International Journal of Clinical Medicine
Vol.2 No.4(2011), Article ID:7546,5 pages DOI:10.4236/ijcm.2011.24066
Clinical Efficacy of Allergen Specific Immunotherapy (ASIT) in Allergic Rhinitis
![]()
Department of Pulmonary Medicine, Yenepoya Medical College, Yenepoya University. P.O. Nithyananda Nagar, Mangalore, Karnataka, India.
Email: sukheshrao@sify.com
Received March 14th, 2011; revised April 28th, 2011; accepted July 15th, 2011.
Keywords: Allergen Specific Immunotherapy, Allergic Rhinitis
ABSTRACT
Though efficacy of Allergen Specific Immunotherapy (ASIT) has been proved in many studies, reports about success in clinical practice and under field conditions in alleviating the suffering or decreasing the morbidity in patients of Allergic Rhinitis are few. 260 patients of Allergic Rhinitis without coexisting diseases were included. Skin prick test was done on all patients. ASIT was initiated with common inhalant indoor allergens as per standard protocol and patients were assessed at the start and at 2 m, 6 m and 18 months of ASIT. ASIT was able to significantly reduce the symptom score in all the three groups namely sneezing, rhinorrhoea and nasal itching (p < 0.001). Concurrently it was also able to produce a significant reduction in the usage of concomitant drug intake (p < 0.001) thereby implying a decrease in morbidity. When assessed regarding clinical efficacy, ASIT was found to be satisfactory or highly effective in more than 75% patients. ASIT has got a role in clinical practice in polysensitized patients in field conditions. This is based on the evidence that besides decrease in hypersensitivity/symptoms, it also has an effect on minimizing the necessity of taking drugs to relieve the symptoms, which has strong implications of economics and toxicity, while treating patients.
1. Introduction
Since its introduction by Noon & Freeman [1] almost a century ago, Allergen Specific Immunotherapy (ASIT) remains one of the most important tools in the management of allergy diseases. Over time, knowledge about ASIT has increased in terms of allergens against which it is effective [2], route of administration [3], method of administration [3]. Review of literature revealed many studies on these aspects [4]. Unfortunately majority of them are lacking in one significant aspect and that is effect of Immunotherapy (IT) in clinical practice. This is significant, as a large number of studies are on effect of IT against a single allergen or a group of similar allergens [5,6]. The situation is not always the same in clinical practice. Moreover the analysis of data in these studies present statistical figures which may not be of much use in day to day practice. Hence, this study was undertaken to study the efficacy of ASIT in Allergic Rhinitis in alleviating the sufferings of the patients in clinical practice.
2. Methods
2.1. Patients
260 patients of Perennial Allergic Rhinitis formed the subject group. All of them were thoroughly investigated to rule out coexistent morbidity like diseases of nose & paranasal sinuses, bronchial asthma, diabetes, etc. Skin testing by prick method was performed in all the subjects. Only those patients who showed positivity to indoor inhalant allergens like house dust, paper dust, cotton dust, dust mites, cockroach, housefly, mosquito were included. These allergens were selected because of anecdotal evidence and prior reports [7] about the causal relationship between them and development of rhinitis and asthma. Also the fact that these are the most common amongst indoor allergens and are compatible when mixed together, but not with other allergens [8], made it prudent to use them in clinical practice.
2.2. Allergen Specific Immunotherapy (ASIT)
After the testing, ASIT was initiated as per the standard protocol, starting with 0.1 ml of 1:5000 dilution subcutaneously, thrice a week, gradually increasing at fixed intervals. Generally the maintenance dose of 0.7 cc to 0.9 cc of 1:50 dilution was administered once every 4 weeks. The total duration was a minimum period of 18 months years, extended as required depending on the response. For the purpose of this study, the assessment was stopped at 18 months years.
2.3. Assessment and Grading of ASIT
The patients were evaluated based on a symptom & medications score. The symptoms assessed were sneezing, rhinorrhoea and nasal itching and were graded on a scale of 0 - 3 with 0 = absent, 1 = mild, 2 = moderate, 3 = severe [9]. Similarly, patients were allowed to use medications as and when required and a score was maintained as follows [9] 1 = one dose of oral or nasal antihistamines. 2 = one dose of nasal corticosteroids. 3 = one dose of oral corticosteroids. These scores were analyzed at the beginning of the study and at 2 m, 6 m and 18 months years. Depending on the reduction in symptom/ medication score the efficacy of IT was categorized as follows: 1) Not effective (reduction in score by less than 30%). 2) Mildly effective (reduction in score between 31% - 50%). 3) Satisfactory (reduction in score between 51% - 70%). 4) Highly effective (reduction in score by more than 71%).
