Open Journal of Medical Psychology
Vol.3 No.1(2014), Article ID:41654,6 pages DOI:10.4236/ojmp.2014.31006
Oxytocin but Not Testosterone Modulates Behavioral Patterns in Autism Spectrum Disorders
1Institute of Physiology, Comenius University in Bratislava, Bratislava, Slovakia
2Mailman Segal Center, Nova Southeastern University, Fort Lauderdale, USA3College of Osteopathic Medicine, Nova Southeastern University, Fort Lauderdale, USA4College of Pharmacy, Nova Southeastern University, Fort Lauderdale, USAEmail: *silvia.lakatosova@gmail.com, nurit@nova.edu, anna.pivovarciova@gmail.com, nika.husarova@gmail.com, rozenfeld@nova.edu, daniela.ostatnikova@fmed.uniba.sk, castejon@nova.edu
Copyright © 2014 Silvia Lakatosova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Silvia Lakatosova. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received October 29, 2013; revised November 29, 2013; accepted December 6, 2013
Keywords:Autism; Oxytocin; Testosterone; Maladaptive Behavior
ABSTRACT
Autism spectrum disorder (ASD) is a neurodevelopmental disorder of unknown etiology. Social deficits represent one of the core symptoms of the diagnosis. The aim was to reveal possible correlations among peripheral levels of oxytocin and testosterone with behavioral and symptom characteristics in patients with ASD. 8 children with ASD were recruited and underwent psychological profiling. Blood oxytocin and testosterone levels were analyzed using ELISA method. Oxytocin levels positively correlated with Adaptation to change category of CARS-2 (P = 0.008, R = 0.848) and Vineland-II maladaptive behavior scores (P = 0.004, R = 0.884). No significant correlations were found among testosterone levels and behavioral parameters. Higher oxytocin levels were connected with more severe adaptive behavior in ASD patients. Increased oxytocin levels in children with more severe phenotype could be a result of compensatory mechanism of impaired oxytocin signaling. Oxytocin seems to employ distinct mechanisms in regulating social behavior in autism and healthy population.
1. Introduction
Oxytocin is a neuropeptide widely promoting high variety of effects on brain functions. One of the most studied is the prosocial effect of the hormone. Oxytocin was found to improve several aspects of human social behavior such as interpersonal trust [1], eye contact [2], empathy measured as the ability to infer mental states of the others [3].
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with core symptoms including social deficits, impaired communication and repetitive behaviors.
Several studies have shown altered plasma levels of oxytocin. Modahl et al. [4] has found reduced oxytocin levels in 29 prepubertal boys with autism compared to 30 age-matched controls in US population. Green et al. [5] has found an alteration in oxytocin peptide processing in this sample, oxytocin plasma levels were decreased and levels of oxytocin C-terminal extended peptides were increased in autism boys compared to healthy subjects. Oxytocin and vasopressin levels were found to be reduced in 77 autistic boys from central Saudi Arabia compared to controls [6]. Andari et al. [7] found profoundly decreased oxytocin levels in 13 high functioning autism patients relative to controls in French population. However, Miller et al. [8] found no differences in oxytocin and vasopressin levels among 75 autistic patients compared to age-matched controls in US population.
Not surprisingly, oxytocin administration is tested as a main or supportive treatment of mental disorders with social deficits, stress-related behaviors and anxiety. Intranasal and intravenous oxytocin administration was shown to improve some of the clinical autism symptoms, such as repetitive behaviors [9], to increase social learning [10], to increase social recognition in reading the mind in eyes test and emotionality [11,12], and to improve social interaction in high functioning autism patients [7].
There are sex differences in the oxytocin system. A trend to increased plasma oxytocin levels in females was found [13], probably since the expression of oxytocin and its receptor is estrogen dependent [14]. Active mechanisms employing estrogens together with oxytocin and oxytocin receptors may provide a protective milieu partially explaining lower incidence of autism spectrum disorders in females.
ASD is 3 - 4 times likely to occur in males than in females. Sex differences in autism incidence also suggest that sex hormones may play a role in ASD etiology. Testosterone and other androgens were found to be increased in plasma or saliva of autism patients [15-17]. Moreover, Baron Cohen in his hypermale brain theory of autism suggests that autism traits develop as a result of increased prenatal testosterone influence [18]. Other evidences supporting this theory involve girls with congenital adrenal hyperplasia who were prenatally exposed to high testosterone levels, display more autistic traits and male types of behavior [19]. Nevertheless, the ratio of length of second to fourth digit, an indicator of increased prenatal testosterone influence, was found to be lower (more masculine) in ASD subjects [20]. In addition, elevated rates of hyperandrogenism symptoms were found in women with ASD [21].
