American Journal of Analytical Chemistry
Vol. 4 No. 7A (2013) , Article ID: 34004 , 8 pages DOI:10.4236/ajac.2013.47A006
Adsorption by Liquid-Liquid Extraction of Hg(II) from Aqueous Solutions Using the 2-Butyl-imidazolium Di-(2-ethylhexyl) Phosphate as Ionic Liquid
Laboratory of Separation and Purification Technology, Department of Chemistry, Faculty of Sciences, Tlemcen University, Tlemcen, Algeria
Email: *madidi13@yahoo.fr
Copyright © 2013 Mohamed Amine Didi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 14, 2013; revised May 16, 2013; accepted June 10, 2013
Keywords: Mercury; Ionic Liquid; Solvent Extraction; Speciation; Butylimidazole; Di-(2-Ethylhexyl) Phosphoric Acid
ABSTRACT
In this work, a novel room temperature ionic liquid (2-butyl-imidazolium di-(2-ethylhexyl) phosphate) ([C4mim] [D2EHPA]) was synthesized and tested as extractant in the mercury(II) liquid-liquid extraction. The effects of parameters such as aqueous to organic phase’s volume ratio, metal concentration IL concentration, pH levels, ionic strength, and temperature were reported. For the extraction of metal, [C4mim]3[R.HR]3[HgCl2]org and [C4mim]3[R.HR]3 [HgClOH]org species were formed where (H2R2) was D2EHPA. In the case of ionic strength, the results showed that the addition of sodium acetate at 0.302 mmol·L−1 to the aqueous phase strongly increased the mercury extraction yield (R = 100%). The extracted species were investigated by a calculation program using CHEAQS V. L20.1 in order to determine the relation between the percentages of the extracted species and the extraction yield. The results showed that the extracted species in the best conditions were HgCl2 and HgClOH with respective percentages 80.66% and 18.29%.
1. Introduction
Mercury (Hg) is one of the most toxic heavy metals commonly found in the global environment. Its toxicity is related to the capacity of its compounds to bio concentrate in organisms and to bio magnified through food chain.
The removal of toxic heavy metal ions from wastewater is of great concern in the environmental field of waste and pollution reduction [1].
Mercury and its compounds are considered one among the priority hazardous contaminants in the environment which are harmful to human health and natural ecosystems [2-5].
It is bio-accumulative and not degradable so levels in living organisms and the environment augment when the emissions increase. There are worldwide efforts and regulations to decrease the emissions.
Several techniques for the removal of mercury ions from wastewaters have been developed such as reduction [6], chemical precipitation [7], membrane separation [8], ion-exchange [9], solvent extraction [10], coagulation [11], and adsorption [12,13].
Furthermore, the large-scale use of this metal in diverse industrial domains requires its recovery from secondary industrial resources.
Ionic liquids (ILs) show unique properties such as nonvolatility (negligible vapor pressure), thermal stability, nonflammable nature, lower reactivity, strong ability to dissolve a large variety of organic and inorganic compounds. That’s why we should study the potential of it to the extraction of rare earth (lanthanides plus Sc and Y) and heavy metals. Indeed, heavy metals are a big problem for industrial companies who want to recycle the waste like mercury or cadmium to be more economically1 profitable as well as for the environment. There is a much bigger application.
Ionic liquids, also called room temperature ionic liquids (RILS) and ambient temperature molten salts, which are liquids at ambient temperature [14], are completely composed of organic cations and inorganic/organic anions.
Recently, ionic liquids have received increased attention from both industrial and academic communities because of their unusual properties and great potential as “green” solvents for industrial processes [15].
Ionic liquids have lots of properties of conventional organic solvents, such as excellent solvation qualities, a low viscosity, immeasurably low vapor pressure, high ionic conductivity, good thermal and electrochemical stability, and a wide temperature range [16-18]. They are environmentally benign, nonvolatile, and nonflammable with a high thermal stability [19].
Ionic liquids are widely considered as alternatives to classical organic solvents and as such have been applied in many fields such as organic synthesis, electrochemistry, liquid phase extraction, catalysis for clean technology and polymerization processes [20-24].
A number of papers have been devoted to the description of synthesis and properties of various ILs, including imidazolium-based ILs and phosphonium-based ILs focusing on their potential for the extraction of organic and inorganic compounds [25].