2.4. Statistical Analysis
Data was analyzed using the software statistical package for Social Sciences (SPSS) version 17. Descriptive statistics, Median and Inter Quartile Range (IQR) was calculated for all the scores. Wilcoxan sign rank test was used to analyze the scores at various months. p < 0.05 was considered to be statistically significant. As this was a clinical study, IRB review and consent was not required.
3. Results
A total of 260 patients suffering from Allergic Rhinitis formed the subject group. Age ranged from 16 years to 45 years with mean age being 30.6 + 9.5 years. 180 were males and 80 females. The results are depicted in Tables 1-7. Table 1 shows the effect of ASIT on the symptom score. The symptom score for sneezing reduced from 3(1) at the start of ASIT to 1(2) at 2 m, 0(1) at 6 m and 0(1) at 18 m, the scores were 2(1), 1(1), 0(1) & 0(0) for rhinorrhoea and 2(1), 1(0), 0.5(1) and 0(1) for nasal itching at the same intervals. The values are represented as Median and Inter Quartile Range (IQR). The numbers indicates the symptoms score in Median and the numbers in bracket indicate IQR. The reduction in all the symptoms was significant at all points of observation (p < 0.001). Effect of ASIT on symptom of nasal itching is shown in Table 2. It is clear that 170 or 65.4% patients had complete relief from itching at 18 m. Moreover none of the patients had moderate or severe itching at 18 m of ASIT. Only 34.6% patients had residual mild itching at end of 18 months which was clinically insignificant. Table 3 shows the effect of ASIT on sneezing as a symptom. The effect on this symptom is not as good as on nasal itching, because though at the end of 18 m, 61.5% reported complete relief from sneezing but equally important is the observation that 23% patients reported presence of moderate amount of sneezing at the end of 18 m of ASIT. Table 4 shows the effect of ASIT on rhinorrhoea. Here the results are for better because 80% of patients reported complete relief from rhinorrhoea and only 7.7% patients had moderate and 11.5% had mild rhinorrhoea at 18 months. Effect of ASIT on drug intake score is shown in Table 5. ASIT was able to significantly reduce the concomitant drug intake by patients of Allergic Rhinitis with the scores being 8.5(3), 4(4), 2(2) and 1(1) at the start of ASIT, at 2 m, 6 m and 18 m respectively. The values are represented as Median and (IQR). The difference was found to be statistically significant at all stages of observation (p < 0.001). Table 6 shows the perception about clinical efficacy of ASIT in reducing the symptoms and morbidity. It is clear that a significant number i.e. 85% patients found it to be either satisfactory or highly effective in reducing their symptoms, whereas a miniscule number (3.8%) found it to completely ineffective. Table 7 shows the efficacy of ASIT as far as drug intake is concerned. Here the results are more encouraging because all patients found ASIT to be satisfactory or highly effective in helping them to reduce their drug intake.
4. Discussion
Though efficacy of ASIT has been established beyond doubt in various trials across the globe [4,11], these are sometimes not of much help to clinicians in treating patients in clinical practice. The reasons are manifold. Many studies have used the reduction of symptoms and/or drug intake as a criteria but this may have no value if it not accompanied by decrease in morbidity [12]. Also majority of studies are in controlled situations or on a single allergen or a group of similar allergens [5,6]. Again, this is not the case in clinical situations where polysensitization is the rule rather than exception. Also the number and type of allergens vary and hence it is difficult to study the efficacy of ASIT in clinical practice [8]. Moreover the results vary because of excessive dilution (because more allergens have been included) or incompatibility (due to injudicious mixture) [8]. Moreover data on statistical significance are generally provided, but p-value per se do not help the clinician to decide about applicability of the treatment. e.g. –25% reduction in symptom

Table 1. Table showing the effect of ASIT on symptom score.
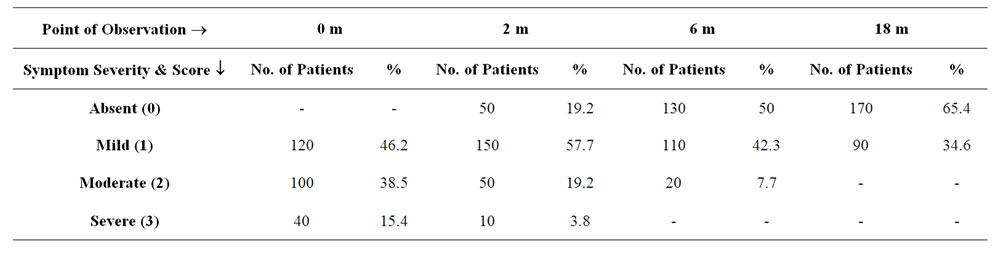
Table 2. Table showing the effect of ASIT on Nasal Itching in terms of severity.
