American Journal of Analytical Chemistry
Vol.07 No.04(2016), Article ID:65777,15 pages
10.4236/ajac.2016.74035
A Theoretical Model of pH-Based Potentiometric Biosensor Based on Immobilized Enzyme Membrane
Jeganathan Saranya1, Lakshmanan Rajendran2*, Mariappan Uma Maheswari3
1Department of Mathematics, Mother Teresa Women’s University, Kodaikanal, India
2Department of Mathematics, Sethu Institute of Technology, Kariapatti, India
3Department of Mathematics, Kamaraj College of Engineering and Technology, Virudhunagar, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 March 2016; accepted 19 April 2016; published 22 April 2016
ABSTRACT
A theoretical model for the non steady-state response of a pH-based potentiometric biosensor immobilizing organophosphorus hydrolase (OPH) is discussed. The model is based on a system of five coupled nonlinear reaction-diffusion equations under non steady-state conditions for enzyme reactions occurring in potentiometric biosensor that describes the concentration of substrate and hydrolysis products within the membrane. New approximate analytical expressions for the concentration of the substrate (organophosphorus pesticides (OPs)) and products are derived for all values of Thiele modulus and buffer concentration using new approach of homotopy perturbation method. The analytical results are also compared with numerical ones and a good agreement is obtained. The obtained results are valid for the whole solution domain.
Keywords:
Mathematical Modeling, Reaction-Diffusion, pH-Based Potentiometric Biosensor, Asymptotic Methods, New Homotopy Perturbation Method

1. Introduction
A potentiometric biosensor is a type of chemical sensor that may be used to find the concentration of some components of the analyte. These sensors measure the electrical potential of an electrode when no voltage is present. The potentiometric biosensors have been widely used in environmental, medical and industrial applications [1] . Also potentiometric biosensor can be used for detection of all OPs but they don’t have low enough limits of detection [2] .
The theoretical modeling of biosensors involves solving the system of linear/non-linear reaction-diffusion equations for substrate and product with a term containing a rate of biocatalytical transformation of substrate. The complications of modeling arise due to solving the partially differential equations with non-linear reaction term and with complex initial and boundary conditions. The modeling of biosensor is analyzed by numerical [1] and analytical method [3] of partial differential equation with various boundary conditions. Recently Meena and Rajendran (2010) discussed a theoretical model of a pH-based potentiometric biosensor immobilizing organophosphorus hydrolase (OPH) for steady state conditions [4] .
Rahamathunissa and Rajendran (2008) implemented He’s variational iteration method in nonlinear boundary- value problems in enzyme substrate reaction diffusion processes in amperometric biosensor [5] . Manimozhi et al. [6] presented the solution of steady-state substrate concentration in the action of biosensor response with mixed enzyme kinetics under a Michalis-Menten scheme. Analytical solutions for the steady-state current at a microdisk chemical sensor have been reported by Dong and Che [7] and by Lyons et al. [7] [8] . Recently, Eswari and Rajendran [9] derived the concentration profile of the product of the enzyme reaction and the electrode current for all values of Michalis-Menten constant using the Homotopy perturbation method.
To our knowledge, no general analytical expressions of the concentrations of the substrate, hydrolysis products, added external buffer and hydrogen ions have been reported for all values of parameters. The purpose of this communication is to derive an analytical expression of non-steady state concentrations of OPs and the deprotonation products for all values of reaction parameter using new homotopy perturbation method.
2. Mathematical Formulation of the Problem
The complete description of the problem is given in [4] [10] . For the sake of completeness the brief description is given in this section and Appendix-A. A schematic diagram of the pH-based potentiometric biosensor immobilizing organophosphorus hydrolase (OPH) is represented in Figure 1.
In this figure, S denotes the substrate of organophosphorus pesticides (OPs). 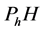 and
and 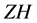 are represent the hydrolysis products of organophosphodiester and alcohol respectively.
are represent the hydrolysis products of organophosphodiester and alcohol respectively.  is the added external buffer and
is the added external buffer and ,
, 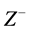 ,
,  ,
, 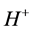 are the deprotonation products. The general scheme that represents an enzyme-cata- lyzed reaction within enzyme membrane can be written as follows:
are the deprotonation products. The general scheme that represents an enzyme-cata- lyzed reaction within enzyme membrane can be written as follows:
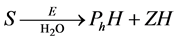 (1)
(1)
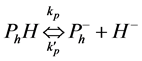 (2)
(2)
Figure 1. Schematic representation of pH-based potentiometric biosensor immobilizing OPs (organophosphorus pesticides).
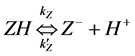 (3)
(3)
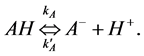 (4)
(4)
The non-linear reaction-diffusion equations for non-steady state condition can be described as follows
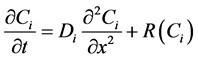 (5)
(5)
where  is the concentration of species,
is the concentration of species, 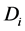 is the diffusion coefficient and
is the diffusion coefficient and 
Table 1. Nomenclature.
3. Dimensionless Form
The dimensionless reaction-diffusion equations for non-steady state condition can be written as follows (Appendix A):