The aim of this work is to investigate the ability of a new ionic liquid, using the 2-butyl-imidazolium di-(2- ethylhexyl) phosphate ([C4mim][D2EHPA]) synthesized in our laboratory, to extract Mercury(II) from aqueous chloride solutions.
The effects of parameters as initial mercury(II) concentration, ionic liquid concentration initial pH of aqueous solution, ionic strength and temperature on the mercury extraction yield were studied.
This study also aims at investigating the extracted species into the organic phase using slope analysis method.
By a calculation program using CHEAQS V. L20.1, the determination of the nature and the percentages of the present species were deducted in organic medium to be able to determine the relation between the nature of the extracted species and the extraction yield [26].
2. Experimental
2.1. Reagents
The reagents used in this study were 1-Butylimidazole (98%, Aldrich), Di-(2-ethylhexyl) phosphate (97%, Fluka), Chloride of mercury (99.5%, Aldrich), Chloride of sodium (99.5%, Aldrich), sodium acetate (99%, Aldrich), 1-(2-Pyridylazo)-2-naphthol (PAN) (97%, Aldrich), chloroform (99%, Fluka), hydrochloric acid (37%, Fluka) and buffer solution at pH = 13.0 (Riedel-Dehaen AG).
2.2. Apparatus
Analytik Jena SPECORD 210 Double Beam UV-VIS was used for spectra recording and absorbance measurements.
Spectra were recorded in the range from 400 to 800 nm with 0.2 nm resolution in 10 mm quartz cells. Data were processed with WinLab software.
pH measurements for all solutions were taken on a potentiometer Consort C831, with combined glass electrode, that was calibrated with pH 4.00, 7.00 and 10.00 buffer standards.
2.3. Ionic Liquid Synthesis
A mixture of D2EHPA (6.448 g, 20 mmol) and 1-butylimidazole (2.483 g, 20 mmol) to a 250 ml round-bottom flask fitted for 24 h at room temperature (20˚C) till the formation of a yellow and viscous liquid.
The viscous liquid was washed three times with 50 ml portion of ethyl acetate in a separation funnel. The washed IL was dried with anhydrous magnesium sulfate and heated under vacuum at 70˚C to remove the solvent. The purity of final product was characterized with 1H NMR, 13C NMR, 31P NMR and FTIR (see Figure 1). The yield was about 98%.
1H NMR: δ/TMS (ppm) = 0.96 (m, 15H, CH3), 1.33 (m, 18H, CH2), 1.56(m, 4H, CH), 3.93 (m, 4H, CH2O P), 5.0 (t, 1H, NH), 5.72 (q; 1H, CHar), 5.89 (q; 1H, CHar), 6.36 (q; 1H, CHar), 7.11 (s, 1 H, P(OH)). 13C NMR: δ/TMS (ppm) = 11.6; 14.1; 68.8; 117.8; 119.1; 142.5, 21.7; 23.0; 23.3; 29.3; 30.4; 30.6; 32.6; 34.2; 22.4; 40.3; 108.1; 108.5; 118.0; 141.5. 31P NMR: δ/H3PO4 (ppm) = 0.02. IR: ν (cm−1) = 895 (w), 1045 (P OC, vS, L), 1240 (P = O, S, L), 1380 (w), 1470 (m), 1685 (w, L), 2855 (S), 2955 (vS).
δ, chemical shift; ν, wave number; s, singulet; d, doublet; t, triplet; q, quadruplet; quint, quintuplet; sext, sextuplet; m, multiplet; J, coupling constante; S, strong; L, large; w, weak.
2.4. Procedure
Experiments were performed by ionic liquid and an

Figure 1. Structure of 2-butyl-imidazolium bis(2-ethylhexyl) phosphate.
aqueous solution of metal ion, shaking for few minutes and then centrifuging to separate the two phases. In our experimental procedure, liquid/liquid extraction was carried out by contacting volumes of ionic liquid and mercury solution (with known concentration and fixed pH), kept under stirring at constant temperature.
The mercury concentrations in the aqueous phase were determined, before and after extraction by Uv-Visible with PAN as ligand for Mercury at pH 13.0. The absorbance of PAN-Hg(II) complex was measured at 580 nm.
2.5. Extraction Efficiencies of Mercury with IL
The biphasic system was shaken to ensure it was fully mixed and then centrifuged to separate the two phases after extraction. The upper aqueous phase was taken out and measured with spectrophotometer to determine the concentration of metal that was left in the aqueous phase. Extraction efficiencies (E) were calculated by
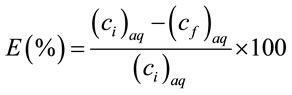 (1)
(1)
and distribution coefficient :
 (2)
(2)
where (Ci)aq and (Cf)aq are the concentration of H(II) ions in aqueous phase before and after extraction, respectively.
