Open Journal of Veterinary Medicine
Vol. 3 No. 2 (2013) , Article ID: 32693 , 10 pages DOI:10.4236/ojvm.2013.32020
Control of Canine Endoparasites, Especially Isospora spp., with Procox® in Naturally Infected Puppies: Parasitological, Bacteriological and Health Parameters*
1Institute of Parasitology, Department of Pathobiology, University of Veterinary Medicine Vienna, Vienna, Austria
2Centre for Artificial Insemination and Embryo Transfer, Department for Small Animals and Horses, University of Veterinary Medicine Vienna, Vienna, Austria
3Functional Microbiology, IBMH, Department of Pathobiology, University of Veterinary Medicine Vienna, Vienna, Austria
4Institute of Virology, Department of Pathobiology, University of Veterinary Medicine Vienna, Vienna, Austria
5Institute of Animal Nutrition, Department for Farm Animals and Veterinary Public Health, University of Veterinary Medicine Vienna, Vienna, Austria
6Clinical Microbiology and Infection Biology, IBMH, Department of Pathobiology, University of Veterinary Medicine Vienna, Vienna, Austria
7Bayer Animal Health GmbH, Monheim, Germany
Email: #Anja.Joachim@vetmeduni.ac.at
Copyright © 2013 Barbara Anna Rauscher et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 7, 2013; revised March 7, 2013; accepted April 7, 2013
Keywords: Dog; Emodepside; Gut Health; Isospora; Toltrazuril; Toxocara; Vaccination
ABSTRACT
The effect of Procox® (Bayer, emodepside/toltrazuril suspension for dogs) against natural infections with Isospora spp. was investigated. Two groups were treated either with 0.5 ml suspension/kg of body weight (0.45 mg emodepside and 9 mg toltrazuril/kg of body weight) in the 3rd, 5th and 7th week of life (w.o.l.) (Procox® group; n = 28) or with a control anthelminthic (Dewormed Control group; n = 26). Animals were surveyed weekly from the 3rd w.o.l. by coproscopy and clinical examination. Faecal samples were examined microbiologically from the 4th to the 8th w.o.l. and faecal inflammatory markers canine calprotectin and canine S100A12 were measured in the 8th w.o.l. Specific antibody titres were evaluated in serum samples from five litters before and after vaccination against canine distemper virus and canine parvovirus 2. The prevalence of Isospora-positive animals increased to 67% in the Dewormed Control group (n = 15 puppies from four parasite-positive litters), while in the Procox® group (n = 15 puppies) it was less than 34% with significantly lower excretion (p < 0.01). Procox® was easily applied and effective; adverse effects did not occur. The level of seroconversion or titre increase upon vaccination was higher in parasite-free animals (91%) compared to Procox® -treated puppies (30%) and the Control animals (10%). Animals from parasite-free litters showed significantly different excretion patterns for haemolytic Escherichia coli and Clostridium perfringens, while there was no difference between Procox® -treated and Control animals. In some animals kept under poor hygienic conditions diarrhoea was noted in association with C. perfringens, E. coli or Salmonella. Concentrations of inflammatory markers in the faeces did not significantly differ between the Procox® and the Control group. Adequate control of parasitic and bacterial infections in suckling puppies requires both antiparasitic treatment and hygiene. Even when parasites do not cause overt effects treatment is recommended in cases with a history of parasite infections.
1. Introduction
Coccidia are important intestinal pathogens of puppies and heavy infections can lead to impaired development and poor health. Canine intestinal coccidia belong to the genus Isospora and include Isospora canis, the more pathogenic species, and the Isospora ohioensis-complex embracing several species (Isospora ohioensis, Isospora burrowsi and Isospora neorivolta) that have morphologically indistinguishable oocysts [1]. Infections occur worldwide and are usually more prevalent in dogs younger than 4 months [2]. They take place by ingestion of sporulated oocysts from the environment. Although transmission via paratenic hosts has been described [3], in young puppies, the infection via ingestion of such hosts is unlikely to play a role in the epidemiology of the infection. Invasion of the intestinal epithelium and reproduction of the parasite induces enteritis with diarrhoea (sometimes haemorrhagic), abdominal pain, anorexia and vomiting [4,5]. Heavy infections can be lethal due to dehydration. After the endogenous development of Isospora is completed, oocysts are excreted with the faeces and sporulate in the environment within a few days [1]. Prepatent periods are 10 - 12 days for I. canis and 6 - 7 days for I. ohioensis-complex [2].
Besides coccidia, nematodes are common in puppies, the most frequent one being Toxocara canis [2,6]. The eggs are not infectious upon excretion with the faeces but require temperature-dependent development of three weeks to several months [7]. Infection can take place via ingestion of infective eggs containing L3 larvae, prenatal or transmammary transmission from the dam to the offspring or by ingestion of paratenic hosts [8]. In puppies, the most common migration route is via liver, lung, trachea and oesophagus to the small intestines, while in older dogs the somatic route with haematogenous dissemination into the inner organs followed by hypobiosis is the most common. In pregnant dams reactivation of somatic hypobiotic larvae and transplacental and transmammary transmission occurs. Enteritis, rarely associated with diarrhoea or vomitus, as well as dehydration, anaemia, anorexia, and bloated abdomen have been described in clinical canine toxocarosis [6].
Procox® (emodepside/toltrazuril suspension for dogs) is a combination of two drugs registered for the treatment of parasitic infections in animals [8,9]. Emodepside is a semi-synthetic derivate of PF1022A, a fungal fermentation product from Mycelia sterilia PF1022 which binds to presynaptic latrophilin receptors in nematodes [10]. Toltrazuril is a symmetric triazinone derivate which acts on all intracellular stages of coccidia except the oocyst. It is assumed that the primary mode of action is inhibition of enzymes of the respiratory chain but pyrimidine synthesis is also affected [11]. Several authors have described the successful application of toltrazuril in the control of canine coccidosis [2,12-14]. Procox® is registered for puppies of two weeks or older with a mixed infection of nematodes and coccidia (T. canis, Uncinaria stenocephala, Ancylostoma caninum, I. ohioenis-complex, I. canis; [8,9,15]).
