Food and Nutrition Sciences
Vol. 2 No. 10 (2011) , Article ID: 16354 , 5 pages DOI:10.4236/fns.2011.210144
Simultaneous Detection of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in Raw Salad Vegetables and Vegetarian Burger Patties
![]()
1Center of Excellence for Food Safety Research, Faculty of Food Science and Technology, Universiti Putra Malaysia, Serdang, Malaysia; 2National Public Health Laboratory, Ministry of Health Malaysia, Sungai Buloh, Malaysia; 3Center for Southeast Asian Studies, Kyoto University, Kyoto, Japan.
Email: echen_86@yahoo.com
Received May 10th, 2011; revised August 2nd, 2011; accepted August 10th, 2011.
Keywords: Salmonella spp., Salmonella Enteritidis, Salmonella Typhimurium, Most Probable Number (MPN), Multiplex Polymerase Chain Reaction
ABSTRACT
The health risks posed by Salmonella spp., Salmonella Enteritidis and Salmonella Typhimu-rium through the consumption of raw vegetables and vegetarian burger patties necessitates the needs for the optimization of analytical approach for their detection and enumeration in the raw vegetables, which served as potential vehicles for transmission of these pathogenic microorganisms. We sought to establish a rapid, economic and sensitive method to detect and determine the load of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium using the most probable numbers (MPN) in combination with the multiplex polymerase chain reaction (MPCR). From the naturally contaminated one hundred and seventy five samples tested (n = 175), the overall prevalence of Salmonella spp. was 28%, Salmonella Enteritidis was 20% and Salmonella Typhimurium was 14.3%, respectively. The MPN-MPCR is a quantitative method to determine the density of cell concentration of Salmonella in all the samples (Salmonella spp. ranged from <3 to 53 MPN/g; S. Enteritidis ranged from <3 to 24 MPN/g; and S. Typhimurium ranged from <3 to 15 MPN/g). The combination of the MPN-MPCR is an efficient, simple, fast analytical method for the detection and enumeration of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in vegetables and the vegetarian burger patties since it can significantly reduce time and labour with analysis completed within 2 days, as opposed to the traditional confirmation method that can take up to 5 days for unequivocal identifi-cation of species.
1. Introduction
Adequate fresh fruit and vegetables consumption is a necessary component for healthy diet and due to the changes in dietary habits, the tendency of consumption of raw vegetables has increased. However, raw fruit and vegetables have been known as vehicle for transmission of human disease. There are outbreak cases of the contamination of bacteria in watercress grown in soil, lettuce and radishes [1] contaminated by Salmonella Typhi, uncooked rhubarb, mature lettuce and radish recover typhoid bacilli [2]. A currently ongoing Escherichia coli O104:H4 bacteria outbreak began in Germany in May 2011. Certain strains of E. coli are a major cause of foodborne illness. In addition 3228 cases and 35 deaths had been reported on 13 June 2011 and a handful of cases have been reported in several countries including Switzerland, Poland, Netherlands Sweden, Denmark, United Kingdom, Canada and the USA. According to Wikimedia (2011), the all affected people had been in Germany shortly before becoming ill. German official issues the outbreak is suspected when their identifying an organic cucumber, bean sprouts and others raw vegetables contaminated by E. coli O104:H4. Therefore, it is possible this Salmonella will cause any high infection if there are no further investigation on this issue in the raw salad food as well.
Salmonella are among the most important causes of foodborne gastroenteritis world-wide. Salmonella infections are known as Salmonellesis and were reported to contaminate vegetables while growing or during harvesting, post harvest handling or distribution [4]. Therefore, the safety of vegetables is of great concerned, since consumption of raw vegetables is actively promoted throughout the world as essential component of healthy diet including in Malaysia.
Polymerase chain reaction (PCR) has been proven to be useful in detecting pathogen in food sample rapidly and accurately. To reduce the time needed to for the detection of Salmonella, multiplex PCR is applied which involves the simultaneous amplification of more than one target genes by mixing multiple primer pairs with different specificities. Multiplex PCR methods have been developed for specific detection of Salmonella, however they are limited to qualitative determination of the organism unless they are used in conjunction with Most Probable Number (MPN) procedure for quantitative determination of this pathogen in foods [3]. The MPN method is commonly used to measure the concentration of a target microbe in samples [4]. The paper reports for the first time the combination of the MPN-MPCR method to simultaneously detect and enumerate Salmonella spp., Salmonella Typhimurium and Salmonella Enteritidis and provide quantitative data that can be use in the risk assessment of this pathogen in vegetables.