2.6. Optimization Experiments
Experiments were conducted to find the effect of different process conditions on the performance of the system and to obtain best conditions for maximum separation. Experiments are conducted to find the effect of:
1) Phase volume ratio;
2) Hg(II) initial concentration;
3) Ionic liquid concentration;
4) Initial pH of aqueous solution;
5) Ionic strength (salt concentration);
6) Temperature.
3. Results and Discussion
3.1. Effect of Volume Ratio
The effect of the aqueous organic volume ratio (Aq/Org ratio) was investigated by varying the Aq/Org ratio from 1 to 6 keeping the total volume of phases constant and initial pH of 6.07. The results are presented in Figure 2.
The results showed that the extraction yield decreases with the increase of the Aq:Org volume ratio. The maximum of extraction yield is reached for a value equal to 1 (84.31%).
The phase volume ratio equal to 1 was chosen for the rest of the experiments.
3.2. Effect of Contact Time
Figure 3 showed UV-Vis absorption spectra of PANHg(II) complex in aqueous phase at different contact time.
The results presented in Figure 4 showed that a minimum of 20 minutes of shaking time is needed for extraction of mercury(II). The prolonged shaking had no positive effect on the extraction of Hg(II).
Extraction of Hg(II) with [C4mim][D2EHPA] is fast and the equilibrium time was 20 min at different conditions of initial concentration of ionic liquid and mercury aqueous solution.
3.3. Effect of Hg(II) Initial Concentration
The effect of the initial mercury concentration on the

Figure 2. Effect of phase volume ratio on the extraction of Hg(II) [Hg2+]0 = 10−3 M, [IL]0 = 5 × 10−3 M, pHi = 6.07, T = 20˚C.
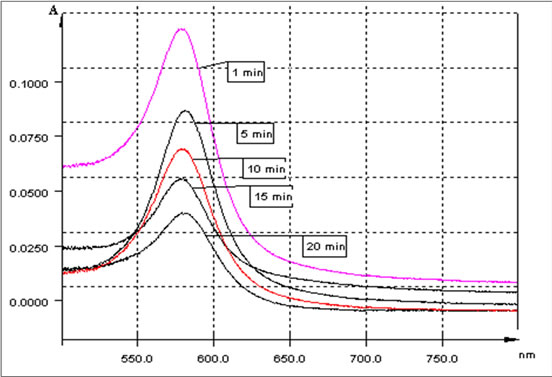
Figure 3. UV-Vis absorption spectra of PAN-Hg(II) complex in aqueous phase at different contact time, λ (nm) = f(Absorbance).
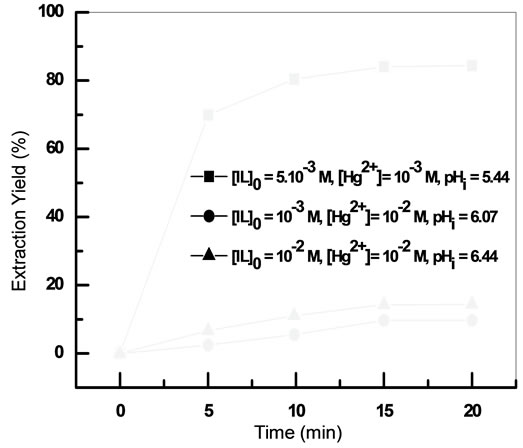
Figure 4. Extraction kinetics at different initial concentrations of IL and Hg(II) Vaq/Vorg = 1, T = 20˚C.
extraction yield was studied in the range 10−3 M - 10−2 M.
From the results given in Figure 5, we notice that the quantity of extracted Mercury(II) decreases with the increase of the initial concentration of metal aqueous phase.
A value of 10−3 M of initial concentration of Hg(II) was chosen for the rest of the experiments.
3.4. Effect of IL Concentration
In order to evaluate the ionic liquid role as a simple medium or as an active partner in a molecular interaction, a series of experiments were performed by varying amount of the IL in the range 10−3 to 7 × 10−3 M at 293 K and 900 rpm stirring speed. The results are shown in Figure 6 witch it was observed that the extraction yield of Hg (II) increases strongly with an increase in the IL concentration in the range 2 × 10−3 to 7 × 10−3 M.