The aim of the present preliminary study was to reveal possible connections among peripheral levels of oxytocin and testosterone and different aspects of ASD symptomathology and behavioral characteristics.
2. Patients and Methods
The study was approved by the Institutional Review Board at Nova Southeastern University (NSU), Fort Lauderdale, Florida, USA. After signed consent 8 children with ASD were recruited into the study (7 boys, 1 girl, age 4.12 ± 0.64 years). Diagnosis of Autism Spectrum disorder was determined by clinical psychologist at Mailman Segal Center , Fort Lauderdale, FL according to DSM-V manual. Children also underwent behavioral testing by trained specialists at Mailman Segal Center. The behavioral and diagnostic tools involved: Autism Diagnostic Observation Schedule (ADOS), a semi-structured assessment that consists of various activities that allows the observation of social and communication behaviors related to the diagnosis of ASD; Childhood Autism Rating Scale, 2nd Edition (CARS-2), a 15-item behavior rating scale used to identify children with autism and to distinguish severity of the disorder, Autism Diagnostic Interview-Revised (ADI-R), a comprehensive interview administered to parents that provides a thorough assessment of individuals with ASD; Preschool Language Scales, Fifth Edition (PLS-5), a language measure that assesses auditory and expressive communication skills; Mullen Scales of Early Learning (MSEL), a developmentally integrated system that assesses language, motor, and perceptual abilities for children ages birth to 68 months of age; Vineland Adaptive Behavior Scales, Second Edition (Vineland-II), used to assess children’s adaptive behaviors and includes the following subdomains: communication, daily living skills, socialization, and motor skills; Child Behavior Checklist for Ages 1.5 - 5 (CBCL), an instrument used to rate a child’s difficult behaviors and competencies and Social communication questionnaire (SCQ), a brief instrument that evaluates communication skills and social functioning in children with ASD. Demographic data of the patients as well as their ADOS scores are displayed in Table 1.
Venous blood samples were drawn from all children at Pediatric Clinic at Sanford L. Ziff Health Care Center, NSU. Plasma oxytocin and testosterone levels were measured using an ELISA method (Enzo Life Sciences, DRG-diagnostics, respectively) at a laboratory of college of Pharmacy, NSU. Correlations of hormonal levels and behavioral scores were assessed using Pearson correlation test. Subsequently, children were divided into two groups—low oxytocin group (below 200 pg/mL) and high oxytocin group (above 200 pg/mL) (low OT, high

Table 1. Demographic data and plasma oxytocin and testosterone levels of ASD subjects. F = female, M = male.
OT group). Differences in behavioral scores among these groups were evaluated using unpaired t test.
3. Results
Oxytocin (OT) and testosterone (T) levels in our patients are depicted in Table 1.
Oxytocin levels positively correlated with Adaptation to change category of CARS-2 (P = 0.008, R = 0.848) and Vineland-II maladaptive behavior scores (P = 0.004, R = 0.884). Maladaptive behavior in Vineland-II scale consists of Internalizing and Externalizing scores. Oxytocin levels correlated positively with both of these scores (P = 0.008, R = 0.847, P = 0.025, R = 0.770, respectively) (Figure 1).
No significant correlations were found among testosterone levels and behavioral parameters.
To focus more on the relationship among oxytocin levels and behavioral scores we have divided patients into two groups: low oxytocin (low OT) and high oxytocin (high OT) group (below and above 200 pgl/mL), four patients referred to each group. Differences in all parameters among these groups were tested using parametric test (unpaired t test). Oxytocin levels were significantly lower in low OT than in high OT groups (mean ± SEM: Low OT: 142.3 ± 3.34 pg/mL, High OT: 254.6 ± 7.925 pg/mL, P < 0.0001). Maladaptive behavior score of Vineland-II scale was significantly lower in low OT than in high OT group (P = 0.005). Adaptation to change category of CARS-2 showed significantly lower scores in low OT than in high OT group (P = 0.003) (Figure 2).
4. Discussion
In the present study, we have found that oxytocin levels positively correlated with CARS-2 category of Adaptation to change. This means that more OT is associated with more abnormal adaptation to change abilities. This finding was confirmed by parametric testing of differences among low and high OT groups, where high OT group scored significantly higher in this category than low OT group.
