This difference suggests that the choice of the two districts was indeed correct, since the purpose was to investtigate lettuce washing and disinfection protocols in different realities. Most of the food services presented specific kitchen utensils for leaf vegetable washing and disinfection, as well as for their display on the buffet.
There was no statistical difference (p = 0.302) between the districts in terms of the kind of lettuce disinfectant chosen by the restaurants. The most popular disinfectants were vinegar (31%) and sodium hypochlorite (27%). The treatment by washing and dipping in potable tap water (T2) proved to be the most popular (39%) among the establishments that were visited. None of the food services used more than one disinfectant in the lettuce treatment. It is worth mentioning that 88% of the food services visited that used any kind of disinfectant could not specify the concentration of the disinfecting agent used. Those that followed a predetermined concentration did not have measuring tools for dilution, which might influence treatment effectiveness. The disinfection time was another factor that was difficult to assess. Many restaurants stated that they would let the leaf vegetables sit “for a while”, whereas others reported dipping them in disinfecting solution while other activities were being carried out and thus they were unable to say exactly how long they were treated.
3.2. Treatment Results
When comparing the mean and median total mesophilic aerobic and coliform populations after testing the washing and disinfection protocols, it was noticed that their numbers were similar, which indicates that there was no great variation among the counts of the ten repetitions performed for the treatments (Table 1). All treatments showed a decrease in both total mesophilic aerobic and coliform counts compared to the initial populations on the samples of lettuce analysed.
The lettuce samples purchased from outlets in Porto Alegre city that were not washed or disinfected presented mean mesophilic aerobic counts that varied from 5.4 × 105 CFU/g to 5.6 × 106 CFU/g, leading to a mean count of 1.6 × 106 CFU/g (Table 1). For the same samples, the total coliform counts varied from 1.1 × 104 CFU/g to 1.4 × 106 CFU/g. Thermotolerant coliforms were confirmed in three samples (2.8 × 104, 1.8 × 104 and 1.3 × 104 CFU/g), and two samples treated with vinegar and sodium hypochlorite did not meet the coliform levels required by legislation (thermotolerant coliforms ≤ 102 CFU/g and the absence of Salmonella sp. [18] for vegetables ready for consumption.
The greatest reductions in total mesophilic aerobic bacteria and coliform populations were found for the sodium hypochlorite (200 ppm of free chlorine) treatment for 30 min, with reductions of 2.46 log10 CFU/g and 2.35 log10 CFU/g, respectively. The treatment with the same disinfectant for 15 min, as suggested by Brazilian legislation [15], showed a reduction of 2.06 log10 CFU/g for total mesophilic aerobic microorganisms and 2.29 log10 CFU/g for total coliform counts. Based on the fact that the microbial reductions obtained with 15 min and 30 min of exposure were not significantly different (p < 0.05), the shorter period of disinfection is an advantage, considering the hurry routines of food services.
On the other hand, the smallest reductions in total mesophilic aerobic microorganisms (0.67 log10 CFU/g) and the coliform population (1.09 log10 CFU/g) were found in the treatments that used potable tap water only (T1 and T2 respectively). The mean mesophilic aerobic counts for these treatments did not significantly differ (p > 0.05) from the counts of the untreated sample (T0). However, regarding the total coliform counts, there was a statistically significant difference (p < 0.05) between the values found for T0 and the treatments using tap water only (Table 2).
According to Mossel [19], the maximum acceptable mesophilic aerobic count in vegetables is 5.0 log10 CFU/g. Therefore, the treatments using only tap water were below the proposed standards regarding the mesophilic aerobic population count. It is important to note that 39% of the restaurants visited washed lettuce with tap water only, while most of the restaurants dipped it in solutions of an insufficient disinfectant concentration, which may compromise the disinfection effectiveness and the microbiological quality of the vegetables they serve.