3.5. Effect of pH
For any extraction process, initial pH of the feed is an important parameter which governs the efficiency of the separation process. Extraction of metal from aqueous solution depends on the strength of the acid/base.
Effect of pH in the aqueous phase on extraction of mercury was studied by varying initial pH in the range 2.10 - 6.44, pH was adjusted by adding necessary moieties of HCl or NaOH 0.01 mol·L−1 (Figure 7). As can be seen, extraction of mercury increases with decrease in the acidity and is found to be maximum in the range pH = 4.12 to 5.18.
This could be explained by the fact that in the pH range (2.10 - 4.12), the extraction was governed by the cation exchange reaction, in which the protons are released [27,28].
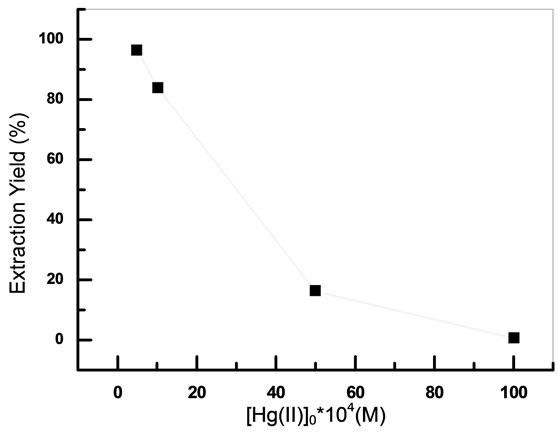
Figure 5. Effect of initial concentration of Hg(II); Vaq/Vorg = 1, [IL]0 = 5 × 10−3 M, teq = 20 min, T = 20˚C, pHi = 6.07.

Figure 6. Effect of initial concentration of ionic liquid. [Hg2+]0 = 10−3 M, pHi = 6.07, teq = 20 min, Vaq/Vorg = 1, T = 20˚C.
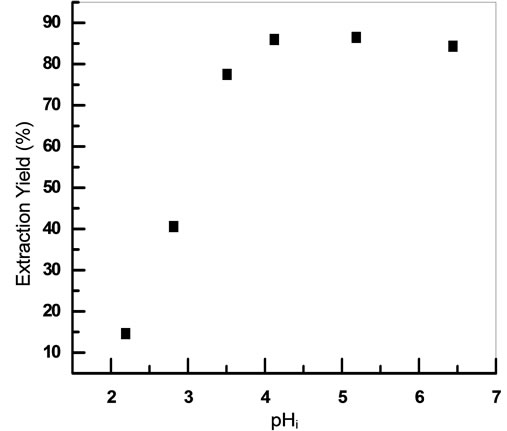
Figure 7. Effect of initial pH of aqueous solution on the extraction efficiencies of Hg(II); [Hg2+]0 = 10−3 M, [IL]0 = 5 × 10−3 M, teq = 20 min, Vaq/Vorg = 1, T = 20˚C.

Figure 8. Distribution diagrams of Hg(II) (1 mmol·L−1) in chloride media using Medusa and Hydra programs [27].
From (Figure 8) it can be seen that maximum mercury extraction obtained at pH 4.12 could be due to the presence of HgCl2 with a high percentage.
The increase of the yield of extraction with an increase of initial pH was related with a relative increase of percentage of HgClOH species.
3.6. Determination of the Metal-Organic Complex Species
The stochiometric coefficients for the extraction reaction can be determined from the intercept of the plot of logD vs. log[IL]0.
Figure 9 shows a plot of log D vs. log [IL]0 at initial pH 6.07. The slope value was found to be ~3.
The extraction mechanism in acetate medium by D2EHPA diluted in chloroform may be represented as:
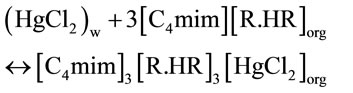
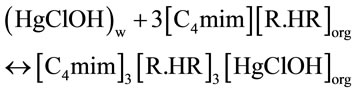
where (H2R2) is D2EHPA which dimerizes in aliphatic solvent as reported by various authors [29,30].
3.7. Effect of Ionic Strength
The influence of the ionic strength on the extraction yields of Hg(II) using ionic liquid, was studied by adding sodium chloride and sodium acetate to the aqueous phase. Firstly, effect of chloride sodium on the extraction of mercury ions was studied by varying concentration of this salt in the range (0.0215 mmol - 0.2300 mmol).