2. Materials and Methods
2.1. Samples Collection and Preparation
One hundred seventy five samples (n = 175) were collected randomly over a period of May to June 2009 from hypermarket and wet market in Serdang, Selangor. This study include the analysis of vegetarian burger patties (n = 25) and common vegetables eaten in raw, Tomato (n = 25), Capsicum (n = 25), Cabbage (n = 25), Lettuce (n = 25), Cucumber (n = 25) and Carrot (n = 25). The samples were transferred to sterile plastic and were transported immediately to the laboratory for analysis.
2.2. Most Probable Number (MPN)
Ten gram portion of each sample was placed in stomacher bag with 90 ml of a Buffered peptone water (BPW) (Merck Dermstadt, Germany) and homogenized in a stomacher machine (Interscience, France) for 60 s. A serial dilution of fresh BPW was prepared and used in Most Probable Number (MPN) method, in which the samples were serially diluted to 1:10, 1:100 and 1:1000 and a portion of each dilution were transferred into three tubes containing 1 ml of Selenite Cystine Broth (SC; Merck, Darmstadt, Germany) and then these tubes were incubated at 37˚C for 18 to 24 h for enrichment stage. After overnight incubation, the MPN tubes were served as positive or negative for turbidity to determine the most probable number for the organisms.
2.3. Multiplex Polymerase Chain Reaction
MPN tubes showing turbid growth were then subjected to multiplex PCR to determine the presence of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium. DNA extraction was carried out using a boiled-cell method [8]. Briefly, 500 ml of the enrichment culture in the turbid MPN tubes were centrifuged at 12,000 rpm for 5 min and then the pellets were resuspended in 500 ul of sterile distilled water and the mixture was boiled in heat block for 10 min and immediately cooled at –20˚C for 10 min. The supernatant was obtained for use as the template DNA solution in the m-PCR. The optimization of the multiplex PCR was established before being used for the detection of Salmonella spp., Salmonella Enteritidis and Salmonela Typhimurium in naturally contaminated samples (Figure 1). Multiplex-PCR amplication was performed in a final volume of 50 µl using 4.0 µl of extracted DNA as template and the reaction mixture containing 10 µl of 5x PCR buffers, 10 µl of 3 mM of deoxynucleaside triphosphate mix (dNTP), and 1.5 µ/µl Taq polymerase. The primers pairs for detection of Salmonella spp. (ST11, forward: 5’-GCCAACCATTGCTAAATTGGCGCA-3’) and ST15, reverse: 5’-GGTAGAAATTCCCAGCGGGT ACTGG-3’), 3 µM) [9], Salmonella Enteritidis (Sef A-1, forward: 5’-GCAGCGGTTACTATTGCAGC-3’ and Sef A-2, reverse: 5’-TGTGACAGGGACATTTAGCG-3’, 2 µM), (Salmonella Typhimurium (Fli5, forward: 5’-CGG TGTTGCCCAGGTTGGTAAT-3’ and TypO4 reverse: 5’-ACTGGTAAAGATGGCT-3’, 7 µM), producing amplified fragment of 429 bp, 330 bp and 620 bp, respectively (Figure 1). The reaction mixture were heated at 94˚C for 2 min in the initial pre-denaturation step followed by 35 cycles of denaturation at 94˚C for 2 min, annealing at 53˚C for 1 min, extension at 72˚C for 1 min, and terminated at 72˚C after 7 min extension period. A 5 µl PCR product were run on 1.0% agarose at 90 V and the gel was then stained with ethidium bromide and visualised under ultra-violet light in a gel documentation system (SynGene).
2.4. Statistical Analysis
Normally distributed data for prevalence by Salmonella spp., Salmonella Enteritidis and Salmonenella Typhimurium over was analyzed for differences between groups using two-way analysis of variance (ANOVA) from SPSS software (version 17.0). The level of significance was set at P < 0.05.
3. Results and Discussion
The numbers of positive samples for Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium are summarized in Table 1. For the MPN-MPCR method, the density for cell concentration of all these pathogen ranged from <3 to 54 MPN/g (Table 2). Though foods of animal origin have frequently been implicated as sources of Salmonella infection [5], there are reports indicating raw vegetables may harbor potential foodborne pathogens such as Salmonella or Escherichia coli 0157:H7 [6].