There was no significant difference between the mean populations after the four treatments using the disinfecttants, both for the total mesophilic aerobic bacteria and coliform counts. The treatments with sodium hypochlorite yielded an approximate reduction of 2 log10 for both the total mesophilic aerobic bacteria and coliform counts, which is in agreement with studies [20,21,14] which showed that the disinfection of lettuce with chlorine reduced the counts by 1 to 2 log10 CFU/g (Table 3). However, there were greater reductions in mesophilic aerobic bacterial counts according to Nascimento et al. [14] and López-Gálvez et al. [22] after disinfection with this product.

Table 1. Mean and median numbers of mesophilic aerobic and total coliform bacteria found in leaf lettuce samples after the different treatments.
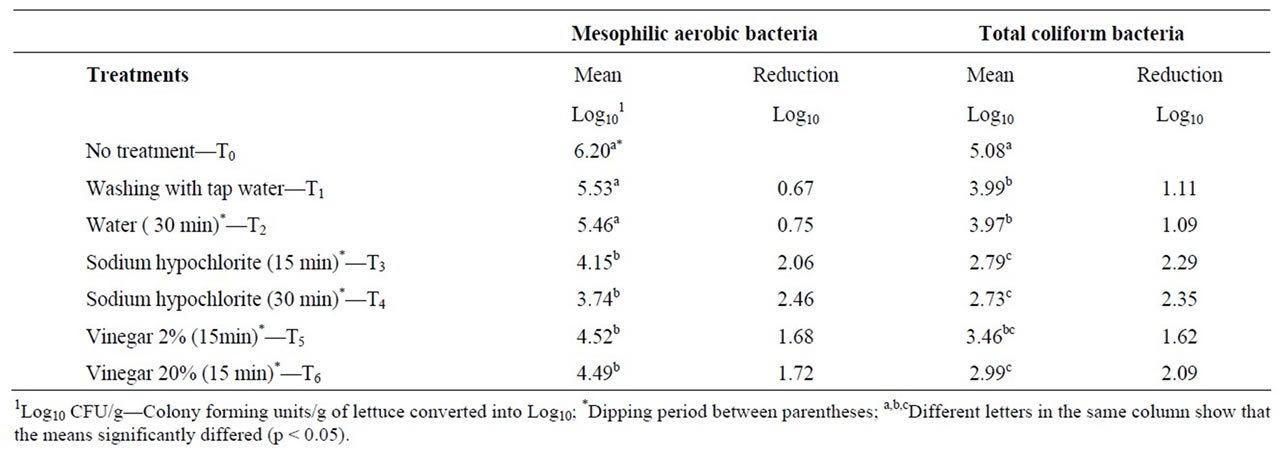
Table 2. Mean (log10 CFU/g) and reduction (log10) of total mesophilic aerobic and coliform bacteria in leaf lettuce samples after the different treatments.
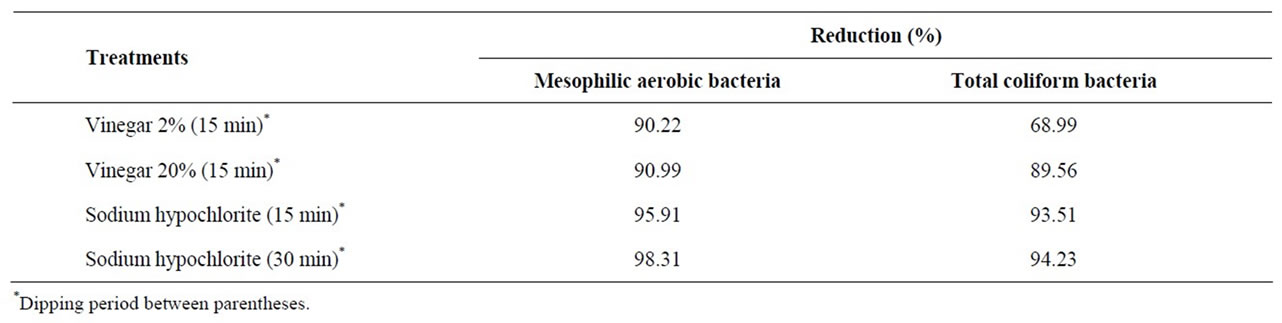
Table 3. Mesophilic aerobic and total coliform bacteria reduction percentages for each treatment in terms of the counts after T1 (washing with tap water), in order of increasing effectiveness.
