Advances in Nanoparticles
Vol.05 No.01(2016), Article ID:63420,7 pages
10.4236/anp.2016.51006
Preparation and Characterization of SnO2 Nanofibers via Electrospinning
Rashmi Rani, Seema Sharma*
Ferroelectric Research Laboratory, Department of Physics, A. N. College, Patna, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 September 2015; accepted 12 February 2016; published 15 February 2016
ABSTRACT
Tin oxide (SnO2) nanofibers are successfully prepared by electrospinning homogeneous viscous solutions of tin acetate in polyvinylpyrrolidone (PVP). The electrospinning is carried out by applying a DC voltage to the tip of a syringe and maintaining the tip to collector distance (TCD), i.e. at DC electric field of 1.25 kV∙cm−1. The electrospun nanofibers are calcined between 550˚C and 650˚C for 4 h. Both spun and heat treated nanofibers are characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), Fourier transform infra red spectroscopy (FTIR) etc. XRD analysis of calcined nanofibers confirms the formation of pure tin oxide. TEM study showed that fibers have a polycrystalline structure with multiple nano-grains.
Keywords:
Naonofibers, TEM, Electrospinning, PVP

1. Introduction
One-dimensional (1D) nanomaterials have stimulated great interest due to their importance in basic scientific research and potential technological applications [1] - [3] . It is generally accepted that 1D nanostructures are ideal systems for exploring a large number of novel phenomena at the nanoscale and investigating the size and dimensionality dependence of structure properties for potential applications [4] . 1D nanomaterials are also expected to play an important role as both interconnects and functional units in fabricating electronic, optoelectronic, electrochemical, and electromechanical devices with nanoscale dimensions [5] . Among the inorganic se- miconductor nanomaterials, 1D metal oxide nanostructures are the focus of current research efforts in nanotechnology since they are the most common minerals on the Earth due to their special shapes, compositions, and chemical, and physical properties.
Tin oxide is an n-type wide band gap semiconductor and it is being used in a variety of applications such as gas sensors [6] [7] and optoelectronic devices [8] [9] . SnO2 gas sensors give a conductance change according to the chemical interaction between the reducing/oxidizing gases and surface adsorbed species. To enhance the gas response, the particle size needs to be decreased to the thickness level of the electron depletion layer [10] [11] . However, the strong agglomeration between primary particles often hampers the diffusion of analyte gas toward the entire sensing surface, which decreases the gas response [12] [13] . The properties of SnO2 in various forms such as nanoparticles [14] , nanowires [15] , nanobelts [16] and other one-dimensional nanostructures have been extensively studied. In comparison with solid one-dimensional nanomaterials, nanotubes gain the advantages in practical applications to catalysts and gas sensors, owing to their higher surface-to-volume ratio. Moreover, the previous studies have been suggested that, the nanostructure’s surface plays a significant role in defining their conductivity. Generally, the conventional methods for preparing SnO2 nanotubes by self assembly [17] and templates directed process [18] often suffer from strict synthesis conditions or tedious procedures. Electrospinning has been considered as a simple and efficient method for producing polycrystalline nanofibers from a rich variety of materials including TiO2 [19] , SnO2 [20] , WO3 [21] , SnO2-TiO2 bi-component nanowires [22] , MoO3 [23] , ZnO [24] , and metal modified TiO2 nanofibers [25] . However, templates and the co-electrospinning technique [26] had to be used for forming inorganic nanotubes. A one-step method for the fabrication of SnO2 nanofibers by directly annealing electrospun composite nanofibers has been presented in this work.
2. Experiment
2.1. Chemicals and Materials
Stannic chloride pentahydrate (SnCl4∙5H2O), ethanol and N,N-dimethylformamide (DMF), Polyvinylpyrrolidone (PVP, Mw = 1,300,000 g∙mol−1) were purchased from Sigma Aldrich.
2.2. Instruments
The X-ray diffraction was from RigakuMinifiex, Japan and the transmission electron microscopy (TEM) was from JEOL, JEM-2010. The FT-IR spectra were PerkinElmer, USA.