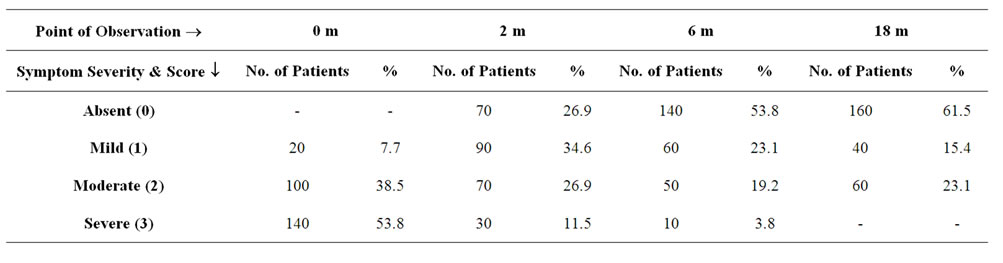
Table 3. Table showing the effect of ASIT on Sneezing in terms of severity.
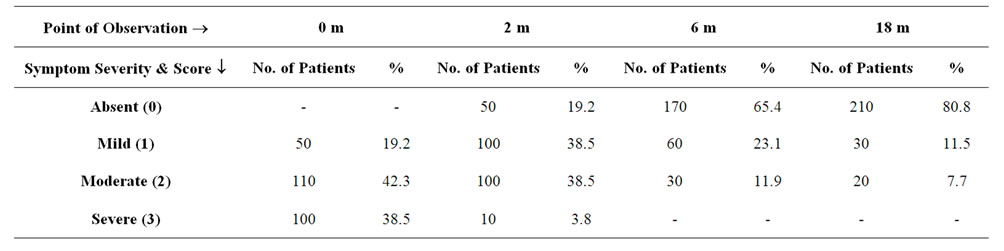
Table 4. Table showing the effect of ASIT on rhinorrhoea in terms of severity.

Table 5. Table showing the effect of ASIT on drug intake score.
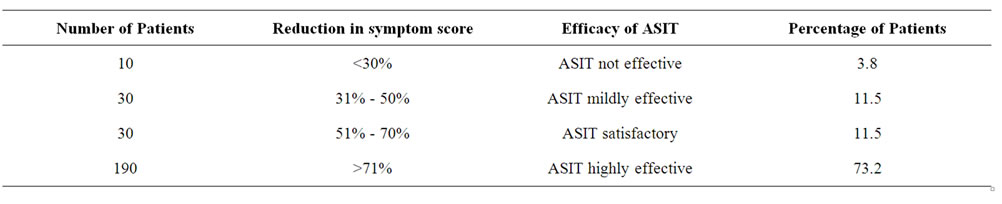
Table 6. Table showing the clinical efficacy of ASIT when symptom score reduction was considered.
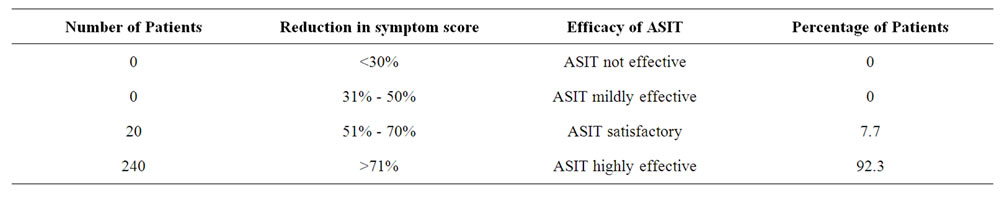
Table 7. Table showing the clinical efficacy of ASIT in relation to concomitant drug intake.