where







The initial and boundary conditions for the above equations becomes



A graphical representation of the boundary conditions of this system conditions can be seen in Figure 2.
4. Analytical Expression of Concentration of Substrate and Products Using New Homotopy Perturbation Method (New HPM) and Laplace Transform Technique
With the rapid development of nonlinear science, there appears an ever-increasing interest of scientists and engineers in the approximate analytical asymptotic techniques for nonlinear problems [11] . It is very difficult to solve nonlinear problems either numerically or theoretically. Perturbation methods provide the most versatile tools available in nonlinear analysis of engineering problems, and they are constantly being developed and applied to ever more complex problems. Homotopy perturbation method was first proposed by the He [12] . Recently, a new approach to HPM is presented to solve the nonlinear problem and this gives a simple approximate solution in the zeroth iteration [13] . By using this new homotopy perturbation method and Laplace transform technique (Appendix B), the concentrations of substrate and products can be obtained as follows:

Figure 2. Boundary conditions employed in the pH-based potentiometric biosensor for the substrate






where

5. Results and Discussion
Equations (15) to (19) represents the general new closed-form of analytical expression for the concentrations of substrate




The kinetic response of a pH-based potentiometric biosensor depends on the concentration of substrate. How- ever, substrate concentration depends on two factors, 




1) Influence of time on the concentration of species. Figures 3(a)-(f) represent concentration of the substrate
Figure 3. (a)-(f) Plot of dimensionless non-steady state concentration profiles of the substrate 



versus dimensionless distance for fixed values of a and 




2) Influence of Thiele modulus on the concentration of species. The influence of Thiele modulus on the concentration of the substrate for some values of other parameters is shown in Figures 4(a)-(f). The concentration of the substrate strongly depends on Thiele modulus a. From this figure, it is observed that the concentration of substrate decreases when Thiele modulus increases. The concentration of substrate is in uniform or in steady state when Thiele modulus
Figure 4. (a)-(f) Plot of dimensionless non-steady state concentration profiles of the substrate 



3) Influence of added buffer concentration on the concentration of species. The influence of added buffer concentration on the concentration of the substrate for some values of other parameters is shown in Figures 5(a)-(e). From this figure, it is inferred that the concentration of the substrate increases when added buffer concentration increases. Solid lines represent the Equation (15) and the dotted lines represent the numerical simulation. Satisfactory agreement is noted. The MATLAB program also given in Appendix B.
Figure 5. (a)-(e) Plot of dimensionless non-steady state concentration profiles of the substrate 



Figures 6(a)-(h) show the dimensionless non-steady state concentration profiles of products








Figure 7(a) & Figure 7(b) show the dimensionless non-steady state concentration of substrate 