While an interaction of coccidia with Clostridium (C.) perfringens has been described for suckling piglets [16] virtually nothing is known about the interaction between the protozoa and the gastrointestinal flora or the influence of antiparasitic treatment in puppies. Similarly, it is frequently postulated by veterinary clinicians that parasitic infections have an influence on the immune status and the immune response to vaccination and therefore deworming before vaccination is recommended; however, no data are available to test this hypothesis. The most important canine anti-viral vaccinations are used to control canine distemper virus and canine parvovirus 2 infections [17]; however, any interactions between maternal antibodies, intestinal parasites (especially nematodes) and the immune system have not been described for young puppies.
The aims of this study were 1) to evaluate the effect of Procox® application in naturally infected puppies from the 3rd week of life (w.o.l.) on the excretion of Isospora oocysts and nematode eggs and on and clinical parameters from the 3rd to the 8th w.o.l.; 2) to evaluate the influence of patent infections on the development of antibody titres after vaccination against parvovirus and distemper virus; 3) to determine the concentration of intestinal inflammation markers in the faeces of puppies with parasitic infections under different treatment schemes; 4) to evaluate possible differences between puppies of different parasitological status and treatment scheme on the composition of the intestinal flora.
2. Materials and Methods
2.1. Animals and Treatment
A total of 54 puppies from 7 litters of different breeds (Table 1) from eastern Austria were examined from June to November 2011. The litters were kept with the bitch (indoor or outdoor) and animals were identified individually via microchip or collar. At each sampling (3rd to 8th w.o.l.) the bitches and their litters underwent a clinical examination. Individual body weight was determined weekly for litters D, E and F (for litter description, see Table 1).
Puppies were randomly assigned to two groups. One half of the puppies from each litter was treated with Procox® (Procox® group), whilst the other half was treated with Banminth® or Drontal® (Dewormed Control group). The Procox® group (n = 28) received an oral dose of 0.5 ml Procox®/kg body weight, corresponding to 0.45 mg/ emodepside and 9 mg toltrazuril/kg body weight in the 3rd, 5th and 7th w.o.l. The Control group (n = 26) received either Drontal® (5 mg praziquantel and 57.5 mg pyrantel embonate/kg body weight) or Banminth® (5 mg pyrantelpamoate/kg body weight) according to the manufacturers’ recommendations (Table 1) at the same time except for two litters where the animals of the Control group received the first anthelminthic treatment in the 5th w.o.l. (Table 1).
All procedures involving dogs were conducted with the owners’ informed consent and approved by the institutional ethics committee and the national authorities
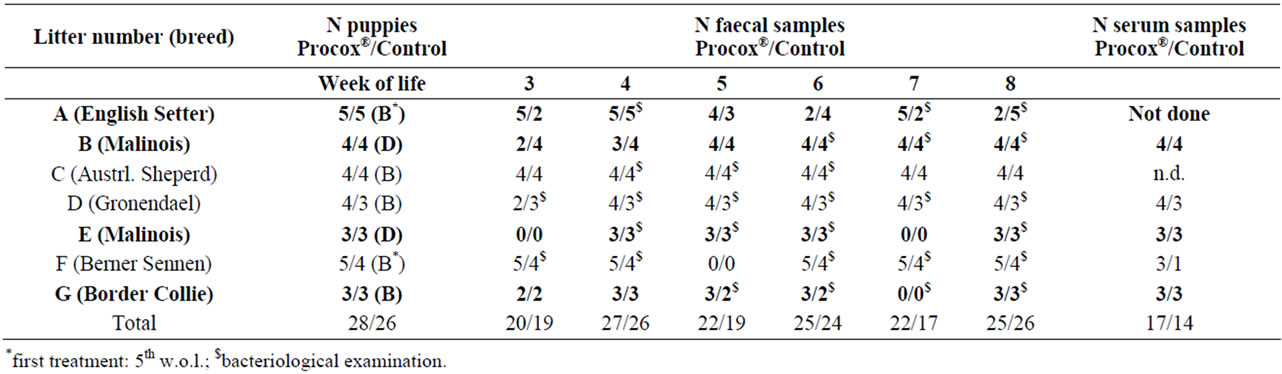
Table 1. Description of litters in the study. B = Banminth®, D = Drontal®. In bold: litters that were positive at least once during the first sampling and therefore included in the efficacy evaluation. Serum samples were obtained as paired samples before vaccination in the 6th/8th w.o.l. and two weeks after that.
(Federal Minstery of Science and Research: GZ 68.205/ 0081-II/3b/2011) according to § 8ff of the Austrian Law for Animal Experiments (Tierversuchsgesetz-TVG).
2.2. Parasitology
Faecal samples from individual puppies were taken weekly from the 3rd to the 8th w.o.l. and examined by flotation (sugar solution, specific gravity: 1.28) and, if positive for coccidia, by McMaster counting (lower detection limit: 50 oocysts per gram of faeces, OPG; flotation with zinc sulphate solution, specific gravity: 1.3.) Results were graded into negative (−), low grade (+), medium grade (++) and high grade (+++).
2.3. Serology
Puppies were vaccinated s.c. twice with a vaccine against canine parvovirus 2 (CPV2), canine distemper virus (CDV), canine adenovirus and canine leptospirosis (Virbagen canis SHPPi/L; Virbac, Vienna, Austria). Serum samples were obtained as paired samples immediately before vaccination in the 6th w.o.l. (8th w.o.l. for litter F) and 2 weeks after that at the follow-up vaccination. Samples obtained from litters B, E and G were divided into samples from the Procox® (n = 10) and the Control (n = 10) group and the samples from the parasite-free litters D and F were defined as samples from parasite-free puppies (n = 11) (Table 1). Serum samples were examined for specific antibodies (IgG) against canine distemper virus (CDV) using an indirect immunofluorescence test (MegaScreen FLUO C.DV-IgG®, MegaCor, Hörbranz, Austria) according to the manufacturer’s instructions. Antibody titres against canine parvovirus 2 (CPV2) were determined by haemagglutination inhibition test as described by Schoder et al. [18] .