2.3. Preparation of SnO2 Nanofibers
Transparent spinning solution was prepared by adding 3 g of SnCl4∙5H2O into 10 wt% PVP in ethanol/DMF solvent mixture (weight ratio 1:1), and the weight ratio of PVP and SnCl4∙5H2O was also 1:1, followed by magnetic stirring at ambient temperature for 24 h. Later, as-prepared solution was introduced in 10 ml syringe with a hypodermic needle (dia. 2 mm) in a controlled electrospinning setup. The flow rate and applied electric field was varied to obtain the optimal conditions for the electrospun fibers. The obtained optimal condition was as follows: flow rate 0.2 ml/h and the applied electric field 1.25 kV/cm. High electric field strength (1.4 kV/cm) was employed to enable for the high stretch rates of the electrospun jet. The distance between the needle tip and the collector against the applied electric field was set as 18 cm. The longer distance between the needle tip and the collector aided the stretching of the jet due to the increase in the distance covered by the spiraling electrospun jet before being deposited on the collector. The fiber mesh obtained was then annealed to obtain SnO2 nanofibers. The electrospun fibers were calcined at 550˚C - 650˚C for 4 h.
2.4. Characterization of the Fabricated Materials
The fine calcined powders were used to characterize the structural and microstructural properties of the compound. The X-ray diffraction pattern of the compounds were recorded at room temperature using X-ray powder diffractometer with CuKα (α = 1.5418 Å) radiation in a wide range of Bragg angles 2θ (20˚ ≤ 2θ ≤ 70˚) at a scanning rate of 2˚ min−1. The structures of the electrospun nanofibers were observed by transmission electron microscopy, Spectroscopic characterization has been investigated by a Fourier-transform infrared (FT-IR) spectrophotometer.
3. Results and Discussion
3.1. Characterization of the Fabricated Materials
3.1.1. XRD Analysis
Figure 1 shows that the XRD pattern of the SnO2 nanofiber calcined at ~600˚C for 4 h. It can be seen that all the
Figure 1. XRD pattern of SnO2 nanofibers calcined at 600˚C for 4 h.
diffraction lines are assigned to tetragonal cassiterite crystalline phase of tin oxide (JCPDS card No. 77- 0452).
No characteristic peaks of impurities were observed, indicating the high purity of the products. This figure shows the electrospun SnO2 fibers crystallized into the cassiterite structure with primary (110), (101), and (200) crystallite orientations. For the as-prepared SnO2 nanofiber, by using Scherrer’s formula,
 (1)
(1)
(where D is the mean grain size, K (=0.94) is the Scherrer’s constant related to the shape and index (h.k.l) of the crystal, θ is diffraction angle and λ is the X-ray wavelength used CuKα, 1.54056 Å, β2θ is broadening of diffraction lines measured at half of its maximum intensity (in radian)). We estimated that the average grain size was about 11 nm. The lattice parameters were calculated from POWD software for the tetragonal crystal structure and were found to be
A = 7.5650 Å, c = 15.0642 Å, c/a = 1.9913 Å
3.1.2. TEM Analysis
Figure 2(a) shows the transmission electron microscopy (TEM) image of the nanofibers after calcination, indicating that the fibers were formed through the agglomeration in small beadforms. The average particle size of SnO2 nanofiber observed in TEM image was computed to be 11.731 nm (Figure 2(b)) through the statistical distribution calculation.
The selected area electron diffraction (SAED pattern Figure 2(c)) shows characteristic crystalline planes of the SnO2 nanofibers indicated the polycrystalline nature of SnO2nanofiber. Inter-planar spacing (also known as d-spacing) was calculated using Bragg equation (Bragg and Bragg 1931):
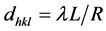 (2)
(2)
where, R (in mm) is the radius of diffraction pattern, L (320 mm) is the distance between specimen and photographic film and λ (0.02736 Å) is the wavelength of the electron based on the accelerating voltage (200 kV).
The calculated values for d-spacings (dhkl) (3.27 and 2.69 Å) were found consistent with the values from standard JCPDS File No. 77-0452 for SnO2 (Table 1). Subsequently, two distinct diffraction planes were indexed as (110) and (101) facets which correspond to the tetragonal phase of tin oxide.