Oxytocin levels positively correlated with The Vineland Adaptive Behavior Scales—subscale of maladaptive behavior. This subscale consists of internalizing and externalizing factors. Oxytocin positively correlated with both subscales as well as with total maladaptive scores. Children possessing either internalizing or externalizing maladaptive behavior are generally not popular among their peers and their behavior is a strong handicap in initializing and maintaining any form of positive social bonds. Total maladaptive behavior scores were higher in high OT group in contrast to low OT group.
These results seem to be somehow controversial, since OT does not seem to be protective against one autism trait which is adaptation. The exact opposite trend seems
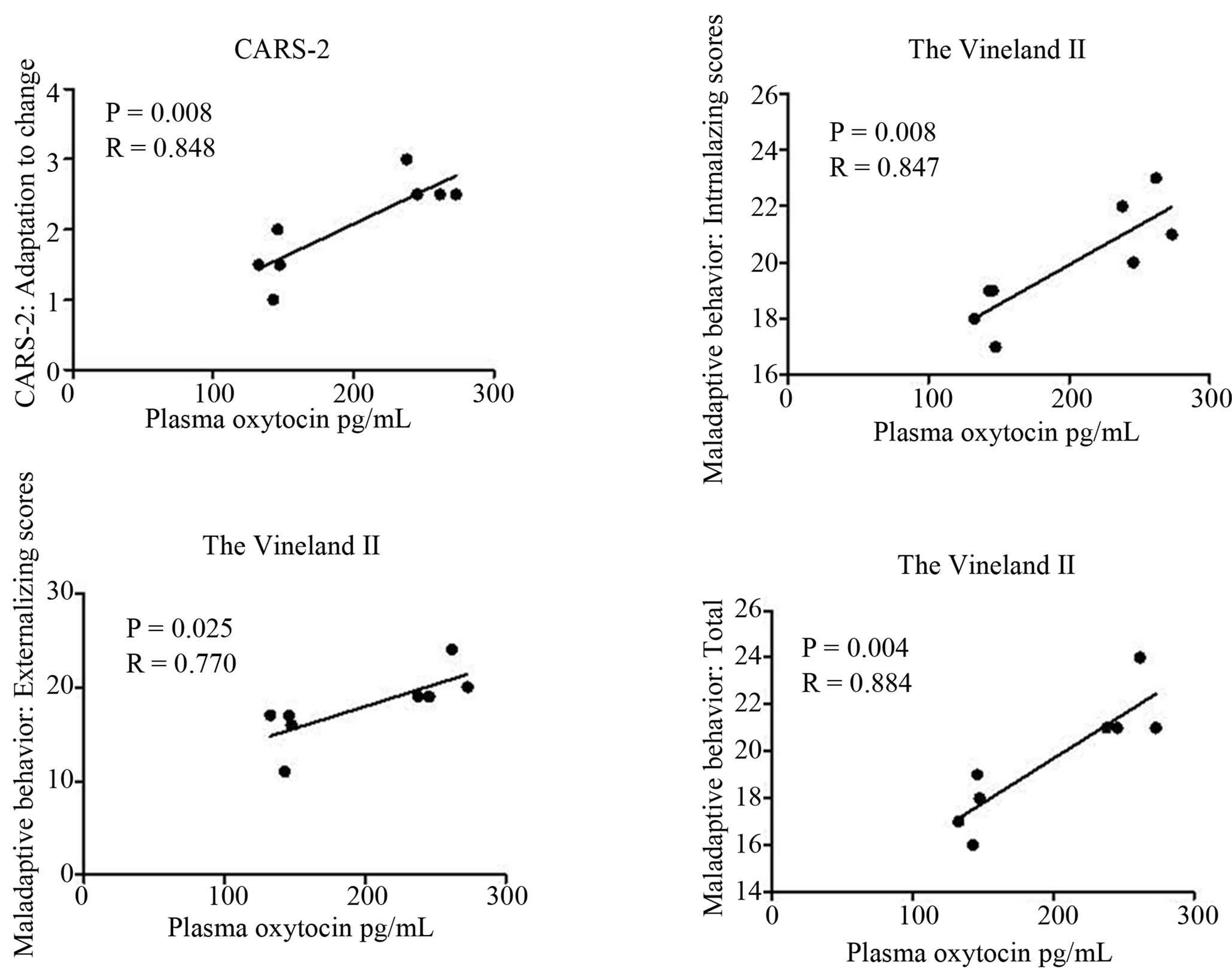
Figure 1. Positive correlations of plasma oxytocin levels and subscores of CARS-2 and The Vineland-II scales.