2.4. Bacteriology
Bacteriological examination of faecal samples was carried out from the 3rd to the 8th w.o.l. (for details on the samples available (see Table 1); only litters with data sets from all puppies on that day were included), focusing on bacteria commonly associated with canine diarrhoea, i.e. haemolytic Escherichia coli, C. perfringens, Campylobacter jejuni, and Salmonella enterica. Samples were collected and either processed immediately or stored at −80˚C until further analysis.
Faecal samples were streaked on Columbia blood agar, MacConkey agar, and Campylobacter selective agar (CCDA) and incubated for 24 - 48 h at 37˚C under aerobic (Columbia blood agar, MacConkey agar), microaerophilic (CCDA) or anaerobic (Columbia blood agar) conditions. For the isolation of Salmonella, samples were inoculated into Selenite-cysteine broth, incubated at 42˚C for 24 h, and subsequently passaged onto xylose-lysinedesoxycholate (XLD) agar (37˚C, 24 h). Species identification of bacteria isolated was performed using standard bacteriological techniques. Relative numbers of bacteria present on agar plates were determined using semiquantitation (low, moderate, high; see section Parasitology) on streak area.
2.5. Faecal Inflammatory Markers
Pooled faecal samples from the 8th w.o.l. were taken for the measurement of canine calprotectin and canine S100A12 which was performed at the Gastrointestinal Laboratory, Texas A&M University (College Station, TX) using validated radioimmunoassays [19,20].
2.6. Statistical Evaluation
Statistical evaluations were made in Microsoft Excel 2003 and SPSS 17.0 (SPSS Inc., Chicago, USA).
Samples were evaluated for normal distribution using the Kolmogorov-Smirnov-test. Since data were not normally distributed, the Mann-Whitney-U-test was chosen for comparison between groups. Significance was set at p < 0.05.
3. Results
3.1. Parasitological and Clinical Examination
54 puppies from seven litters were included in the study, and 272 faecal samples could be obtained during the examination period, 141 from Procox®-treated puppies, 131 from the Dewormed Control group (Table 1). Overall, 38 samples (14.0%) contained oocysts of Isospora (all I. ohioensis-complex) and 12 (4.4%) eggs of T. canis (Table 2). In four litters, puppies excreted oocysts or eggs during the first visit and were included in the study (litters A, B, E, and G). The remaining three litters were used as a parasite-free group for the bacteriological and serological examinations.
Consequently, for the comparison of the treatment regimes, 30 puppies from the positive litters were available, 15 in the Procox® group and 15 in the Dewormed Control group which were treated with Banminth® or Drontal® (Table 1).
In the Procox® group 9/70 (12.9%) samples were positive for Isospora oocysts, and high excretion intensities were not observed. Oocyst excretion was detected from the 5th w.o.l. until the end of the study. The highest excretion was 18,500 OPG, the average in the positive samples was 2167 OPG. In the Dewormed Control group 29/69 (42%) samples were positive, with excretion from the 3rd to the 8th w.o.l. 12 samples (17%) were highly positive (Table 2). The highest observed excretion was 95,250 OPG, the average in the positive samples was 10,510 OPG (Figure 1). Nine puppies excreted oocysts more than once, eight of which were in the Dewormed Control group. Three puppies that excreted oocysts prior to treatment were negative one week after treatment. For comparing the overall excretion prevalence for Isospora, all samples of the Procox® group and all samples of the Control were summed up. This prevalence was significantly reduced (p = 0.000) in the Procox® group compared to the Control. When comparing groups on each sampling time-point the difference was significant on week five and seven only (p = 0.021 and 0.031, respectively).
Toxocara was detected in one animal in the Procox® group in the 3rd w.o.l. (day of first treatment/sampling) but no more after that. By contrast, in the Dewormed Control group eggs were detected from the 3rd to the 6th w.o.l. in 11/69 samples (15.9%); six samples (8.7%) were highly positive. Mixed infections with Isospora and Toxocara were detected in four samples from four puppies in the Dewormed Control group.
Weekly weight gain was very variable due to breed differences; there were no differences in weight gain from the 3rd to the 8th week between the Procox®-treated animals and the Dewormed Control group (data not shown).
