Open Journal of Applied Sciences
Vol.4 No.6(2014), Article ID:46010,6 pages DOI:10.4236/ojapps.2014.46033
Synthesis of Cardanol Sulfonate Gemini Surfactant and Enthalpy-Entropy Compensation of Micellization in Aqueous Solutions
Weiguang Shi, Pengxiang Wang, Cuiqin Li*, Jie Li, Haiyan Li, Zhiqiu Zhang, Song Wu, Jun Wang
Provincial Key Laboratory of Oil & Gas Chemical Technology, College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing, China
Email: *licuiqin78@163.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 5 April 2014; revised 7 May 2014; accepted 15 May 2014
ABSTRACT
A novel cardanol sulfonate Gemini surfactant with high surface properties was synthesized by cashew phenol, 1,3-dibromopropane and chlorosulfonic acid through three steps procedure of etherification, sulfonation and neutralization. A surface tension method was employed to investigate the thermo-dynamic properties of micellization in aqueous solution for cardanol sulfonate Gemini surfactant synthesized in laboratory. As a result, the micellization of cardanol sulfonate Gemini surfactant in aqueous solutions is spontaneous and entropy-driven. The micellization process is enthalpy-entropy compensated with the compensation temperature (Tc) of 308 ± 1 K.
Keywords:Cardanol, Gemini Surfactant, Micellization, Enthalpy-Entropy Compensation

1. Introduction
As a kind of green environmental protection raw material and biomass resources, cashew phenol is widely used as polymer materials, adhesives, coatings and composite materials [1] [2] . In recent years, many surfactants with high activity, lower cost, wide application and no pollution green features [3] were synthesized using cashew phenol as the starting material. Cashew-based carboxylate surfactant was synthesized by Cesar Scorzza [4] , and its ability to reduce the surface tension is fairly with the traditional carboxylic acid salt surfactants. While Passapan Peungjitton [5] was synthesized cashew-based sulfonate surfactant, which has good surface activity and biodegradability; Wang and his group [6] [7] synthesized a kind of cashew-based surfactant with good surface activity and emulsification properties, which can be used in enhanced oil recovery (EOR). But most of the previous researches have focused on cashew-based monomeric surfactant, few studies about the Gemini surfactant are reported.
In this paper, a designed cardanol sulfonate Gemini surfactant was synthesized by three-step reactions. The surface tension (γcmc) and critical micelle concentrations (CMC) were obtained by a drop-volume method, and the surface properties in aqueous solution, including the efficiency of decreasing the surface tension (pC20), the maximum surface adsorption (Γmax) and the saturation adsorption area per surfactant molecule (Amin) were calculated by Gibbs adsorption equation. And the thermodynamic parameters of micellization (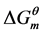 ,
, ) were investigated, which may provide some information for the relationships between the formation ability, the stability of the micelles and the molecular structure of cardanol sulfonate Gemini surfactant.
) were investigated, which may provide some information for the relationships between the formation ability, the stability of the micelles and the molecular structure of cardanol sulfonate Gemini surfactant.
2. Experimental
2.1. Materials
Cardanol was purchased from Mei Dong Bio-Logical Material Co., Ltd. in Shanghai, China. 1,3-dibromo propane was obtained from Yuan Li chemical reagent company in Tianjin, China. Chlorosulfonic acid, sodium hydroxide and methylene chloride were provided by Hua Dong chemical reagent company in Shenyang, China. Te-traethylammonium bromide was supplied by chemical engineering technology research and development center in Guangdong, China. Diethyl ether and ethylic acid was obtained from Kaitong chemical reagent factory in Tianjin, China. All solvents were analytical grade and purified before using them. And deionized water was employed for all experiments.
2.2. Preparation of Cardanol Sulfonate Gemini Surfactant
In order to synthesize cardanol sulfonate Gemini surfactant, several steps of chemical reaction and treatments were employed. In the first procedure, the etherification reaction was carried out to generate cardanol Gemini surfactant. And in the next procedure, sulfonation reaction is undergoing to form cardanol sulfonic acid Gemini surfactant with the products obtained from the first procedure. In the final procedure, the product generated from the last two steps, was neutralized using sodium hydroxide solution, and then filtered, distillated and obtained the purity of the final product is 82.1% by two-phase titration methods. The schematic steps for synthetic route of cardanol sulfonate Gemini surfactant are shown in the Figure 1, and all the experimental details are recorded in our previous work [7] .