The results showed that the addition of amount of the chloride sodium to the aqueous phase has an antagonistic effect on the yield of extraction at different values of initial pH (Figure 10).
The Figure 11 shows a comparative study on the extraction behavior of mercury(II) from different salt medium using (sodium chloride and sodium acetate) at the same initial pH of aqueous solution.
From Figure 11 it can be seen that the addition of sodium acetate at 0.03 mmol·L−1 in aqueous solution of mercury increases the extraction yield to 100%, however the increase of this concentration decreases the extraction yield.
The results found by calculation program using CHEAQS V. L20.1 are summarized in Table 1. From this table it can be seen that the increase of the extraction yield is related with the decrease of percentage of HgCl2 species and the increase of percentage of HgClOH species.
Figure 12 shows the variation of extraction yield with percentages ratio of extracted species HgCl2 and HgClOH
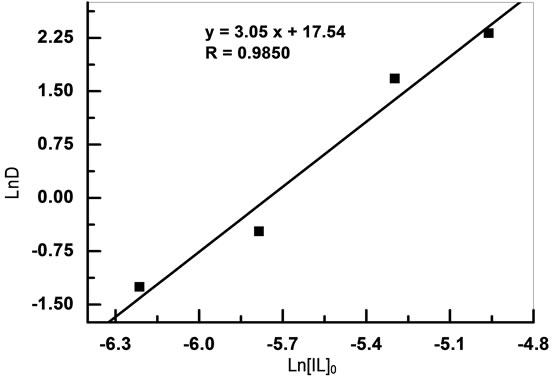
Figure 9. Effect of ionic liquid concentration on the distribution coefficient of mercury. [Hg2+]0 = 10−3 M, pH = 6.07, T = 20˚C, Vaq/Vorg = 1.

Figure 10. Effect of NaCl on extraction yield; [Hg2+]0 = 10−3 M, [IL]0 = 5 × 10−3 M, teq = 20 min, Vaq/Vorg = 1, T = 20˚C.
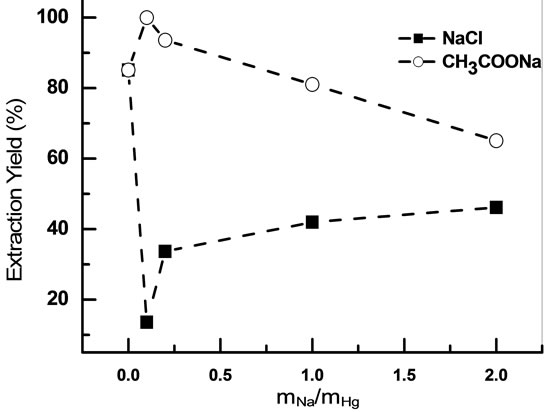
Figure 11. Comparative study to effect of [CH3COONa] and [NaCl] on extraction yield [Hg2+]0 = 10−3 M, [IL]0 = 5 × 10−3 M, pHi = 5.41, teq = 20 min, Vaq/Vorg = 1, T = 20˚C.
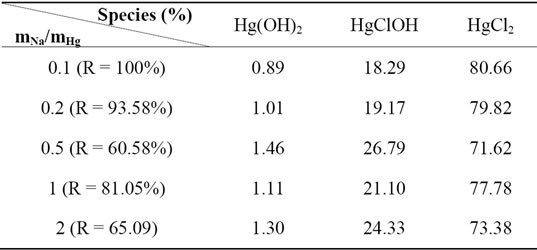
Table 1. Nature and percentages of extracted species at different concentration of CH3COONa.
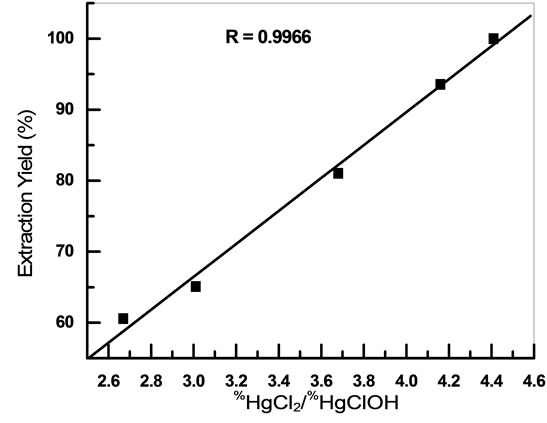
Figure 12. Variation of extraction yield with percentages ratio of extracted species HgCl2 and HgClOH.