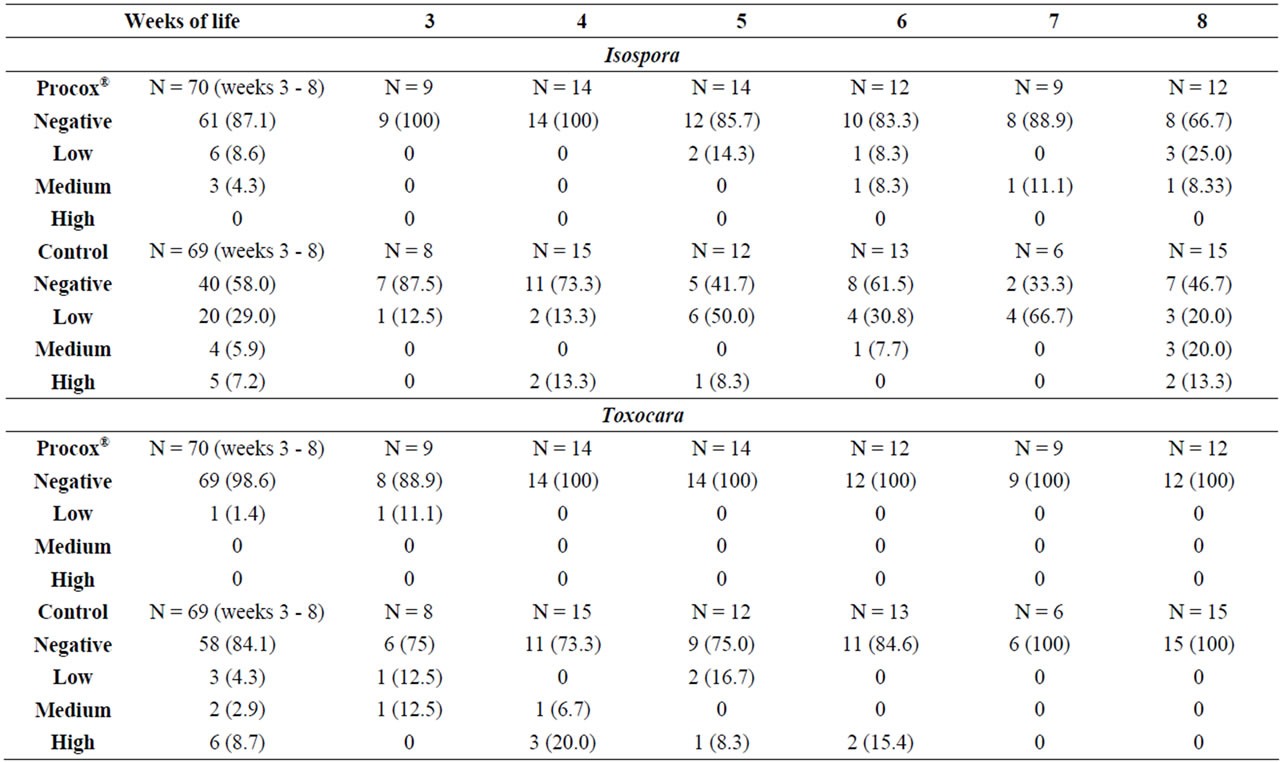
Table 2. Excretion intensity (semi-quantitative) in the puppies of the positive litters (n = 15 in the Procox® group, n = 15 in the Control group). N = number of samples; in brackets: percentage when n > 0.
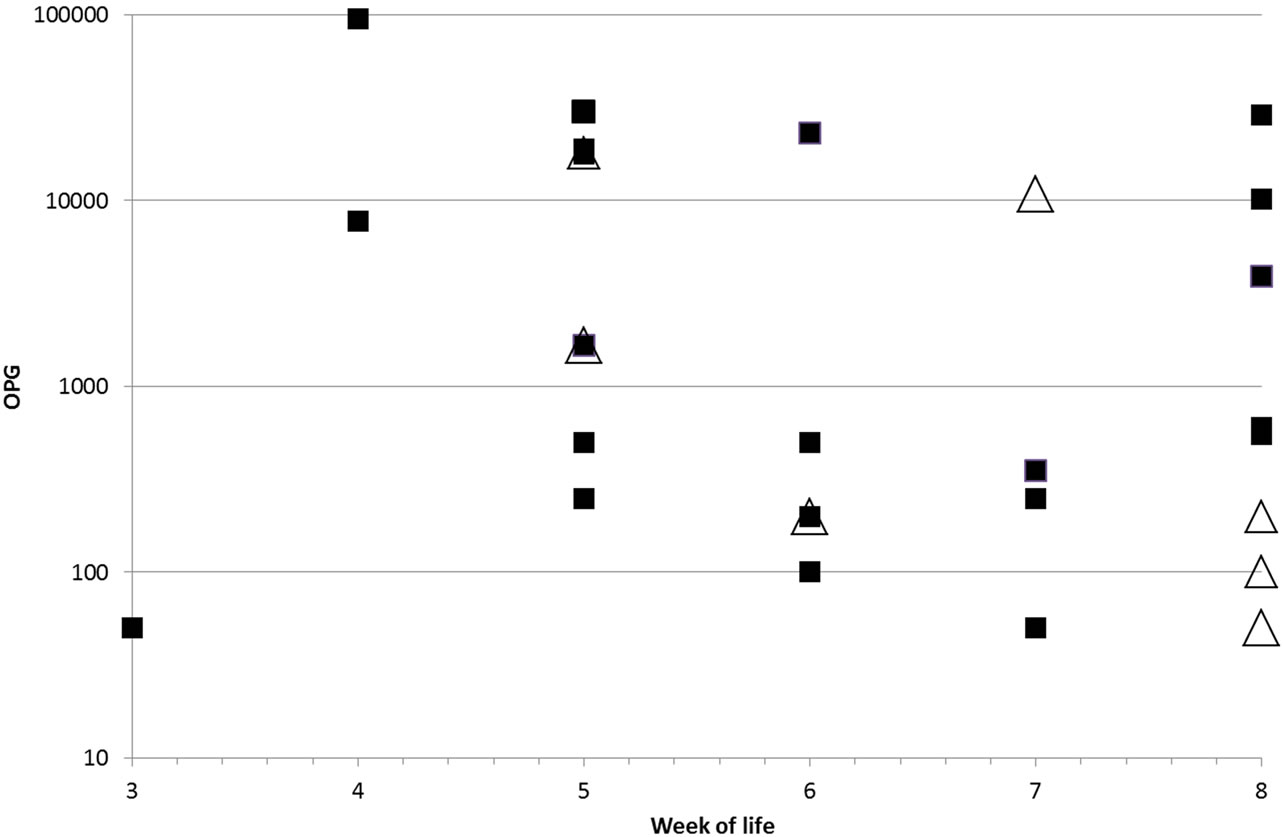
Figure 1. Individual OPG (oocysts per gram of faeces) values of McMaster positive animals in the control group (black squares) and the Procox® group (white triangles).
Diarrhoea was present in litter A (two animals from the Dewormed Control group in the 4th w.o.l. and three control animals and one Procox®-treated puppy in the 6th w.o.l.) and in all animals from litter G (5th w.o.l.). Softer faeces were observed in all puppies from litter C (which was free of parasites during the observation period). These changes in faecal consistency were associated with the presence of C. perfringens in all cases. In litter A Salmonella and haemolytic E. coli could be found in some cases.