The populations of both groups of microorganisms found after treatment with 20% vinegar were signifycantly different from those found after treatment with tap water only. Although treatment with 20% vinegar showed microbial reductions close to those achieved by treatment with 200 ppm sodium hypochlorite, it was also observed that the exposure of lettuce to 20% vinegar for 15 min caused a darkening of the leaves 1 h post-treatment. Therefore, this treatment can be ruled out for food service procedures, since leaf vegetables remain on display for more than 2 h at the counter, especially when used as a garnish for other dishes served by the establishment.
Most of the food services that were investigated only washed lettuce with potable tap water, without applying any disinfecting agent, and this was the treatment that yielded the lowest microbial reduction. The protocol using 200 ppm sodium hypochlorite for 30 min proved to be the most effective in terms of microbial count reducetions, followed by the protocols using sodium hypochlorite for 15 min and the 20% vinegar solution. The latter, however, caused darkening of the lettuce leaves and the former consume a lot of time, considering the daily routines of food services.
Based on the results it can be concluded that protocol which used 200 ppm sodium hypochlorite for 15 min (T3) was the most recommended to be used in food services for lettuce decontamination, and it was based on its microbial reduction capacity, short time to be perform and no influence on the darkening of the leaves.
REFERENCES
- S. T. Philippi, “Nutrição e Técnica Dietética,” Manole, Barueri, 2003.
- L. R. R. Santana, R. D. S. Carvalho, C. C. Leite, L. M. Alcântara, T. W. S. Oliveira and B. M. Rodrigues, “Qualidade Fisica, Microbiológica e Parasitológica de Alfaces (Lactuca sativa) de Diferentes Sistemas de Cultivo,” Ciência e Tecnologia de Alimentos, Vol. 26, No. 2, 2006, pp. 264-269. doi:10.1590/S0101-20612006000200006
- M. Abadias, J. Usall, M. Anguera, C. Solsona and I. Viñas, “Microbiological Quality of Fresh, Minimally-Processed Fruit and Vegetables, and Sprouts from Retail Establishments,” International Journal Food Microbiology, Vol. 123, No. 1-2, 2008, pp. 121-129. doi:10.1016/j.ijfoodmicro.2007.12.013
- N. Holden, L. Pritchard and I. Toth, “Colonization outwith the colon: plants as an alternative environmental reservoir for human pathogenic enterobacteria,” FEMS Microbiology Reviews, Vol. 33, No. 4, 2009, pp. 689-703. doi:10.1111/j.1574-6976.2008.00153.x
- J. P. Nourti, T. Niskanen, S. Hallanvuo, J. Mikkola, E. Kela, M. Hatakka, M. Fredriksson-Ahomaa, O. Lyytikäinen, A.Siitonen, H. Korkeala and P. Ruutu, “A widespread outbreak of Yersinia pseudotuberculosis O:3 infection from iceberg lettuce,” Journal Infectious Deseas Vol. 189, No. 5, 2004, pp.766-774. doi:10.1086/381766
- S. R. P. Silva, S. E. F. Verdin, D. C. Pereira, A. M. Schatkoski, M. B.Rott and G. Corção, “Microbiological quality of minimally processed vegetables sold in Porto Alegre, Brazil,” Brazilian Journal of Microbiology, Vol. 38, No. 4, 2007, pp. 594-598. doi:10.1590/S1517-83822007000400003
- E. D. Hilborn, J. H. Mermin, P. A. Mshar, J. L. Hadler, A. Voetsch, C. Wojtkunski, M. Swartz, R. Mshar, M. A. Lambert-Fair, J. A. Farrar, M. K. Glynn and L. Slutsker, “Multistate outbreak of Escherichia coli 0157:H7 infections associated with consumption of mesclum lettuce,” Archives of Internal Medicine, Vol. 159, No. 15, 1999, pp. 1758-1764. doi:10.1001/archinte.159.15.1758
- G. Kapperud, L. M. Rorvik, V. Hasseltvedt, E. A. Hoiby, B. G. Iversen, K. Staveland, G. Johnsen, J. Leitao, H. Herikstad, Y. Andersson, G. Langeland, B. Gondrosen and J. Lassen, “Outbreak of Shigella sonnei Infection Traced to Imported Iceberg Lettuce,” Journal of Clinical Microbiology, Vol. 33, No. 3, 1995, pp. 609-614.