Figure 3 shows the Williamson Hall plot of the SnO2nanofiber indicating crystalline strain of the material is about 0.04221. It relies on the principle that the approximate formulae for size broadening, βL, and strain broadening, βe, vary quite differently with respect to Bragg angle, θ:
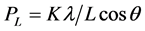 (3)
(3)
 (4)
(4)


Figure 2. (a) TEM image of SnO2 nanofiber calcined at 600˚C for 4 h. Inset: nanofiber in dark scattered surface; (b) Particle size distribution of SnO2 nanofibers; (c) SEAD pattern of SnO2 nanofibers (denotes diffraction facets for SnO2).
Table 1. Comparison of inter-planer spacings (dhkl) from standard tin oxide diffraction data (JCPDS file No. 77-0452) with the calculated and experimentally observed values from SAED and XRD diffractogram.
One contribution varies as 1/cosθ and the other as tanθ. If both contributions are present then their combined effect should be determined by convolution. The simplification of Williamson and Hall is to assume the convolution is either a simple sum or sum of squares (see previous discussion on Sources of Peak Broadening within this section). Using the former of these then we get:
Figure 3. Williamson-Hall plot of SnO2 nanofibers.
Figure 4. FT-IR spectrum of SnO2 nanofibers.
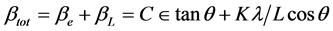 (5)
(5)
If we multiply this equation by cosθ we get:
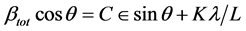 (6)
(6)
and comparing this to the standard equation for a straight line (m = slope; c = intercept)
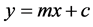
We see that by plotting βtot cosθ versus sinθ we obtain the strain component from the slope (Cε).
3.1.3. FT-IR Analysis
Figure 4 sows the FTIR spectra of the prepared SnO2 samples dried at 80˚C. The peaks around 1645 and 3421 cm−1 correspond to the bending vibrations of absorbed molecular water and the stretching vibrations of−OH groups respectively. The weak peaks at 2367 and 2907 cm−1 belong to the stretching vibrations of-C-H-bonds, and the ones at 1405, 1257 and 1023 cm−1 correspond to the bending vibrations of -CH2 and -CH3, which shows that a few organic groups are absorbed on the surfaces of SnO2nanoparticles [27] . From this spectrum, it can be observed apparently at 663 cm−1 that strong band associated with the antisymmetric Sn-O-Sn stretching mode of the surface-bridging oxide formed by condensation of adjacent surface hydroxyl groups.
4. Conclusion
In conclusion, SnO2 nanofibers have been produced by an electrospinning method and characterized by X-ray powder diffraction (XRD) and transmission scanning electron microscopy (TEM). The XRD result showed that SnO2 nanofibers have tetragonal cassiterite crystalline phase. The best annealing temperature is about 600˚C. The sizes of the nanoparticles in the nanofibers estimated by TEM were found be about 11 nm while those of the nanofiber lie in the range of about 100 - 200 nm.
Cite this paper
RashmiRani,SeemaSharma, (2016) Preparation and Characterization of SnO2 Nanofibers via Electrospinning. Advances in Nanoparticles,05,53-59. doi: 10.4236/anp.2016.51006
References
- 1. Yang, P.D., Yan, H.Q., Mao, S., Russo, R., Johnson, J., Saykally, R., Morris, N., Pham, J., He, R. and Choi, H. (2002) Controlled Growth of ZnO Nanowires and Their Optical Properties. Journal of Advanced Materials, 12, 323.
http://dx.doi.org/10.1002/1616-3028(20020517)12:5<323::aid-adfm323>3.0.co;2-g - 2. Zhai, T.Y., Zhong, H.Z., Gu, Z.J., Peng, A.D., Fu, H.B., Ma, Y., Li, Y.F. and Yao, J.N. (2007) Manipulation of the Morphology of ZnSe Sub-Micron Structures Using CdSe Nanocrystals as the Seeds. Journal of Physical Chemistry C, 111, 2980-2986.