The body temperature was in the normal range without differences between the different groups (details not shown).
All administered drugs were well tolerated; adverse effects of treatment, such as salivation or emesis, were not observed. Procox® was applied by the researchers involved in this study and side effects were neither observed nor reported by the owners.
3.2. Serology
Before vaccination at six (litter F eight) weeks of age most puppies were negative or had low to moderate titres (maternal antibodies) in the range of <1:80 (negative) to 1:320 against CDV and <1:8 (negative) to 1:512 against CPV2 with no noticeable differences between the treatment groups (data not shown). Two weeks after vaccination, 30% of the Procox®-treated puppies, 10% of the Dewormed Control animals and 91% of the parasite-free animals seroconverted or exhibited significant rises in titres against CDV and 40%, 50% and 91% against CPV2, respectively.
3.3. Microbiota
The animals were divided into four groups, namely Procox®-treated from parasite-positive litters, A, B, E and G (parasites + Procox), Control animals from parasitepositive litters (parasites + Control), and the same groups from parasite-free litters C, D, and F (parasites-Procox® and parasites-Control), to test for possible effects of the parasitological status and different antiparasitic treatment schemes on the shedding of bacterial pathogens commonly associated with canine diarrhoea. For each sampling date, 6 - 15 animals were available for each group from week 4 to 8 of life (the 3rd w.o.l. was omitted because too few data were available).
Haemolytic E. coli was present at all sampling dates in all groups except for the 5th w.o.l. in the parasites + Control group. While there were no differences in relation to Procox®-treatment, the excretion of haemolytic E. coli was significantly increased in the parasite-free groups during the 4th and 5th w.o.l. and significantly decreased in the 8th w.o.l. (p = 0.003; 0.033 und 0.013, respectively) compared to the parasite infected groups; the total excretion was not different between the parasitized and the parasite-free groups (Figure 2(a)).
C. perfringens was detected in all groups throughout the study without differences in relation to Procox®- treatment. In the 5th w.o.l. parasite-free groups excreted significantly fewer C. perfringens (p < 0.001) and in the 8th w.o.l. significantly more (p = 0.039) (Figure 2(b)).
Overall, intensities and extensities of excretion were similar between the groups for E. coli and C. perfringens.
Salmonella enterica serovar Typhimurium (1, 4, 5, 12:i:1, 2 U277) was detected through enrichment in one animal each of the parasites + Procox and the parasites + Control group from litter A the 4th w.o.l., two animals
(litter F) from the parasite-Procox group in the 6th w.o.l. and one from the parasite-Control group (litter F) in the 8th w.o.l.
Campylobacter jejuni was not detected in any sample.
3.4. Faecal Inflammatory Markers
The median concentration of canine calprotectin in the pooled faeces from the puppies taken at the 8th w.o.l. was 0.7 µg/g (range: 0.04 - 7.68 µg/g) in the Procox® group and 1.96 µg/g (range: 0.11 - 8.92 µg/g) in the Dewormed Control group. The median concentration of S100A12 in the same samples was 48.7 ng/g (range: 5.7 - 363.5 ng/g) in the Procox® group and 95.4 ng/g (range: 5.7 - 203.9 ng/g) in the Dewormed Control group. No significant difference between animals treated with Procox® and controls was found for either marker (p = 0.456 and 0.701, respectively).


Figure 2. Excretion patterns (average excretion intensities) for haemolytic E. coli (a) and C. perfringens (b). parasites+: animals from litters where parasites were detected; parasites−: animals from parasitized litters; each divided into a Control and a Procox®-group. Intensity is given as relative intensity (0 = negative; 1 = low; 2 = medium; 3 = high).
4. Discussion
4.1. Clinical Outcome
During the examination period, diarrhoea occurred in some litters in association with C. perfringens excretion. Other signs of disease such as fever or retarded growth were not noticed. The dog breeders were aware of the impact of Toxocara infections and treated puppies routinely with Drontal® or Banminth® if they were in the Dewormed Control group. This explains why no overt clinical toxocarosis was seen in the involved litters. According to the owners, clinical coccidiosis was not considered a major health problem and the incidence of oocyst shedding was not very high, probably because the examined litters all belonged to small private breeders with mostly a high hygiene standard and a small number of litters reared at the same time. Large, commercial breeders seem to have a higher prevalence for Isospora (e.g. [2]) since more susceptible young animals are present simultaneously.
4.2. Parasitological Results
For this field study seven litters of different dog breeds were examined for coccidia and other intestinal parasites and treated repeatedly, either with Procox® or Banminth®/Drontal® (Control group) from the 3rd w.o.l. with weekly follow-up examinations until the 8th w.o.l. In four litters, parasites (Isospora and/or Toxocara) could be detected at least once; these were included in the comparative study for the efficacy of the different antiparasitic drugs.
Isospora was already detected at high prevalence rates at the first visits in the 3rd w.o.l. Similarly, Buehl et al. [2] described patent infections in dogs of that age in high rates, indicating that many puppies had become infected during the 2nd w.o.l. when they were still in the litter box. Other authors describe a much later onset of infection in the 4th to the 6th w.o.l. [12,13]. A re-occurrence of excretion was described by Buehl and co-authors [2] in untreated animals and could also be noted in the present study during the 6th to 8th w.o.l., indicating that immunity is not sterile or requires repeated infections for complete protection against re-infection. In the Procox®-treated puppies re-shedding also occurred after some weeks, although in lower numbers, and the maximum excretion rate was 33% in the 8th w.o.l., compared to the untreated Control group with a maximum prevalence of 67%. I. ohioensis-complex has been described to re-occur after treatment [8], supporting the hypothesis of incomplete immunity. Nevertheless, Procox® effectively suppressed the shedding of oocysts one week after application, as described before by Altreuther et al. [8]. Overall, both the extensity and intensity of oocyst excretion in treated puppies could be reduced significantly with the applied treatment scheme. Repeated treatment was chosen to combat infections that take place at any time in the first weeks of life, as control in an early phase of infection is essential for efficacy [2,13]. Clinical coccidiosis was not observed, probably due to the absence of the more pathogenic I. canis in the examined litters.