Figure 2. Differences in subscores of CARS-2 and the Vineland-II scales among low and high oxytocin level groups. Values are expressed as mean ± SEM.
to be found in our sample; oxytocin was related to more severe adaptation abilities confirmed by two powerful psychological tools. These associations were significant by employing more stringent parametric tests despite the small sample size. Furthermore, we have found a positive association of peripheral OT levels with reciprocal interaction and communication subscales of ADI in Slovak children with autism, more severe communication and interaction difficulties were connected to higher OT in patients with autism. However, OT levels in patients with autism were lower than in healthy age-matched controls (unpublished data). A more severe phenotype in children with autism measured by CARS-2 and Vineland-II scales could reflect impaired OT signaling due to decreased expression and/or sensitivity of the oxytocin receptor. As a compensatory mechanism OT levels could be elevated in these patients, however, they are still lower than OT levels in healthy individuals. In addition, OT signaling may be altered in patients with autism due to a different development of neuronal circuits and thus reflect distinctive patterns of behavior in these children compared to healthy individuals as discussed below. Nevertheless, a small sample size and absence of a control group are strong limitations of the present study.
There are a limited number of studies which examined hormonal levels in connection to behavior in ASD. Modahl [4] showed that OT was negatively associated with Daily Living Skills, Interpersonal Relations, and tended to be negatively associated with Communication and overall Adaptive Level of Vineland standard scores. Thus in this study, overall adaptive level seemed to be negatively correlated with OT levels, which is in partial agreement with our results. On the other hand, OT was positively associated with these characteristic in control group [4]. It is possible that oxytocin employs distinct mechanisms in regulating social behavior in children with autism and in healthy individuals.
In the study by Green et al. [5], oxytocin C-terminal extended peptide which was abundant in ASD sample was correlated positively with stereotype behavior measured by Autistic Disorders Checklist (ADC). Al-Ayadhi [6] found no significant correlation between the degree of autism and plasma levels of oxytocin or vasopressin. Finally, the last study by Miller [8] showed no differences in OT levels between ASD and control subjects. However, he found significant gender differences. Girls had higher levels of OT, while boys had significantly higher levels of vasopressin. Higher OT correlated positively with greater anxiety in girls, and with better pragmatic language in all boys and girls.
These findings suggest that there may exist distinct mechanisms of OT action in boys and girls in modulating the different patterns of behavior.
However, it remains questionable how precisely the peripheral levels of oxytocin reflect its central levels and central actions.
In addition, different autism traits may have several etiologies, which may differ between sexes. Nevertheless, profound social deficits represent a core symptom in all ASD patients. Several studies show a correlation of social deficits with oxytocin system, i.e. polymorphisms in oxytocin receptor gene [22]. Moreover, these social deficits seem to be at least partially improved after oxytocin administration in autism patients [7,10-12]. All of these findings strengthen the importance of the oxytocin system in ASD etiology. However, exact mechanisms of OT actions remain to be clarified.
In our sample we have not found any correlations among testosterone levels and symptoms or behavioral characteristics in ASD subjects. One of the causes may be the small sample size.
Information about correlations of actual sex steroid levels in autism and behavioral parameters and autism symptoms are lacking. However, a recent study in healthy children showed that higher postnatal testosterone levels in early infancy were predictive of more male-typical behaviors in the second year of life with more autism spectrum behaviors [23]. In addition, number of studies connects prenatal testosterone levels measured in amniotic fluid or predicted from 2D:4D ratio and various types of autism spectrum behaviors in healthy population [24]. In fact, actual circulating hormones are possible predictors of special types of behavior, however, this effect seems to be an activation of early prenatal brain organization. Thus it is important to consider also prenatal organizational hormonal influences. However, this is very difficult to study and only indirect measures are usually available in narrow populations like ASD.
This pilot study has several limitations including small sample size and both sexes evaluated together (7 males, one female), facts that may have affected the analyses. However, since the age of subjects was 3 to 5 years, we have decided not to exclude one girl from the cohort. Enlargement of the sample is needed to confirm our preliminary results.
In conclusion, we have found that OT levels negatively affect adaptation abilities in all ASD subjects. No relationship among T and autism symptoms or behavioral characteristics was observed. Both examined hormones are related to social bonding properties in human and nonhuman species. According to the steroid/peptide theory of social bonds, specifically defined behavioral patterns affect levels of steroids and neuropeptides and these in turn facilitate or inhibit social bonds [25]. Further studies are needed to define these behavioral patterns and the way they may facilitate or inhibit expression of particular phenotypes underlying ASD.