http://dx.doi.org/10.1021/jp067498x - 3. Lieber, C.M. and Wang, Z.L. (2007) Functional Nanowires. MRS Bulletin, 32, 99-108.
http://dx.doi.org/10.1557/mrs2007.41 - 4. Li, Z., Wang, X. and Lin, T. (2014) Highly Sensitive SnO2 Nanofiber Chemiresistors with a Low Optimal Operating Temperature: Synergistic Effect of Cu2+/Au Co-Doping. Journal of Materials Chemistry A, 2, 13655-13660.
http://dx.doi.org/10.1039/C4TA01926A - 5. Xia, Y.N., Yang, P.D., Sun, Y.G., Wu, Y.Y., Mayers, B., Gates, B., Yin, Y.D., Kim, F. and Yan, H.Q. (2003) One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Advanced Materials, 15, 353-389.
http://dx.doi.org/10.1002/adma.200390087 - 6. Song, X., Qi, Q., Zhang, T. and Wang, C. (2009) A Humidity Sensor Based on KCl-doped SnO2 Nanofibers. Sensors and Actuators B: Chemical, 138, 368-373.
http://dx.doi.org/10.1016/j.snb.2009.02.027 - 7. Zhang, Y., Li, J.P., An, G.M. and He, X. (2010) Highly Porous SnO2 Fibers by Electrospinning and Oxygen Plasma Etching and Its Ethanol-Sensing Properties. Sensors and Actuators B: Chemical, 144, 43-48.
http://dx.doi.org/10.1016/j.snb.2009.10.012 - 8. Zhang, Z., Shao, C., Li, X., Zhang, L., Xue, H., Wang, C. and Liu, Y. (2010) Electrospun Nanofibers of ZnO-SnO2 Heterojunction with High Photocatalytic Activity. Journal of Physical Chemistry C, 114, 7920-7925.
http://dx.doi.org/10.1021/jp100262q - 9. Yang, Z., Du, G., Feng, C., Li, S., Chen, Z., Zhang, P., Guo, Z., Yu, X., Chen, G., Huang, S. and Liu, H. (2010) Synthesis of Uniform Polycrystalline Tin Dioxide Nanofibers and Electrochemical Application in Lithium-Ion Batteries. Electrochimica Acta, 55, 5485-5491.
http://dx.doi.org/10.1016/j.electacta.2010.04.045 - 10. Yamazoe, N., Sakai, G. and Shimanoe, K. (2003) Oxide Semiconductor Gas Sensors. Catalysis Surveys from Asia, 7, 63-75.
http://dx.doi.org/10.1023/A:1023436725457 - 11. Yamazoe, N. (1991) New Approaches for Improving Semiconductor Gas Sensors. Sensors and Actuators B: Chemical, 5, 7-19.
http://dx.doi.org/10.1016/0925-4005(91)80213-4 - 12. Korotcenkov, G. (2005) Gas Response Control through Structural and Chemical Modification of Metal Oxide Films: State of the Art and Approaches. Sensors and Actuators B: Chemical, 107, 209-232.
http://dx.doi.org/10.1016/j.snb.2004.10.006 - 13. Williams, D.E. and Pratt, K.F.E. (2000) Microstructure Effects on the Response of Gas-Sensitive Resistors Based on Semiconducting Oxides. Sensors and Actuators B: Chemical, 70, 214-221.
http://dx.doi.org/10.1016/S0925-4005(00)00572-4 - 14. Gong, S., Liu, J., Xia, J., Quan, L., Liu, H. and Zhou, D. (2009) Gas Sensing Characteristics of SnO2 Thin Films and Analyses of Sensor Response by the Gas Diffusion Theory. Materials Science and Engineering B: Advanced Functional Solid-State Materials, 164, 85-90.
http://dx.doi.org/10.1016/j.mseb.2009.07.008 - 15. Xu, L., Dong, B., Wang, Y., Bai, X., Liu, Q. and Song, H. (2010) Electrospinning Preparation and Room Temperature Gas Sensing Properties of Porous In2O3 Nanotubes and Nanowires. Sensors and Actuators B: Chemical, 147, 531-538.