Table 1 indicates the prevalence data that referring the other Salmonella serovars besides S. Enteritidis and S. Typhimurium in the samples analyzed. The detection of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in vegetarian burger patties indicates that this product required monitoring to ensure the safety for vegetarian consumers. The vegetarian burger patties contained a patties made by a soy sources and other vegetables product. Microbial contamination of vegetarian burger patties can occur from the environment since there is various handling procedure to produce the burger patties such as close contact with the contaminated container, equipment and utensil [7-9]. Though temperature for storage of these vegetarian burger patties were maintained at 4˚C, Salmonella is a facultative anaerobes which is able to grow in temperature range of 4˚C - 8˚C [10].
The result in this study indicates that raw salad vegetables examined can be a potential source of Salmonellosis. In this study, Salmonella Enteritidis was the most predominant serotype recovered from the leafy vegetables, namely lettuce and cabbage. This is aligned with the finding by reference [11] where leafy vegetables may allow more surface attachment that contribute to the high rate of Salmonella survival. These vegetable are top soil creeper and soil may be one of source of contamination if animal waste are used as fertilizer. In local eateries in Malaysia, Lettuce is normally used in the raw state as salad in chicken rice, hamburger, sandwiches and other ready to eat foods. Lettuce leaves should be washed thoroughly before use as salad and should be washed leaf by leaf to eliminates any potential pathogen attached on the leaves.
In this study, 14.28% of raw vegetables and vegetarian burger patties examined were contamina-ted with Salmonella Typhimurium. According to reference [12] vegetables which have a close contact with soil may have a higher possibility of contamination. The contaminated irrigation water, animal waste fertilizers and post harvest
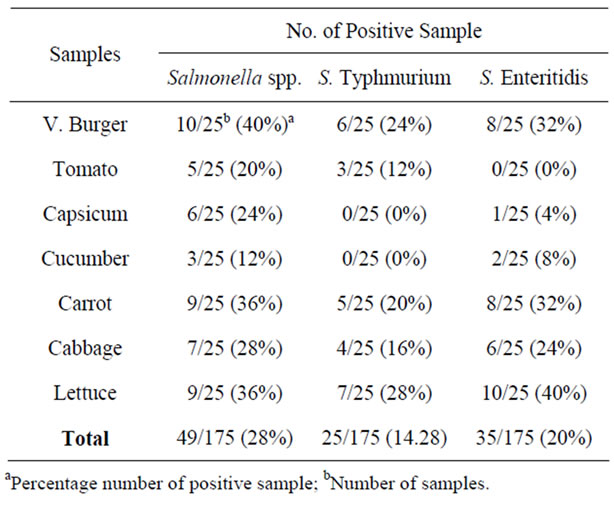
Table 1. Prevalence of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in raw vegetables and vegetarian burger patties.
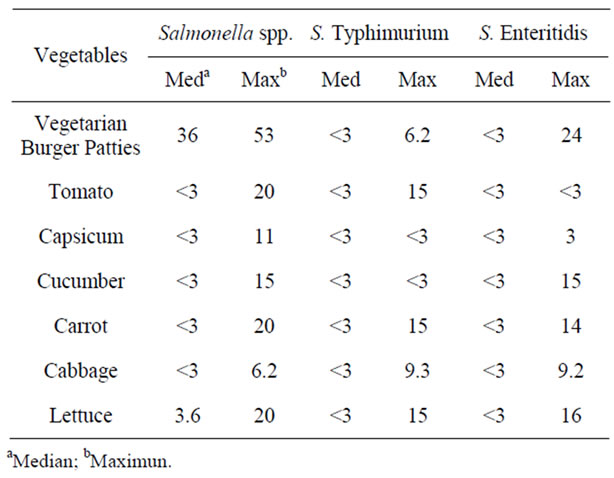
Table 2. Most probable number-multiplex PCR results of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in raw vegetables and vegetarian burger patties.
washing can be the sources of contamination in vegetables. This should be a concern since these serotypes have been implicated with several large outbreaks of Salmonellosis [12,13].
Cross contamination could occur during handling and from unhygienic surrounding environment. From the random observation in this study, vegetables were place at location nearer the poultry, egg, meat or dairy product which are common vehicle of infection for Salmonella outbreak [14]. Salmonella is able to grow under extreme environment and capable of adhesion to initiate biofilm formation on the abiotic surface. It has been reported that an isolate of Salmonella Newport grew to significant cell number and its growth was not affected by the frequency of replacement of the irrigation water, suggesting that Salmonella adhered tightly to the sprout [15].