2.3. Analytical Methods
The structure for designed cardanol sulfonate Gemini surfactant was confirmed by spectral data. 1 H NMR (400 MHz) spectra were recorded with solutions in CDCl3 by Bruker DRX 400 spectrometer using tetramethylsilane as a standard. IR spectrum was obtained by pellets in KBr methods using a Bruker Vector 22 FTIR spectrometer. Surface tensions of surfactant aqueous solutions at different concentrations were measured at 25.0˚C ± 0.1˚C by a drop-volume method [8] . The surface tensions were averaged values by three times measurements. The estimated error of the surface tension measurements was in the 0.01 mN/m. The surface tension value was decreased gradually with increasing the concentration of cardanol sulfonate Gemini surfactant and exhibited an inflection point, the concentration corresponding to the inflection point is the CMC. The thermo-dynamic properties of micellization in aqueous solution were studied by surface tension method.
3. Results and Discussion
3.1. Characterization of Cardanol Sulfonate Gemini Surfactant
The structure of the designed cardanol sulfonate Gemini surfactant was identified by IR spectrum and 1H NMR spectra. The IR frequency of synthesized cardanol sulfonate Gemini surfactant are 2970 cm−1, 2871 cm−1, 1115 cm−1, 1014 cm−1, 929 cm−1, 864 cm−1, 723 cm−1, and 662 cm−1, which are the characteristic peaks of functional
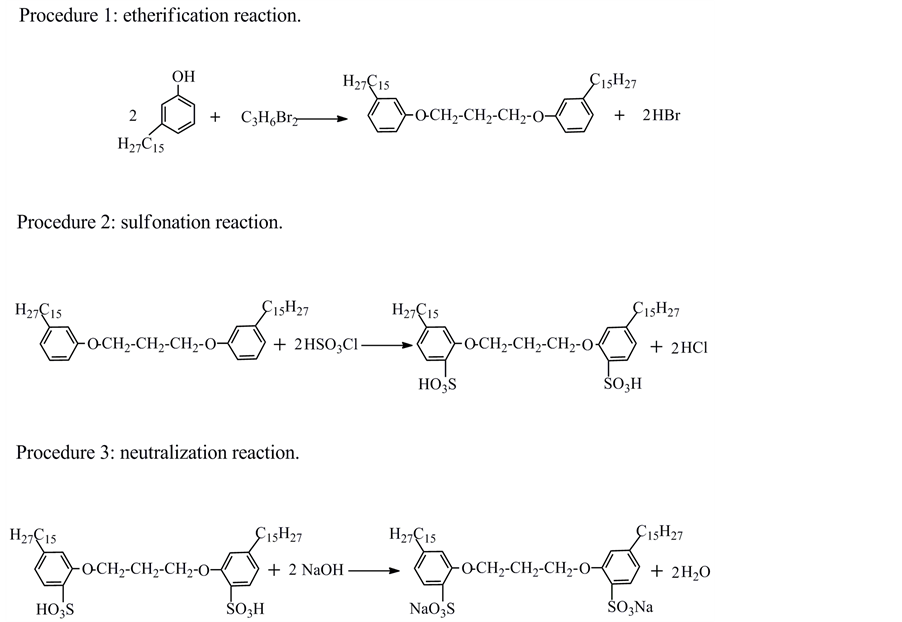
Figure 1. Synthetic route of cardanol sulfonate Gemini surfactant.
groups for -CH=CH-, -S=O, -C-O-, and Ph-H. And 1H NMR (400 MHz, CDCl3), δ: 7.77 ~ 6.71 (d, 3H) ArH; 5.77 (m, 8H) CHCH2; 4.02 (m, 4H) OCH2CH2CH2O; 2.77 (m, 4H) CHCH2CH; 2.50 (m, 4H) CH2Ar; 2.04 (m, 2H) OCH2CH2CH2O; 1.58 ~ 1.3 (m, 32H) CH2; 0.90 (t, 6H) CH3.
3.2. Surface Properties in Aqueous Solution
As is shown in Table 1, the CMC for cardanol sulfonate Gemini surfactant was 6.20 × 10−2 mmol·L−1, and γcmc was 36.92 mN·m−1. It displays a lower surface tension as a kind of Gemini surfactants, and the critical micelle concentration is two orders of magnitudes lower than the cardanol sulfonate single chain surfactant synthesized before [7] . And the value of Γmax of Gemini surfactant is 6.16 mol·cm−2 and the Amin is 0.27 nm2. Owing to two hydrophobic chains, it increases the distortion of the hydrophilic group. At the same time, the electrostatic interaction is weaken, which can restrain the hydrophilic group divorced, close and regular the arrangement of molecules. High concentration of micelle leads to higher surface activity, increase the maximum surface adsorption and reduce the saturation adsorption area per surfactant molecule that compared to the conventional surfactants.