- M. S. Nascimento, N. Silva, M. P. L. Catanozi and K. C. Silva, “Avaliação comparativa de diferentes desinfetantes na sanitização de uva,” Brazilian Journal Food Technology, Vol. 6, 2003, pp. 63-68.
- S. Rubino, P. Cappuccinelli and D. J. Kelvin, “Escherichia coli (STEC) serotype O104 outbreak causing haemolytic syndrome (HUS) in Germany and France,” The Journal Infection Developing Countries, Vol. 5, No. 6, 2011, pp. 437-440.
- World Health Organization Regional Office for Europe, 2011. http://www.euro.who.int/en/what-we-do/health-topics/emergecies/international-health-regulations/news/news/2011/ 06/ehec-outbreak-update-22
- M. Pirovani, A. Piagentini, D. Güemes and S. Arkwright, “Reduction of chlorine concentration and microbial load during washing-disinfection of shredded lettuce,” International Journal of Food Science & Technology, Vol. 39, No. 3, 2004, pp. 341-347. doi:10.1111/j.1365-2621.2004.00791.x
- Brasil, Ministério da Saúde, Agência Nacional de Vigilância Sanitária, Resolução—RDC No. 216 de 15 de Setembro de 2004. http://www.anvisa.gov.br
- M. S. Nascimento, N. Silva, M. P. L. M. Catanozi and K. C. Silva, “Effects of Different disinfectition treatments on natural microbiota of lettuce,” Journal Food Protection, Vol. 66, No 9, 2003, pp. 1697-1700.
- Rio Grande do Sul, Secretaria da Saúde, Portaria No. 78/ 2009. http://www.saude.rs.gov.br
- N. Silva, V. C. A. Junqueira and N. F. A. Silveira, “Manual de métodos de análise microbiológica de alimentos,” Varela, São Paulo, 1997.
- SPSS—Statistical Package for the Social Sciences (Computer program), Version 8.0, McGraw-Hill Book Company, Chicago, 1998.
- Brasil, Ministério da Saúde, Agência Nacional de Vigilância Sanitária, Resolução—RDC No. 12, de 02 de Janeiro de 2001. http://www.anvisa.gov.br
- Mercosul/GMC, Grupo Mercado Comum, Resolução No. 28 de 20 de Junho de 2002. http://www.anvisa.gov.br
- A. Allende, E. Aguayo and F. Artés, “Microbial and sensory quality of commercial fresh processed red lettuce throughout the production chain and shelf life,” International Journal Microbiology, Vol. 91, No. 2, 2004, pp. 109-117. doi:10.1016/S0168-1605(03)00373-8
- L. A. Keskinen, A. Burke and B. A. Annous, “Efficacy of chlorine, acidic electrolysed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157:H7 from Lettuce Leaves,” International Journal Food Microbiology, Vol. 132, No. 2-3, 2009, pp. 134- 140.
- F. López-Galvez, A. Allende, M. V. Selma and M. I. Gil, “Prevention of Escherichia coli cross-contamination by different commercial sanitizers during washing of freshcut lettuce,” International Journal Food Microbiology, Vol. 133, No. 1-2, 2009, pp. 167-171. doi:10.1016/j.ijfoodmicro.2009.05.017