http://dx.doi.org/10.1016/j.snb.2010.04.003 - 16. Soares, A.J. and Perry, R.J. (2010) Modeling and Simulation of a Single Tin Dioxide Nanobelt FET for Chemical Sensors. IEEE Sensors Journal, 10, 235-242.
http://dx.doi.org/10.1109/JSEN.2009.2032154 - 17. Qiu, Y., Chen, P. and Liu, M. (2010) Evolution of Various Porphyrin Nanostructures via an Oil/Aqueous Medium: Controlled Self-Assembly, Further Organization, and Supramolecular Chirality. Journal of the American Chemical Society, 132, 9644-9652.
http://dx.doi.org/10.1021/ja1001967 - 18. Park, J.Y., Choi, S.-W. and Kim, S.S. (2010) A Synthesis and Sensing Application of Hollow ZnO Nanofibers with Uniform Wall Thicknesses Grown Using Polymer Templates. Nanotechnology, 21, Article ID: 475601.
http://dx.doi.org/10.1088/0957-4484/21/47/475601 - 19. Kim, I.D., Rothschild, A., Lee, B.H., Kim, D.Y., Jo, S.M. and Tuller, H.L. (2006) Ultrasensitive Chemiresistors Based on Electrospun TiO2 Nanofibers. Nano Letters, 6, 2009-2013.
http://dx.doi.org/10.1021/nl061197h - 20. Wang, Y., Ramos, I. and Santiago-Aviles, J.J. (2007) Detection of Moisture and Methanol Gas Using a Single Electrospun Tin Oxide Nanofiber. IEEE Sensors Journal, 7, 1347-1348.
http://dx.doi.org/10.1109/JSEN.2007.905045 - 21. Wang, G., Ji, Y., Huang, X., Yang, X., Gouma, P.I. and Dudley, M.J. (2006) Fabrication and Characterization of Polycrystalline WO3 Nanofibers and Their Application for Ammonia Sensing. The Journal of Physical Chemistry B, 110, 23777-23782.
http://dx.doi.org/10.1021/jp0635819 - 22. Liu, Z., Sun, D.D., Guo, P. and Leckie, J.O. (2007) An Efficient Bicomponent TiO2/SnO2 Nanofiber Photocatalyst Fabricated by Electrospinning with a Side-by-Side Dual Spinneret Method. Nano Letters, 7, 1081-1085.
http://dx.doi.org/10.1021/nl061898e - 23. Li, S., Shao, C., Liu, Y., Tang, S. and Mu, R. (2006) Nanofibers and Nanoplatelets of MoO3 via an Electrospinning Technique. Journal of Physics and Chemistry of Solids, 67, 1869-1872.
http://dx.doi.org/10.1016/j.jpcs.2006.04.017 - 24. Kim, I.D., Hong, J.M., Lee, B.H., Kim, D.Y., Jeon, E.K., Choi, D.K. and Yang, D.J. (2007) Dye-Sensitized Solar Cells Using Network Structure of Electrospun ZnO Nanofiber Mats. Applied Physics Letters, 91, Article ID: 163109.
http://dx.doi.org/10.1063/1.2799581 - 25. Formo, E., Lee, E., Campbell, D. and Xia, Y. (2008) Functionalization of Electrospun TiO2 Nanofibers with Pt Nanoparticles and Nanowires for Catalytic Applications. Nano Letters, 8, 668-672.
http://dx.doi.org/10.1021/nl073163v - 26. Chen, H., Wang, N., Di, J., Zhao, Y., Song, Y. and Jiang, L. (2010) Nanowire-in-Microtube Structured Core/Shell Fibers via Multifluidic Coaxial Electrospinning. Langmuir, 26, 11291-11296.
http://dx.doi.org/10.1021/la100611f - 27. Gnanam, S. and Rajendran, V. (2010) Preparation of Cd-Doped SnO2 Nanoparticles by Sol-Gel Route and Their Optical Properties. Journal of Sol-Gel Science and Technology, 56, 128-133.
http://dx.doi.org/10.1007/s10971-010-2285-7
NOTES
*Corresponding author.