Toxocara was detected before treatment in both the Procox® and the Dewormed Control group, albeit in lower prevalences compared to Isospora. In contrast to the coccidia, nematodes did not re-appear in the Procox® group during the examination period due to repeated treatment within the prepatent period, indicating a high efficacy of emopdepside against Toxocara [21,22]. Despite treatment with Banminth® or Drontal® some animals in the Dewormed Control group still excreted eggs until the 6th w.o.l. The treatment schemes of the Control group varied between litters; this could be the reason for egg shedding in this group.
Procox® was applied by the researchers involved in this study and side effects were neither observed nor reported by the owners. It can be concluded that it is suitable and effective for the control of both Isospora and Toxocara in puppies from the 3rd w.o.l.
4.3. Serum Titre Development upon Vaccination
The evaluation of specific antibodies against CDV or CPV2 in three parasite-positive and two negative litters showed that seroconversion or significant titre increase occurred mostly in the parasite-free animals while no obvious differences could be noted in puppies from parasitized litters, irrespective of treatment. Since animals were observed by litter for this part of the study, this effect could be due to the low pre-vaccination titres in the two parasite-free litters. A difference between the Procox® and the Control groups was not observed, probably because both groups received anthelmintic treatment and infections were subclinical. Initially high pre-vaccination titres indicate that maternal antibodies prevail for several weeks. Since they can interfere with active immunisation, pre-vaccination titre testing is indicated [23].
4.4. Microbiota
Gut health in puppies can be expected to be influenced by many factors including maternal antibodies, maternal flora, hygiene, feed composition, bacterial, viral as well as parasitic agents and individual immunological status. In this study, a number of those factors and the potential influence of parasite infection were investigated under field conditions. These effects cannot easily be dissected in a field trial, and one may mask the other. Irrespective of the antiparasitic treatment, parasite-free litters shed higher amounts of haemolytic E. coli and less C. perfringens during the early stage of life than parasitized litters. Profound differences in the dynamics of shedding patterns for E. coli and C. perfringens were noted between parasitized and parasite-free litters, both of which can lead to enteric or systemic disease during intestinal flora imbalances. In some cases diarrhoea occurred together with high excretion of C. perfringens, and occasionally haemolytic E. coli and S. enterica was seen in diseased animals. Generally, there was a strong litter effect on the excretion pattern of pathogenic bacteria that may at least partially be explained by differences in maternal flora, hygiene, food composition and the general immunological status of the litters. Feed composition and immunity strongly influence the gut flora of young puppies [24], which could mask potential effects of antiparasitic treatment.
Although commonly considered as normal constituents of the canine gut flora [25], haemolytic E. coli (ETEC, NTEC) and C. perfringens (enterotoxigenic type A and beta2-toxin producing strains) have been frequently incriminated as cause of diarrhoea in dogs [26-28]. In addition, both bacteria are well known for their zoonotic potential and shedding dogs may pose a risk for zoonotic transmission. S. enterica serovar Typhimurium (1, 4, 5, 12:i:1, 2 U277), a serovar associated with salmonellosis in wild-living birds [29], was occasionally isolated from non-diarrhoeic puppies underscoring the lack of an association between the isolation of Salmonella and clinical diarrhoea. However, as Salmonella organisms have strong zoonotic implications, risk of transmission to people has to be minimized by implementing appropriate hygienic strategies in the household [27]). The application of probiotics is supposed to support the undisturbed development of the gut flora in young puppies in the first w.o.l. and reduce the number of pathogenic bacteria, although until now studies have only been carried out in adult dogs [30,31]. This study also implies that the presence of parasites may influence the composition of the gut flora in puppies. More refined methods for detection of mixed bacterial flora in puppies might elucidate the association between the presence of certain microbial pathogens and clinical disease.
4.5. Inflammatory Markers
Although the mean values of both markers were reduced in the Procox®-treated group compared to the Dewormed Control group, significant differences were not seen in the faecal concentration of the inflammatory markers canine calprotectin and canine S100A12. The values of the faecal calprotectin concentration and S100A12 concentration were all within the published reference intervals for adult dogs of <2.9 - 137.5 µg/g and 24 - 745 ng/g, respectively (reference intervals for puppies are currently not available). To our knowledge, there is only one other study reporting canine calprotectin in puppy faeces, which was within the same order of magnitude [32]. In contrast to Grellet et al. [32], we did not find a significant difference between puppies positive or negative for Isospora, neither in calprotectin concentration nor in S100A12 concentration (p = 0.950 and 0.560, respectively; data not shown). Since none of the dogs showed clinical symptoms, infestation with Isospora sp. did obviously not cause clinically relevant intestinal inflammation. This is supported by the lack of correlation between Isospora infections and diarrhoea.
4.6. Conclusions
In this study the antiparasitic efficacy of a combination product containing emodepside against roundworms and toltrazuril against coccidia was demonstrated under the conditions of a field trial. The potential influence of such an antiparasitic treatment regime on shedding of pathogenic bacteria was studied. In addition inflammatory markers and the development of serum antibody titre development following vaccination (CDV and CPV2) were investigated.
Treatment against parasites significantly reduced the prevalences of Toxocara and Isospora in comparison to the controls. The data obtained for the microbiota, inflammation markers and serum antibodies showed some positive influence of the tested treatment regimen; however, they could not prove significant differences in relation to treatment. Under field conditions there may be too many factors influencing the investigated parameters. Further studies under controlled experimental conditions are required to further analyse a potential influence of parasitic infections on those parameters.