(%HgCl2/%HgClOH). As can be seen, extraction of mercury increases with increased%HgCl2/%HgClOH ratio in the range of 2.67 - 4.41.
3.8. Effect of Temperature
The effect of temperature on the extraction of the Hg(II) using ionic liquid was examined at 293, 303 and 318 K respectively. The experimental results are collected in Figure 13.
The effect of temperature on the extraction of Mercury (II) ions was studied under optimum conditions.
Different thermodynamic parameters were computed using Van’t Hoff equation in the form [31]:
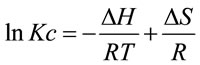 (3)
(3)
 (4)
(4)
where ΔH, ΔS, ΔG, and T are the enthalpy, entropy, Gibbs free energy, and temperature in Kelvin, respectively. The values of equilibrium ratio (Kc), were calculated at each temperature using the relationship.
 (5)
(5)
where Fe is the fraction of Hg(II) extracted at equilibrium.
The plot of logKc vs 1/T is a straight line as shown in Figure 14 with correlation coefficient r = 0.9870. The numerical values of ΔH, ΔS are computed from the slope. The negative value of Gibbs free energy as shown in Table 2, indicates the spontaneous nature of extraction, while negative value of ΔH reflects the exothermic extraction behavior. The negative value of ΔS indicates the complex stability.
4. Conclusions
The extraction of Hg(II) ions in a biphasic system consisting of an ionic liquid and an aqueous chloride phase was demonstrated. This novel extraction system can be employed for the separation and preconcentration of metal ions. RTILs are environmentally benign because of their nonvolatile and nonflammable properties.
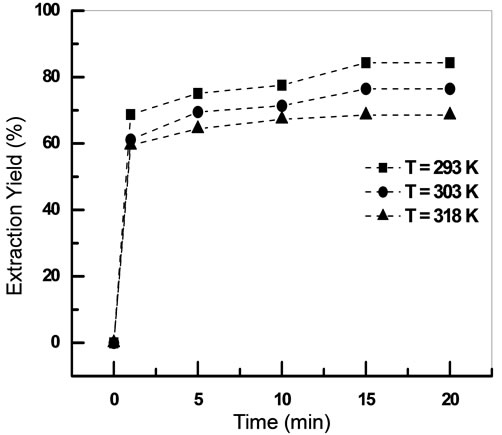
Figure 13. Effect of temperature on the extraction of Hg(II) [Hg2+]0 = 10−3 M, [IL]0 = 5 × 10−3 M, teq = 20 min, Vaq/Vorg = 1, T = 20˚C.

Table 2. Thermodynamic constants of the extraction of mercury ions.

Figure 14. Variation of logKc with 1/T for the extraction of Hg(II) using ionic liquid.
The higher capacity for Hg(II) of [C4mim][D2EHPA] indicates that ionic liquid synthesized may act as extractant.
A study of the extraction kinetics of Mercury(II) from an aqueous solution with a new synthesized ionic liquid showed that the extraction equilibrium was reached after 20 min at different initial concentrations of mercury and ionic liquid.
The best extraction of Mercury(II) was obtained for IL concentration of 0.005 M and a mercury concentration of 0.001 M. The extraction percentage increases with an increase of initial pH of the aqueous phase and is found to be maximum in the range pH = 4.12 to 5.18.
Investigation with slope method concludes that the extraction of mercury(II) [C4mim]3[R.HR]3[HgCl2]org and [C4mim]3[R.HR]3[HgClOH]org species was formed.
In the case of salt addition, the best performance was reached (100%) with addition of sodium acetate at the concentration of 0.03 mmol·L−1 in aqueous chloride solution of mercury in the following conditions: initial pH = 5.41, [Hg2+]0 = 10−3 M, [IL]0 = 5 × 10−3 M.
The extracted species in organic phase were HgCl2 and HgClOH, their percentages were respectively 80.66% and 18.29%.
The negative value of Gibbs free energy (∆G) indicates the spontaneous nature of extraction.
5. Acknowledgements
We gratefully acknowledge the ATRST (Agence Thématique de Recherche en Sciences & Technologie-Algérie) (ATRST) (ex ANDRU) for their financial support.
REFERENCES
- OSPAR, “Directive 2008/105/EC of the European Parliament and of the Council of the European Union,” OJEC, Vol. 348, 2008, pp. 84-97.