Acknowledgements
Authors thank to all participants and their families for their cooperation. The study was supported by the following grants: APVV-0253-10, APVV-0254-11, VEGA- 1/0066/12.
REFERENCES
- M. Kosfeld, M. Heinrichs, P. J. Zak, U. Fischbacher and E. Fehr, “Oxytocin Increases Trust in Humans,” Nature, Vol. 435, No. 7042, 2005, pp. 673-676. http://dx.doi.org/10.1038/nature03701
- A. J. Guastella, P. B. Mitchell and M. R. Dadds, “Oxytocin Increases Gaze to the Eye Region of Human Faces,”Biological Psychiatry, Vol. 63, No. 1, 2008, pp. 3- 5. http://dx.doi.org/10.1016/j.biopsych.2007.06.026
- G. Domes, M. Heinrichs, A. Michel, C. Berger and S. C. Herpertz, “Oxytocin Improves ‘Mind-Reading’ in Humans,” Biological Psychiatry, Vol. 61, No. 6, 2007, pp. 731-733. http://dx.doi.org/10.1016/j.biopsych.2006.07.015
- C. Modahl, L. Green, D. Fein, M. Morris, L. Waterhouse, C. Feinstein and H. Levin, “Plasma Oxytocin Levels in Autistic Children,” Biological Psychiatry, Vol. 43, No. 4, 1998, pp. 270-277. http://dx.doi.org/10.1016/S0006-3223(97)00439-3
- L. Green, D. Fein, C. Modahl, C. Feinstein, L. Waterhouse and M. Morris, “Oxytocin and Autistic Disorder: Alterations in Peptide Forms,” Biological Psychiatry, Vol. 50, No. 8, 2001, pp. 609-613. http://dx.doi.org/10.1016/S0006-3223(01)01139-8
- L. Y. Al-Ayadhi, “Altered Oxytocin and Vasopressin Levels in Autistic Children in Central Saudi Arabia,” Neurosciences (Riyadh), Vol. 10, No. 1, 2005, pp. 47-50.
- E. Andari, J. R. Duhamel, T. Zalla, E. Herbrecht, M. Leboyer and A. Sirigu, “Promoting Social Behavior with Oxytocin in High-Functioning Autism Spectrum Disorders,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 107, No. 9, 2010, pp. 4389-4394. http://dx.doi.org/10.1073/pnas.0910249107
- M. Miller, K. L. Bales, S. L.Taylor, J. Yoon, C. M. Hostetler, C. S. Carter and M. Solomon, “Oxytocin and Vasopressin in Children and Adolescents with Autism Spectrum Disorders: Sex Differences and Associations with Symptoms,” Autism Research, Vol. 6, No. 2, 2013, pp. 91-102. http://dx.doi.org/10.1002/aur.1270
- E. Hollander, S. Novotny, M. Hanratty, R. Yaffe, C. M. DeCaria, B. R. Aronowitz and S. Mosovich, “Oxytocin Infusion Reduces Repetitive Behaviors in Adults with Autistic and Asperger’s Disorders,” Neuropsychopharmacology, Vol. 28, No. 1, 2003, pp. 193-198. http://dx.doi.org/10.1038/sj.npp.1300021
- E. Hollander, J. Bartz, W. Chaplin, A. Phillips, J. Sumner, L. Soorya, E. Anagnostou and S. Wasserman, “Oxytocin Increases Retention of Social Cognition in Autism,” Biological Psychiatry, Vol. 61, No. 4, 2007, pp. 498-503. http://dx.doi.org/10.1016/j.biopsych.2006.05.030
- A. J. Guastella, S. L. Einfeld, K. M. Gray, N. J. Rinehart, B. J. Tonge, T. J. Lambert and I. B. Hickie, “Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders,” Biological Psychiatry, Vol. 67, No. 7, 2010, pp. 692-694. http://dx.doi.org/10.1016/j.biopsych.2009.09.020
- E. Anagnostou, L. Soorya, W. Chaplin, J. Bartz, D. Halpern, S. Wasserman, A. T. Wang, L. Pepa, N. Tanel, A. Kushki and E. Hollander, “Intranasal Oxytocin versus Placebo in the Treatment of Adults with Autism Spectrum Disorders: A Randomized Controlled Trial,” Molecular Autism, Vol. 3, No. 1, 2012, pp. 3-16.