Adequate control of parasitic and bacterial infections in suckling puppies requires both antiparasitic treatment and hygiene. Even in litters or breeding units where parasites do not cause overt effects treatment is recommended in cases with a history of parasite infection and/ or parasitological diagnosi.
5. Acknowledgements
The authors thank Dr. Muna Latif for excellent technical assistance with the serological examinations. This study was financially supported by Bayer Animal Health, Germany.
REFERENCES
- D. S. Lindsay, J. P. Dubey and B. L. Blagburn, “Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic Animals,” Clinical Microbiology Reviews, Vol. 10, No. 1, 1997, pp. 19-34.
- I. E. Buehl, H. Prosl, H. C. Mundt, A. G. Tichy and A. Joachim, “Canine Isosporosis—Epidemiology of Field and Experimental Infections,” Journal of Veterinary Medicine B, Vol. 53, No. 10, 2006, pp. 482-487. doi:10.1111/j.1439-0450.2006.00973.x
- J. P. Dubey and H. Mehlhorn, “Extraintestinal Stages of Isospora ohioensis from Dogs in Mice,” Journal of Parasitology, Vol. 64, No. 4, 1978, pp. 689-695. doi:10.2307/3279961
- J. P. Dubey, “Life-Cycle of Isospora ohioensis in Dogs,” Parasitology, Vol. 77, 1978, pp. 1-11. doi:10.1017/S0031182000048654
- J. P. Dubey, “Pathogenicity of Isospora ohioensis Infection in Dogs,” Journal of the American Veterinary Medical Association, Vol. 173, No. 2, 1978, pp. 192-197.
- C. Epe, “Intestinal Nematodes: Biology and Control,” Veterinary Clinics of North America Small Animal Practice, Vol. 39, No. 6, 2009, pp. 1091-1107. doi:10.1016/j.cvsm.2009.07.002
- M. Stoye, “Spul und Hakenwürmer des Hundes—Entwicklung, Epizootiologie, Bekämpfung,” Berliner und Münchner Tierärztliche Wochenschrift, Vol. 96, No. 23, 1979, pp. 109-111.
- G. Altreuther, N. Gasda, K. Adler, K. Hellmann, H. Thurieau, A. Schimmel, D. Hutchens and K. J. Krieger, “Field Evaluations of the Efficacy and Safety of Emodepside Plus Toltrazuril (Procox® Oral Suspension for Dogs) against Naturally Acquired Nematode and Isospora spp. Infections in Dogs,” Parasitology Research, Vol. 109, No. 1, 2011, pp. 21-28. doi:10.1007/s00436-011-2399-z
- G. Altreuther, N. Gasda, I. Schroeder, A. Joachim, T. Settje, A. Schimmel, D. Hutchens, K.J. Krieger, “Efficacy of Emodepside Plus Toltrazuril Suspension (Procox® Oral Suspension for Dogs) against Prepatent and Patent Infection with Isospora canis and Isospora ohioensis-Complex in Dogs,” Parasitology Research, Vol. 109, No. 1, 2011, pp. 9-20. doi:10.1007/s00436-011-2398-0
- A. Harder, L. Holden-Dye, R. Walker and F. Wunderlich, “Mechanisms of Action of Emodepside,” Parasitology Research, Vol. 97, No. 1, 2005, pp. 1-10. doi:10.1007/s00436-005-1438-z
- G. Scholtysik and S Steuber, “Antiparasitäre Chemotherapie,”Enke Verlag, Stuttgart, 2002, pp. 401-456.
- K. Bode, “Endoparasitenbefall in lommerziellen Hundezuchten unter besonderer Berücksichtigung der Isosporose,” Dr. Med. Vet. Dissertation, Tierärztliche Hochschule Hannover, Hannover, 1999.
- U. Seeliger, “Feldstudie zur Epidemiologie und Bekämpfung der Isosporose des Hundes,” Dr. Med. Vet. Dissertation, Tierärztliche Hochschule Hannover, Hannover, 1999.
- A. Daugschies, H. C. Mundt and V. Letkova, “Toltrazuril Treatment of Cystoisosporosis in Dogs under Experimental and Field Conditions,” Parasitology Research, Vol. 86, No. 10, 2000, pp. 797-799. doi:10.1007/s004360000217
- A. Schimmel, I. Schroeder, G. Altreuther, T. Settje, S. Charles, S. Wolken, D. J. Kok, J. Ketzis, D. Young, D. Hutchens and K. J. Krieger, “Efficacy of Emodepside Plus Toltrazuril, Procox® Oral Suspension for Dogs against Toxocara canis, Uncinaria stenocephala and Ancylostoma caninum in Dogs,” Parasitology Research, Vol. 109, No. 1, 2011, pp. 1-8. doi:10.1007/s00436-011-2397-1
- H. Mengel, M. Krüger, M.-U. Krüger, B. Westphal, A. Swidsinski, S. Schwarz, H.C. Mundt, K. Dittmar and A. Daugschies, “Necrotic Enteritis Due to Simultaneous Infection with Isospora suis and Clostridia in Newborn Piglets and Its Prevention by Early Treatment with Toltrazuril,” Parasitology Research, Vol. 110, No. 4, 2012, pp. 1347-1355. doi:10.1007/s00436-011-2633-8
- L. E. Carmichael, “Canine Viral Vaccines at a Turning Point—A Personal Perspective,” Advances in Veterinary Medicine, Vol. 41, 1999, pp. 289-307. doi:10.1016/S0065-3519(99)80022-6
- D. Schoder, V. Benetka, I. Sommerfeld-Stur, N. Kopf, E. Weissenbacher, C. Pallan, K. Walk and K. Möstl, “Untersuchungen zum Antikörper-Status gegen HundestaupeVirus und Canines Parvovirus-2 bei Hunden in Niederö- sterreich und Wien nach unterschiedlichen Impfintervallen,” Veterinary Medicine Austria/Wiener Tierärztliche Monatsschrift, Vol. 93, No. 7-8, 2006, pp. 176-182.