- J. C. Clifton, “Mercury Exposure and Public Health,” Pediatric Clinics of North America, Vol. 54, No. 2, 2007, pp. 237-269.
- T. W. Clarkson and L. Magos, “The Toxicology of Mercury and Its Chemical Compounds,” Critical Reviews in Toxicology, Vol. 36, No. 8, 2006, pp. 609-662. doi:10.1080/10408440600845619
- L. D. Hylander and M. E. Goodsite, “Environmental Costs of Mercury Pollution,” Science of the Total Environment, Vol. 368, No. 1, 2006, pp. 352-370. doi:10.1016/j.scitotenv.2005.11.029
- A. Oehmen, J. Fradinho, S. Serra, G. Carvalho, J. L. Capelo, S. Velizarov, J. G. Crespo and M. A. M. Reis, “The Effect of Carbon Source on the Biological Reduction of Ionic Mercury,” Journal of Hazardous Materials, Vol. 165, No. 1-3, 2009, pp. 1040-1048. doi:10.1016/j.jhazmat.2008.10.094
- M. M. Matlock, B. S. Howerton and D. A. Atwood, “Irreversible Precipitation of Mercury and Lead,” Journal of Hazardous Materials, Vol. 84, No. 1, 2001, pp. 73-82. doi:10.1016/S0304-3894(01)00190-X
- J. Barron-Zambrano, S. Laborie, P. Viers, M. Rakib and G. Durand, “Mercury Removal and Recovery from Aqueous Solutions by Coupled Complexation-Ultrafiltration and Electrolysis,” Journal of Membrane Science, Vol. 229, No. 1-2, 2004, pp. 179-186. doi:10.1016/j.memsci.2003.10.028
- T. S. Anirudhan, L. Divya and M. Ramachandran, “Mercury(II) Removal from Aqueous Solutions and Wastewaters Using a Novel Cation Exchanger Derived from Coconut Coir Pith and Its Recovery,” Journal of Hazardous Materials, Vol. 157, No. 2-3, 2008, pp. 620-627. doi:10.1016/j.jhazmat.2008.01.030
- Z. Li, Q. Wei, R. Yuan, X. Zhou, H. Liu, H. Shan and Q. Song, “A New Room Temperature Ionic Liquid 1-Butyl- 3-Trimethylsilylimidazolium Hexafluorophosphate as a Solvent for Extraction and Preconcentration of Mercury with Determination by Cold Vapor Atomic Absorption Spectrometry,” Talanta, Vol. 71, No. 1, 2007, pp. 68-72. doi:10.1016/j.talanta.2006.03.023
- Y. Terashima, H. Ozaki and M. Sekine, “Removal of Dissolved Heavy Metals by Chemical Coagulation, Magnetic Seeding and High Gradient Magnetic Filtration,” Water Research, Vol. 20, No. 5, 1986, pp. 537-545. doi:10.1016/0043-1354(86)90017-5
- S. Basha, Z. V. P. Murthy and B. Jha, “Sorption of Hg(II) onto Carica Papaya: Experimental Studies and Design of Batch Sorber,” Chemical Engineering Journal, Vol. 147, No. 2-3, 2009, pp. 226-234. doi:10.1016/j.cej.2008.07.005
- I. B. Rae, S. W. Gibb and S. Lu, “Biosorption of Hg from Aqueous Solutions by Crab Carapace,” Journal of Hazardous Materials, Vol. 164, No. 2-3, 2009, pp. 1601-1604. doi:10.1016/j.jhazmat.2008.09.052
- Ionic Liquids Today, No. 2-11, 2011.
- R. D. Rogers and K. S. Seddon, “Ionic Liquids Industrial Applications for Green Chemistry ACS Symposium Series,” ACS, Washington DC, 2002, p. 818.