The concentration of Salmonella cells on the raw vegetables and vegetarian burger patties were ranged from <3 to 53 MPN/g (Salmonella spp., <3 to 53 MPN/g, Salmonella Enteritidis, <3 to 16 MPN/g, and Salmonella Typhimurium, <3 to 15 MPN/g). According to reference [16,17] the dose required to cause infection by the Salmonella toxin is approximately 103 viable organisms orally for mice to excess of 1010 for adult cattle and 107 viable organism are normally required to initiate human gastroenteritis [17,18]. All the Salmonella serotypes detected in this study has the maximum concentration of 101 cell in a sample. However, in certain outbreaks, number of cases occur cause by Salmonella Typhimurium PT10 with the infective dose as low as 1(<10 - 100) cells/ml in product containing high level of fat were reported to cause illness [19]. If consumed with liquid food, the infectious dose will decreased and its will transverse stomach rapidly or food such as milk and cheese that able to neutralize the stomach acid [16,17]. Therefore, the result in this study indicates that the raw vegetables can be potential sources of Salmonellosis.
As referring to Figure 1, there are samples which contain Salmonella. Positive control (L1 and L2) refers to the DNA bands of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in size of DNA product at 429 bp, 330 bp and 620 bp. In some cases, when involved the multiplex PCR optimization, there are samples which could be contain more than desired bands. After amplified the MPCR condition from optimization to get the size of desire DNA product, and screen with the samples, there are possibility that cause this results such as pippetting error technique while providing the MPCR reaction and MPCR condition will be different when it was tested with the samples although it has been optimized. In addition, as shown by the Figure 1, MPCR product resulted in DNA bands of different thickness and clearness even they have the same primers. This is because of the cell concentration in each of the samples are different as stated in the Table 2. For example, Samples 5 and 6 have the DNA bands which are not the same thickness and clearness since the concentration of Salmonella is different in those samples.
Although there are reports on the previous studies on the Salmonella in raw vegetables, but there are no microbiological monitoring of these vegetables using combination of MPN-multiplex PCR associated with Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium in raw vegetables and vegetarian burger patties. Multiplex PCR approaches were successful in previous
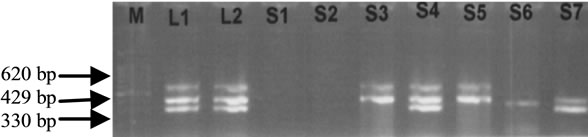
Figure 1. Agarose gel 1.0% electrophoresis of Salmonella spp. (429 bp), Salmonella Typhimurium (620 bp) and Salmonella Enteritidis (330 bp) genes. M: Marker with 100 bp. L1 and L2: Positive control with Salmonella spp. (429 bp), Salmonella Enteritidis (330 bp) and Salmonella Typimurium (620 bp). Sample 1: Tomato (no detection); Sample 2: Capsicum (no detection); Sample 3: Vegetarian burger: Salmonella spp. (429 bp), and Salmonella Typimurium (620 bp); Sample 4: Salmonella spp. (429 bp), Salmonella Enteritidis (330 bp) and Salmonella Typhimurium (620 bp); Sample 5: Cabbage: Salmonella spp. (429 bp), Salmonella Typimurium (620 bp); Sample 6: Cucumber: Salmonella spp. (429 bp); Sample 7: Carrot: Salmonella spp. (429 bp), Salmonella Enteritidis (330 bp).
studies by many researchers such as reference [11] on the simultaneous detection E. coli 0175, Salmonella spp. and Listeria monocytogenes, reference [11] study on the identification of Citrobacter freundii, Salmonella spp., and Salmonella Typhi and reference [3] reported the simultaneous identification of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium from environmental swab of poultry house.
4. Conclusions
The MPN-MPCR method used in this study was very useful and rapid tool to simultaneously detect more than one serovar of Salmonella in the raw vegetables and vegetarian burger patties samples analyzed in this study. Due to it sensitivity, although there may be low number of Salmonella on the naturally contaminated samples (<3 MPN/g), this method was able to allow us to simultaneously detect and enumerate the load of Salmonella in the samples examined. The employment of the MPN-MPCR method is an alternative quantitative method for Salmonella. Monitoring Salmonella in raw salad vegetables is important in the preparation of a risk assessment.
5. Acknowledgements
This study was supported by a Science Fund (Project No. 02-01-04-SF0390) from the Ministry of Science, Technology and Innovation, Malaysia and in part by a Grantin-Aid for Scientific Research (KAKENHI 191010) from the Japan Society for the Promotion of Sciences and by grant-in-aid of Ministry of Health, Labour and Welfare, Japan.