Table 2 and Table 3 represents the comparison of analytical expression of concentration of the substrate 
Figure 6. (a)-(h) Plot of dimensionless non-steady state concentration profiles of products





Figure 7. (a)-(b) Plot of dimensionless non-steady state concentration profiles of substrate 





Table 2. Comparison of analytical expression of concentration of the substrate 
Table 3. Comparison of analytical expression of concentration of the substrate 

error deviation increases, when the reaction diffusion parameter a increases. Similarly in Table 3, average percentage of error deviation decreases, when the time 
6. Conclusion
A non-linear time dependent system of differential equation in pH-based potentiometric biosensor has been solved using the new HPM. New approximate analytical expressions for the concentrations of the substrate and hydrolysis products are derived. The time dependent substrate concentration profiles are also presented using SCILAB program. Concentration of substrate and product depends upon Thiele modulus and initial concentration of substrate which is discussed in this communication.
Acknowledgements
This work was supported by the DST SB/SI/PC-50/2012, New Delhi, India. The authors are thankful to Mr. S. Mohamed Jaleel, The Chairman, Dr. A. Senthilkumar, The Principal, Dr. P. G. Jansi Rani, Head of the Department of Mathematics, Sethu Inistitute of Technology, Kariapatti-626115, Tamilnadu, India for their encouragement.
Cite this paper
Jeganathan Saranya,Lakshmanan Rajendran,Mariappan Uma Maheswari, (2016) A Theoretical Model of pH-Based Potentiometric Biosensor Based on Immobilized Enzyme Membrane. American Journal of Analytical Chemistry,07,363-377. doi: 10.4236/ajac.2016.74035
References
- 1. Baronas, R., et al. (2010) Mathematical Modeling of Biosensors: An Introduction for Chemists and Mathematicians. Springer.
http://dx.doi.org/10.1007/978-90-481-3243-0 - 2. Sahin, A. (2012) Development of Electrochemical Methods for Detection of Pesticides and Biofuel Production. Ph.D. Thesis, Columbia University Academic Commons.
- 3. Rajendran, L. (2013) Chemical Sensors Simulation and Modeling, Electrochemical Sensors. In: Korotchenkov, G., Ed.
- 4. Meena, A. and Rajendran, L. (2010) Analysis of a pH-Based Potentiometric Biosensor Using the Homotopy Perturbation Method. Chemical Engineering & Technology, 33, 1-10.
http://dx.doi.org/10.1002/ceat.200900580 - 5. Rahamathunissa, G. and Rajendran, L. (2008) Application of He’s Variational Iteration Method in Nonlinear Boundary Value Problems in Enzyme—Substrate Reaction Diffusion Processes: Part 1. The Steady-State Amperometric Response. Journal of Mathematical Chemistry, 44, 849-861.
http://dx.doi.org/10.1007/s10910-007-9340-9 - 6. Manimozhi, P., Subbiah, A. and Rajendran, L. (2010) Solution of Steady-State Substrate Concentration in the Action of Biosensor Response at Mixed Enzyme Kinetics. Sensors and Actuators B, 147, 290-297.
http://dx.doi.org/10.1016/j.snb.2010.03.008 - 7. Dong, S. and Che, G. (1991) Electrocatalysis at a Microdisk Electrode Modified with a Redox Species. Journal of Electroanalytical Chemistry, 309, 103-114.
http://dx.doi.org/10.1016/0022-0728(91)87007-Q - 8. Lyons, M.E.G., Bannon, T. and Rebouillat, S. (1998) Reaction/Diffusion at Conducting Polymer Ultramicroelectrodes. Analyst, 123, 1961-1966.
http://dx.doi.org/10.1039/a804039g - 9. Eswari, A. and Rajendran, L. (2010) Analytical Solution of Steady-State Current an Enzyme-Modified Microcylinder Electrodes. Journal of Electroanalytical Chemistry, 648, 36-46.
http://dx.doi.org/10.1016/j.jelechem.2010.07.002 - 10. Wang, L.H., Zhang, L., Huang, J.X. and Chen, H.L. (2006) Model of a pH-Based Potentiometric Biosensor Immobilizing Organophosphorus Hydrolase. Chemical Engineering & Technology, 29, 462-467.
http://dx.doi.org/10.1002/ceat.200500281 - 11. He, J.-H. (2006) Some Asymptotic Methods for Strongly Nonlinear Equations. International Journal of Modern Physics, 20, 1141-1199.
http://dx.doi.org/10.1142/S0217979206033796 - 12. He, J.-H. (1999) Homotopy Perturbation Technique. Computer Methods in Applied Mechanics and Engineering, 178, 257-262.
http://dx.doi.org/10.1016/S0045-7825(99)00018-3 - 13. Rajendran, L. and Anitha, S. (2013) Comments on Analytical Solution of Amperometric Enzymatic Reactions Based on HPM. Electrochimica Acta, 102, 474-476.
http://dx.doi.org/10.1016/j.electacta.2013.03.163
Appendix A. The Dimensionless Reaction-Diffusion Equations
In the enzyme membrane, the reaction-diffusion equations for the concentration of species for non-steady state condition can be represented as follows [4] [10] .