The efficiency of decreasing the surface tension can be characterized by the value of logarithm of the surfactant concentration pC20. The consumption of Gemini surfactant is 0.06 mmol·L−1 while 4.50 mmol·L−1 of monomeric surfactants, which is used to reduce the surface tension of water by 20 mN·m−1 (Table 1). As a result, smaller value of pC20 makes higher tendency of the surfactant to adsorb on the air-water interface, in a mean while, to form micelles easier and efficiently reduce the surface tension. It indicates that the Gemini surfactant is superior in improving the efficiency of reducing surface tension.
3.3. Equations Thermodynamic Parameter for the Micellization in Aqueous Solution
Base on the fundamental principle of aqueous solution thermodynamics and classical mass-action model of micellization, thermodynamic parameters were calculated at 25˚C using the Equation (1)-(3):
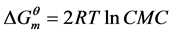 (1)
(1)
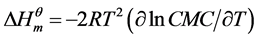 (2)
(2)
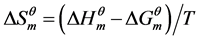 (3)
(3)
And the Figure 2 was plotted with the natural logarithms of CMC of cardanol sulfonate Gemini surfactant shown in Table 2 and temperatures to obtain ∂lnCMC/∂T in Equation (2). Then the thermodynamic parameters ( ,
, and
and ) can be calculated (Table 3).
) can be calculated (Table 3).
Table 1. Parameters of cardanol sulfonate Gemini surfactant.
aCardanol sulfonate singlechain surfactant [7] ; bCardanol sulfonate Gemini surfactant.
Table 3. Thermodynamic parameters of the micellization of designed Gemini surfactant in aqueous solution.
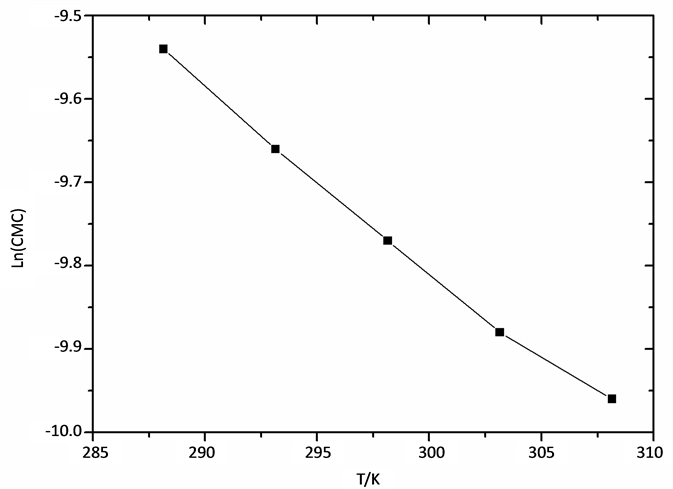
Figure 2. ln(CMC)-T plot of cardanol sulfonate Gemini surfactant in aqueous solution.
As is shown in Table 3, all values of 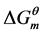 for micellization at 25˚C are negative. It means that the micellization process of Gemini surfactants in aqueous solutions is spontaneous, and the micellization system is stable.
for micellization at 25˚C are negative. It means that the micellization process of Gemini surfactants in aqueous solutions is spontaneous, and the micellization system is stable.  shows that the micellization is endothermic process.
shows that the micellization is endothermic process. 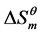 is positive, which indicates that the “iceberg structure” is broken and the water molecules tend to a disordered state. With increasing of temperature,
is positive, which indicates that the “iceberg structure” is broken and the water molecules tend to a disordered state. With increasing of temperature, 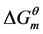 and
and 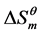 decrease, but the
decrease, but the 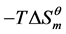 value increases, which illustrate that the contribution of the entropy for changing Gibbs free energy is diminished by increasing of temperature. In another word, high temperature is the disadvantage of micellization because of the driving force of entropy is reduced.
value increases, which illustrate that the contribution of the entropy for changing Gibbs free energy is diminished by increasing of temperature. In another word, high temperature is the disadvantage of micellization because of the driving force of entropy is reduced.
3.4. Enthalpy-Entropy Compensation
The enthalpy-entropy compensation for the micellization process of Gemini surfactants in aqueous solutions can be illuminated by the formula below:
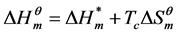 (1)
(1)
where Tc is the compensation temperature, it is the parameter of dehydration in the procedure of molecular aggregates, which represents the interaction between solute and solvent. 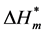 is the value of enthalpy change, it is the parameter of aggregation for hydrophobic group of the Gemini surfactant, which reflects the interaction between the solute and solute. When
is the value of enthalpy change, it is the parameter of aggregation for hydrophobic group of the Gemini surfactant, which reflects the interaction between the solute and solute. When 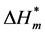 is smaller, the stability of the corresponding aggregate structure is higher. When
is smaller, the stability of the corresponding aggregate structure is higher. When  is zero,
is zero,  is defined as the value of entropy change. Under this situation, the micellization is forced by entropy change. This result is consistent with literature studies [9] .
is defined as the value of entropy change. Under this situation, the micellization is forced by entropy change. This result is consistent with literature studies [9] .