- M. J. Earle and K. R. Seddon, “Ionic Liquids, Green Solvents for the Future,” Pure and Applied Chemistry, Vol. 72, No. 7, 2000, pp. 1391-1398. doi:10.1351/pac200072071391
- A. Noda, K. Hayamizu and M. Watanabe, “Pulsed-Gradient Spin-Echo 1H and 19F NMR Ionic Diffusion Coefficient, Viscosity, and Ionic Conductivity of Non-Chloroaluminate Room-Temperature Ionic Liquids,” The Journal of Physical Chemistry B, Vol. 105, No. 20, 2001, pp. 4603- 4610. doi:10.1021/jp004132q
- H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, “Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation,” The Journal of Physical Chemistry B, Vol. 109, No. 13, 2005, pp. 6103- 6110. doi:10.1021/jp044626d
- J. L. Anderson, J. Ding, T. Welton and D. W. Armstrong, “Characterizing Ionic Liquids on the Basis of Multiple Solvation Interactions,” Journal of the American Chemical Society, Vol. 124, No. 47, 2002, pp. 14247-14254. doi:10.1021/ja028156h
- L. Duchet, J. C. Legeay, D. Carrié, L. Paquin, J. J. Vanden Eynde and J. P. Bazureau, “Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles Using Ionic Liquid-Phase Organic Synthesis (IoLiPOS) Methodology,” Tetrahedron, Vol. 66, No. 4, 2010, pp. 986-994. doi:10.1016/j.tet.2009.11.079
- M. V. B. Zanoni, E. I. Rogers, C. Hardacre and R. G. Compton, “The Electrochemical Reduction of the Purines Guanine and Adenine at Platinum Electrodes in Several Room Temperature Ionic Liquids,” Analytica Chimica Acta, Vol. 659, No. 1-2, 2010, pp. 115-121. doi:10.1016/j.aca.2009.11.026
- N. Fontanals, S. Ronka, F. Borrull, A. W. Trochimczuk and R. M. Marcé, “Supported Imidazolium Ionic Liquid Phases: A New Material for Solid-Phase Extraction,” Talanta, Vol. 80, No. 1, 2009, pp. 250-256. doi:10.1016/j.talanta.2009.06.068
- K. R. Seddon, “Ionic Liquids for Clean Technology,” Journal of Chemical Technology & Biotechnology, Vol. 68, 1997, pp. 351-356. doi:10.1002/(SICI)1097-4660(199704)68:4<351::AID-JCTB613>3.0.CO;2-4
- P. Kubisa, “Ionic Liquids as Solvents for Polymerization Processes—Progress and Challenges,” Progress in Polymer Science, Vol. 34, No. 12, 2009, pp. 1333-1347. doi:10.1016/j.progpolymsci.2009.09.001
- M. Regel-Rosocka, “Extractive Removal of Zinc(II) from Chloride Liquors with Phosphonium Ionic Liquids/Toluene Mixtures as Novel Extractants,” Separation and Purification Technology, Vol. 66, No. 1, 2009, pp. 19-24. doi:10.1016/j.seppur.2008.12.002
- “Program for Calculating Chemical Equilibria in Aquatic Systems,” RIVM, Bilthoven, 2004. http://home.tiscali.nl/cheaqs
- N. Miralles, A. M. Sastre, M. Aguilar and M. Cox, “Solvent Extraction of Zinc(II) by Organophosphorus Acids Compounds from Perchlorate Solutions,” Solvent Extraction and Ion Exchange, Vol. 10, No. 1, 1992, pp. 51-68. doi:10.1080/07366299208918092
- I. Puigdomenech, “HYDRA (Hydrochemical EquilibriumConstant Database) and MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) Programs,” Royal Institute of Technology, Sweden. http://www.kemi.kth.se/medusa
- A. Mellah and D. Benachour, “The Solvent Extraction of Zinc and Cadmium from Phosphoric Acid Solution by Di-2-Ethyl Hexyl Phosphoric Acid in Kerosene Diluents,” Journal of Chemical Engineering & Process, Vol. 45, No. 8, 2006, pp. 684-690. doi:10.1016/j.cep.2006.02.004
- E. K. Alamdari, D. Moradkhani, D. Darvish, M. Askari and D. Behnian, “Synergistic Effect of MEHPA on CoExtraction of Zinc and Cadmium with DEHPA,” Minerals Engineering, Vol. 17, No. 1, 2004, pp. 89-92. doi:10.1016/j.mineng.2003.10.003
- T. Welton, “Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis,” Chemical Reviews, Vol. 99, No. 8, 1999, pp. 2071-2083. doi:10.1021/cr980032t
- A. Kadous, M. A. Didi and D. Villemin, “A New Sorbent for Uranium Extraction: Ethylenediamino Tris(methylenephosphonic) Acid Grafted on Polystyrene Resin,” Journal of Radioanalytical and Nuclear Chemistry, Vol. 284, No. 2, 2010, pp. 431-438. doi:10.1007/s10967-010-0495-7
NOTES
*Corresponding author.