- R. M. Heilmann, J. S. Suchodolski and J. M. Steiner, “Development and Analytic Validation of a radioimmunoassay for the Quantification of Canine Calprotectin in Serum and Feces from Dogs,” American Journal of Veterinary Research, Vol. 69, No. 7, 2008, pp. 845-853. doi:10.2460/ajvr.69.7.845
- R. M. Heilmann, D. J. Lanerie, C. G. Ruaux, N. Grützner, J. S. Suchodolski and J. M. Steiner, “Development and Analytic Validation of an Immunoassay for the Quantification of Canine Calprotectin in Serum and Feces and Its Variability in Serum from Healthy Dogs,” Veterinary Immunology and Immunopathology, Vol. 144, No. 3-4, 2011, pp. 200-209. doi:10.1016/j.vetimm.2011.09.011
- G. Altreuther, I. Radeloff, C. LeSueur, A. Schimmel and K. Krieger, “Field Evaluation of the Efficacy and Safety of Emodepside Plus Praziquantel Tablets (Profender® Tablets for Dogs) against Naturally Acquired Nematode and Cestode Infections in Dogs,” Parasitology Research, Vol. 105, No. 1, 2009, pp. 23-29. doi:10.1007/s00436-009-1492-z
- G. Altreuther, A. Schimmel, I. Schroeder, T. Bach, S. Charles, D. Kok, F. Kraemer, S. Wolken, D. Young and K. Krieger, “Efficacy of Emodepside Plus Praziquantel Tablets (Profender® Tablets for Dogs) against Mature and Immature Infections with Toxocara canis and Toxascaris leonina in Dogs,” Parasitology Research, Vol. 105, No. 1, 2009, pp. 1-8.
- M. J. Day, M. C. Horzinek and R. D. Schultz, “WSAVA Guidelines for the Vaccination of Dogs and Cats,” Journal of Small Animal Practice, Vol. 51, No. 6, 2010, pp. 1-32. doi:10.1111/j.1748-5827.2010.00959a.x
- J. Zentek, B. Marquart, T. Pietrzak, O. Ballèvre, R. Rochat, “Dietary Effects on Bifidobacteria and Clostridium perfringens in the Canine Intestinal Tract,” Journal of Animal Physiology and Animal Nutrition, Vol. 87, No. 11-12, 2003, pp. 397-407. doi:10.1046/j.0931-2439.2003.00451.x
- D. Y. Kil and K. S. Swanson, “Companion Animals Symposium: Role of Microbes in Canine and Feline Health,” Journal of Animal Science, Vol. 89, No. 5, 2011, pp. 1498-1505. doi:10.2527/jas.2010-3498
- J. Prada, G. Baljer, J. de Rycke, H. Steinrück, S. Zimmermann, R. Stephan and L. Beutin, “Characteristics of Alpha-Hemolytic Strains of Escherichia coli Isolated from Dogs with Gastroenteritis,” Veterinary Microbiology, Vol. 29, No. 1, 1991, pp. 59-73. doi:10.1016/0378-1135(91)90110-2
- M. Starcic, J. R. ohnson, A. L. Stell, J. van der Goot, H. G. Hendriks, C. van Vorstenbosch, L. van Dijk and W. Gaastra, “Haemolytic Escherichia coli Isolated from Dogs with Diarrhea Have Characteristics of Both Uropathogenic and Necrotoxigenic Strains,” Veterinary Microbiology, Vol. 85, No. 4, 2002, pp. 361-377. doi:10.1016/S0378-1135(02)00003-2
- S. L. Marks, S. C. Rankin, B. A. Byrne and J. S. Weese, “Enteropathogenic Bacteria in Dogs and Cats: Diagnosis, Epidemiology, Treatment, and Control,” Journal of Veterinary Internal Medicine, Vol. 25, No. 6, 2011, pp. 1195-1208. doi:10.1111/j.1939-1676.2011.00821.x
- T. Refsum, K. Handeland, D. L. Baggesen, G. Holstad and G. Kapperud, “Salmonellae in Avian Wildlife in Norway from 1969 to 2000,” Applied and Environmental Microbiology, Vol. 68, No. 11, 2002, pp. 5595-5599. doi:10.1128/AEM.68.11.5595-5599.2002
- M. L. A. Baillon, Z. V. Marshall-Jones and R. F. Butterwick, “Effects of Probiotic Lactobacillus acidophilus Strain DSM13241 in Healthy Adult Dogs,” American Journal of Veterinary Research, Vol. 65, No. 3, 2004, pp. 338-343. doi:10.2460/ajvr.2004.65.338
- P. M. Sherman, K. C., Johnson-Henry, H. P., Yeung, P. S. Ngo, J. Goulet and T. A. Tompkins, “Probiotics Reduce Enterohemorrhagic Escherichia coli O157:H7- and Enteropathogenic E. coli O127:H6-Induced Changes in Polarized t84 Epithelial Cell Monolayers by Reducing Bacterial Adhesion and Cytoskeletal Rearrangements,” Infection and Immunity, Vol. 73, No. 8, 2005, pp. 5183-5188. doi:10.1128/IAI.73.8.5183-5188.2005
- A. Grellet, R. M. Heilmann, J. S. Suchodolski, A. Feugier, G. Casseleux, V. Biourge, T. Bickel, B. Plack, D. Grandjean and J. M. Steiner, “Evaluation of Canine Calprotectin Infeces from a Large Group of Puppies,” Journal of Veterinary Internal Medicine, Vol. 24, No. 6, 2010, p. 1553.
NOTES
*Parts of the Results were published in Diploma Theses at the Vetmeduni Vienna 2012 (M. Stejskal, “Untersuchung der Auswirkung von Procox® auf die Darmflora von Welpen” and M. S. Konecny, “Auswirkungen der Darmflora gravider Hündinnen auf die Scheidenflora und auf die Welpengesundheit”).
#Corresponding author.