where




where

By introducing the following set of dimensionless variables

and defining the following “composite species” [10]




we obtain the dimensionless form of Equations (6)-(10) for the concentration of species which are given in the text.
Appendix B. Analytical Solutions of Equations (6)-(10) Using Complex Inversion Formula
In this appendix, we indicate how the Equations (15) is derived. Using new homotopy perturbation approach [13] , Equation (6) can be written as


The approximate solution of Equation (B.2) is

Substituting Equation (B.3) into Equation (B.2) and arranging the coefficients of powers p


The initial and boundary conditions for Equations (12)-(14) becomes



Equation (B.4) can be written as

where 

Using residue theorem (Appendix C) we can obtain the Equation (15) in the text.
Appendix C. Inverse of Equation (B. 10) by Using Complex Inversion Formula
In this appendix, we indicate how Equation (B.10) may be inverted using the complex inversion formula. If 


where the integration in Equation (C.1) is to be performed along a line 




where the residues are computed at the poles of the function

From the theory of complex variables, we can show that the residue of a function 


Hence, in order to invert Equation (B.10), we need to evaluate

The poles are obtained from



Hence, we note that

The first residue in Equation (B.17) is given by

The second residue in Equation (B.17) is given by

where 



where 
Appendix D. Scilab/Matlab Program to Find the Numerical Solutions of Equations (15) to (18)
function pdex4
m = 0;
x = linspace(0,1);
t = linspace(0,10);
sol = pdepe(m,@pdex4pde,@pdex4ic,@pdex4bc,x,t);
u1 = sol(:,:,1);
u2 = sol(:,:,2);
figure
plot(x,u1(end,:))
title('u1(x,t)')
xlabel('Distance x')
ylabel('u1(x,2)')
%――――――――――――――――――――――
%figure
%plot(x,u2(end,:))
%title('u2(x,t)')
%xlabel('Distance x')
%ylabel('u2(x,2)')
%――――――――――――――――――――――
function [c,f,s] = pdex4pde(x,t,u,DuDx)
c = [1; 1];
f = [1; 1].*DuDx;
a=5;
F1 = -(a^2*u(1))/(1+u(1));
F2 = -(a^2*u(1))/(1+u(1));
s = [F1;F2];
% ――――――――――――――――――――?
function u0 = pdex4ic(x)
u0 = [0; 0];
% ――――――――――――――――――――?
function [pl,ql,pr,qr] = pdex4bc (xl,ul,xr,ur,t)
pl = [0;0];
ql = [1;1];
pr = [ur(1)-1;ur(2)-0];
qr = [0; 0];
NOTES
*Corresponding author.