REFERENCES
- R. H. Creel, “Vegetables as a Possible Factor in DisSemination of Thypoid Fever,” Journal of Public Health Reports, Vol. 27, No. 6, 1912, pp. 187-193. doi:10.2307/4567429
- L. C. Chai, F. M. Ghazali, F. A. Bakar, H. Y. Lee, L. R. A. Suhaimi, S. A. Talib, Y. Nakaguchi, M. Nishibuchi and S. Radu, “Occurance of Thermophilic campylobacter spp. Contamination on Vegetables Farms in Malaysia,” Journal Microbiology Biotechnology, Vol. 19, 2009, pp. 1415-1420. doi:10.4014/jmb.0901.0002
- Y. C. Su and C. Liu, “Vibrio parahaemolyticus: A Concern of Seafood Safety,” Food Microbiology, Vol. 24, No. 6, 2007, pp. 549-558. doi:10.1016/j.fm.2007.01.005
- Kaysner and Depaola, “Food Drug Administration: Bacteriological Analytical Manual,” 2004. http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacterriologicalAnlyricalManuaBAM/ucm070830.htm.
- A. S. Noorzaleha, R. Gulam, H. Zaiton, R. Abdul, I. Siti, N. Mitsuaki and R. Son, “Incidence of Salmonella spp., in Raw Vegetables in Selangor, Malaysia,” Journal of Food Control, Vol. 14, No. 7, 2002, pp. 475-479. doi:10.1016/S0956-7135(02)00105-6
- L. R. Beuchat, “Pathogenic Microorganisms Associated with Fresh Produce,” Journal of Food Protection, Vol. 59, No. 2, 1996, pp. 204-216.
- C. Soumet, G. Ermel, V. Rose, P. Drouin, G. Salva and P. Colin, “Evaluation of a Multiplex PCR Assay for Simultaneous Identification of Salmonella spp., Salmonella Enteritidis and Salmonella Typhimurium from Environmental Swabs of Poultry Houses,” Letter Applied Microbiology, Vol. 28, No. 2, 1999, pp. 113-117. doi:10.1046/j.1365-2672.1999.00488.x
- D. C. Rodrigue, R. V. Tauxe and B. Rowe, “International Increase of Salmonella Enteritidis. A New Pandemic,” Epidemiology Infection, Vol. 105, No. 1, 1990, pp. 21-27. doi:10.1017/S0950268800047609
- F. L. Bryan, “Risk Associated with Vehicles of Foodborne Pathogens and Pathogens and Toxin,” Journal of Food Protection, Vol. 51, 1988, pp. 498-508.
- K. B. Arun, “Foodborne Microbial Pathogens: Mechanism and Pathogenesis,” India, 2008.
- L. L. Chia, H. C. Cheng, C. Chishih, C. H. Yhu, Y. L. Tzou and T. O. Jonathan, “A Multiplex Polymerase Chain Reaction Method for Rapid Identification of Citrobacter freundii and Salmonella Species, Including Salmonella Typhi,” Journal of Microbiology, Immunology and Infection, Vol. 40, No. 3, 2007, pp. 222-226.
- H. Herikstad, Y. Motarjemi and R. V. Tauxe, “Salmonella Surveillance: A Global Survey of Public Health Serotyping,” Epidemiology Infection, Vol. 129, No. 1, 2002, pp. 1-8. doi:10.1017/S0950268802006842
- C. L. Litter, F. C. Taylor, S. K. Sagoo, L. A. Gillespie, K. Grat and J. McLauchin, “Prevalence and Level of Listeria monocytogenes and Other Listeria Species in Prepacked Mixed Vegetbales Salad in UK,” Journal of Food Microbiology, Vol. 72, 2007, pp. 711-717.
- N. H. Bean and P. M. Griffin, “Foodborne Disease Outbreaks in the United States, 1973-1987: Pathogen, Vehicles and Trends,” Journal of Food Protection, Vol. 53, No. 9, 1990, pp. 804-881.
- Y. B. Ngwai, C. Wambebe and Y. Adachi, “Survivability of Salmonella Typhimurium L1388 and Salmonella Enteritidis L1225 under Stressful Growth Condition,” Online Journal of Health Allied Science, Vol. 6, 2007, p. 2.
- G. Andrea, M. Annalisa, C. Paola and M. Rosangela, “Simulteneous Detection of Escherichia coli 0175:H7, Salmonella spp., and Listeria monocytogenes by Multiplex PCR,” Journal of Food Control, Vol. 20, No. 8, 2009, pp. 733-738. doi:10.1016/j.foodcont.2008.09.010
- A. J. Lax, P. A. Barrow, P. W. Jones and T. S. Wallis, “Current Perspectives in Salmonellosis,” British Veterinary Journal, Vol. 151, No. 4, 1995, pp. 351-377. doi:10.1016/S0007-1935(95)80126-X