- K. M. Kramer, B. S. Cushing, C. S. Carter, J. Wu and M. A. Ottinger, “Sex and Species Differences in Plasma Oxytocin Using an Enzyme Immunoassay,” Canadian Journal of Zoology, Vol. 82, 2004, pp. 1194-1200. http://dx.doi.org/10.1139/z04-098
- C. S. Carter, “Sex Differences in Oxytocin and Vasopressin: Implications for Autism Spectrum Disorders?” Behavioural Brain Research, Vol. 176, No. 1, 2007, pp. 170-186. http://dx.doi.org/10.1016/j.bbr.2006.08.025
- D. A. Geier and M. R. Geier, “A Prospective Assessment of Androgen Levels in Patients with Autistic Spectrum Disorders: Biochemical Underpinnings and Suggested Therapies,” Neuro Endocrinology Letters, Vol. 28, No. 5, 2007, pp. 565-573.
- E. Schwarz, P. C. Guest, H. Rahmoune, L. Wang, Y. Levin, E. Ingudomnukul, L. Ruta, L. Kent, M. Spain, S. Baron-Cohen and S. Bahn, “Sex-Specific Serum Biomarker Patterns in Adults with Asperger’s Syndrome,” Molecular Psychiatry, Vol. 16, No. 12, 2011, pp. 1213- 1220. http://dx.doi.org/10.1038/mp.2010.102
- L. Ruta, E. Ingudomnukul, K. Taylor, B. Chakrabarti and S. Baron-Cohen, “Increased Serum Androstenedione in Adults with Autism Spectrum Conditions,” Psychoneuroendocrinology, Vol. 36, No. 8, 2011, pp. 1154-1163. http://dx.doi.org/10.1016/j.psyneuen.2011.02.007
- S. Baron-Cohen, R. C. Knickmeyer and M. K. Belmonte, Science, Vol. 310, No. 5749, 2005, pp. 819-823. http://dx.doi.org/10.1126/science.1115455
- R. Knickmeyer, S. Baron-Cohen, B. A. Fane, S. Wheelwright, G. A. Mathews, G. S. Conway, C. G. Brook and M. Hines, “Androgens and Autistic Traits: A Study of Individuals with Congenital Adrenal Hyperplasia,” Hormones and Behavior, Vol. 50, No. 1, 2006, pp. 148-153. http://dx.doi.org/10.1016/j.yhbeh.2006.02.006
- J. T. Manning, S. Baron-Cohen, S. Wheelwright and G. Sanders, “The 2nd to 4th Digit Ratio and Autism,” Developmental Medicine & Child Neurology, Vol. 43, No. 3, 2001, pp. 160-164.
- E. Ingudomnukul, S. Baron-Cohen, S. Wheelwright and R. Knickmeyer, “Elevated Rates of Testosterone-Related Disorders in Women with Autism Spectrum Conditions,” Hormones and Behavior, Vol. 51, No. 5, 2007, pp. 597- 604. http://dx.doi.org/10.1016/j.yhbeh.2007.02.001
- A. K.Wermter, I. Kamp-Becker, P. Hesse, G. SchulteKörne, K. Strauch and H. Remschmidt, “Evidence for the Involvement of Genetic Variation in the Oxytocin Receptor Gene (OXTR) in the Etiology of Autistic Disorders on High-Functioning Level,” American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, Vol. 153B, No. 2, 2010, pp. 629-639.
- J. Saenz and G. M. Alexander, “Postnatal Testosterone Levels and Disorder Relevant Behavior in the Second Year of Life,” Biological Psychiatry, Vol. 94, No. 1, 2013, pp. 152-159. http://dx.doi.org/10.1016/j.biopsycho.2013.05.011
- B. Auyeung, J. Ahluwalia, L. Thomson, K. Taylor, G. Hackett, K. J. O’Donnell and S. Baron-Cohen, “Prenatal versus Postnatal Sex Steroid Hormone Effects on Autistic Traits in Children at 18 to 24 Months of Age,” Molecular Autism, Vol. 3, No. 1, 2012, p. 17. http://dx.doi.org/10.1186/2040-2392-3-17
- S. M. van Anders, K. L. Goldey and P. X. Kuo, “The Steroid/Peptide Theory of Social Bonds: Integrating Testosterone and Peptide Responses for Classifying Social Behavioral Contexts,” Psychoneuroendocrinology, Vol. 36, No. 9, 2011, pp. 1265-1275. http://dx.doi.org/10.1016/j.psyneuen.2011.06.001
NOTES
*Corresponding author.