The relationship between the enthalpies of intention (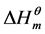 ) and the entropy term (
) and the entropy term (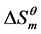 ) was investigated that a linear plots line was obtained with slope of unity (Figure 3). Then a linear fitting was done to calculate the compensation temperature Tc, and it was found to be 308 ± 1 K (Table 4). It illustrates that micellization of cardanol sulfonate Gemini surfactant in aqueous solution is proved to be enthalpy-entropy compensation. In a sense, compensation temperature is the key point for micellization in aqueous solution, the contributions of enthalpy or entropy are equal [10] .
) was investigated that a linear plots line was obtained with slope of unity (Figure 3). Then a linear fitting was done to calculate the compensation temperature Tc, and it was found to be 308 ± 1 K (Table 4). It illustrates that micellization of cardanol sulfonate Gemini surfactant in aqueous solution is proved to be enthalpy-entropy compensation. In a sense, compensation temperature is the key point for micellization in aqueous solution, the contributions of enthalpy or entropy are equal [10] .
4. Conclusion
A designed cardanol sulfonate Gemini surfactant with high surface properties was synthesized successfully. The micellization process of cardanol sulfonate Gemini surfactants in aqueous solutions is spontaneous and the micellization is mainly forced by entropy change. High temperature is the disadvantage for micellization because of the

Figure 3. 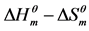 plots of the micellization of cardanol sulfonate Gemini surfactant in aqueous solution.
plots of the micellization of cardanol sulfonate Gemini surfactant in aqueous solution.
Table 4. Fitting result for 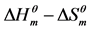 plots of cardanol sulfonate Gemini surfactant.
plots of cardanol sulfonate Gemini surfactant.
r: linear correlation coefficient.
driving force of entropy is reduced. In the meanwhile, there is enthalpy-entropy compensation in the micellization, and the compensation temperature is 308 ± 1 K.
Acknowledgements
We thank the key technologies R & D program of the education department of Hei Longjiang province China for financial support (KY120124).
References
- Hu, Y.M. and Guo, M.L. (1990) The Composition, Chemical Property and Application of CNS. Journal of Chinese Lacquer, 1, 40-41.
- Paramashivappa, R. and Phani Kumar, P. (2002) Synthesis of Sildenafil Analogues from Anacardic Acid and Their Phosphodiesterase-5 Inhibition. Journal of Agricultural and Food Chemistry, 50, 7709-7713. http://dx.doi.org/10.1021/jf0258050
- Rosen, M.J. and Kunjappu, J.T. (2004) Surfactants and Interfacial Phenomena. 4th Edition, Milton J. Ropsen & Joy T. Kunjappu, New York.
- Scorzza, C., Nieves, J. and Vejar, F. (2009) Synthesis and Physicochemical Characterization of Anionic Surfactants Derived from Cashew Nut Shell Oil. Journal of Surfactants and Detergents, 13, 27-31. http://dx.doi.org/10.1007/s11743-009-1143-5
- Peungjitton, P., Sangvanich, P. and Pornpakakul, S. (2008) Sodium Cardanol Sulfonate Surfactant from Cashew Nut Shell Liquid. Journal of Surfactant and Detergents, 12, 85-89. http://dx.doi.org/10.1007/s11743-008-1082-6
- Li, H.Y., Wang, J., Liu, C.H., Han, J., Li, C.Q. and Ning, M.M. (2012) Synthesis and Surface Activity of CashewBased Anion-Nonionic Surfactants. Open Journal of Applied Sciences, 2, 93-97. http://dx.doi.org/10.4236/ojapps.2012.22012
- Wang, J., Wang, Y.W., Li, C.Q., Ma, X.G. and Zhu, X.F. (2011) Synthesis of Biomass Cardanol Sulfonate Surfactant and Its Emulsification Performance. Chemical Research, 22, 48-51. http://dx.doi.org/10.4028/www.scientific.net/AMR.183-185.1534
- Zhu, B.Y. and Zhao, G.X. (1981) Measuring Surface Tension of Liquids-Drop Weight/Drop Volume Method. Chemistry, 6, 21-26.
- Lee, D.J. (1995) Enthalpy-Entropy Compensation in Ionic Micelle Formation. Colloid and Polymer Science, 273, 539-543. http://dx.doi.org/10.1007/BF00658683
NOTES

*Corresponding author.