score/drug intake may be statistically significant but not relevant to the patient [13]. With this in mind the design of the study was scheduled by taking these factors into consideration 1) Group of polysensitized patients. 2) Mixture of compatible allergens. 3) Group of common inhalant allergens. 4) Selecting the symptom/medication score as an outcome measure and extra polating it to determine whether ASIT is clinically effective. As it is clear (Table 1) that ASIT has been able to significantly reduce the symptom score as far as all the three symptoms go, the values being for sneezing from 3(1) to 0(1), Rhinorrhoea from 2(1) to 0(1) and nasal itching from 2(1) to 0(1), (p < 0.001). As already discussed these figures do not matter much to the patient or the clinician in treating allergy patients. Hence, a detailed analysis of these symptoms was done which showed up interesting and encouraging results (Tables 2-5). Table 2 shows the effect of ASIT on symptoms of itching. It is clear that almost 65% patients experienced complete relief from Nasal Itching. To put it in another way almost 55% patients who had moderate to severe itching felt that their symptoms were totally relieved. The results were similar though not as satisfactory when sneezing as a symptom was evaluated, with about 62% had complete relief from it but a good number (23%) had experienced moderate amount of sneezing at the end of 18 m (Table 2). With rhinorrhoea the results are more encouraging with almost 80% persons reporting complete relief (Table 4). These data clearly justify the rationale and objective of this study. Because as it is evident, when we go by the symptom score we can safely say that IT reduces the score and the reduction is statistically significant both in terms of number of patients and also P value. But subsequent analysis reveals that ASIT is more effective in relieving rhinorrhoea as compared to sneezing and nasal itching. Whether this was because of patient selection or the effect of ASIT per se, was beyond the domain of this study and hence not evaluated. But still this observation may have strong implication in clinical practice e.g. if a patient is having rhinorrhoea he may be more satisfied and hence more compliant with ASIT as compared to the patient who is having sneezing or nasal itching though this implication/observation is open to discussion. Now, coming to the medication score, ASIT was able to achieve a statistically significant (p < 0.001) reduction in drug score at all points of study. i.e. at 2 m, 6 m, 18 m (Table 5). It would have been better if this would have been enlarged to include reduction in usage of individual group of drugs namely antihistamines/steroids/topical drugs. But logistical and practical difficulties prevented us from doing it. Nevertheless the significant reduction in drug intake is itself a clinically relevant observation, as detailed below. Next we studied the efficacy of ASIT in terms of patients perception and statistical data analyzed together. We found that ASIT was found/perceived to be highly effective in 73.2% cases when reduction in symptom score was taken into consideration (Table 6) but 92.3% patients found it to be highly effective when reduction in drug intake was studied (Table 7). Similarly ASIT was perceived to be not effective or mildly effective in 3.8% and 11.5% when symptom score was tabulated (Table 6) but in none of the patients when drug score was studied (Table 7). This was an interesting observation and can have strong relevance in the sense that even if ASIT may not be able to achieve symptom free status in all but it can still definitely help reach a situation where symptoms are not severe enough to warrant drug intake. Benefits of this effect can be profound in terms of economic burden/side effect/psycho somatic effects of drug intake. To summarize ASIT is equally effective in clinical practice in treating allergy patients. More so when we just don’t look into reduction in hypersensitivity/symptoms alone but also concomitant use of drugs for relief of symptoms.
5. Conclusions
ASIT has got a significant role in alleviating the suffering of patients of Allergic Rhinitis, because sufferings include both from clinical symptoms and from psychosomatic factors associated with drug intake. Our experience clearly shows that ASIT has been successful in achieving a significant reduction in concomitant drug intake along with decrease in symptoms. This has a strong clinical relevance in the treatment of Allergic Rhinitis, because though the patient may not become symptom free after ASIT, but he may be able to achieve a significant reduction in drug intake which has a huge advantage in terms of economics and lesser side effects. Thus we feel that ASIT should be initiated early in all patients of Allergic Rhinitis to achieve a healthier, drug free clinical status.
6. Acknowledgements
The assistance of Miss. Neevan D’ Souza, Asst. Professor cum Statistician in analyzing the data and Miss. Shiny Jackline in typing the Manuscript is acknowledged and appreciated.
REFERENCES
- J. Freeman and I. Noon, “Further Observation on the Treatment of Hay Fever by Hypodermic Inoculation of Pollen Vaccine,” Lancet, Vol. 2, 1911, pp. 814-816.
- L. Jacobson and E. Valovivta, “How Strong Is the Evidence That Immunotherapy in Children Prevents the Progression of Allergy and Asthma,” Current Opinion in Allergy and Clinical Immunology, Vol. 7, No. 6, 2007, pp. 556-561. doi:10.1097/ACI.0b013e3282f1d67e
- I. Finegold, “Is Immunotherapy Effective in Allergic Diseases?” Current Opinion in Allergy and Clinical Immunology, Vol. 2, No. 6, 2002, pp. 537-540. doi:10.1097/00130832-200212000-00010
- M. Calderon, B. Alves, M. Jacobson, et al., “Allergen Injection Immunotherapy for Seasonal Allergic Rhinitis,” Cochrane Database of Systematic Reviews, Vol. 1, 2007, p. CD001936.
- H.S. Nelson, J. Lahr, R. Rule, A. Bock and D. Leung, “Treatment of Anaphylactic Sensitivity to Peanuts by Immunotherapy with Injections of Aqueous Peanut Extract,” The Journal of Allergy and Clinical Immunology, Vol. 99, No. 6, 1997, pp. 744-751. doi:10.1016/S0091-6749(97)80006-1
- A. Didier, M. Melac, A. Mantagut, M. Lheretier-Barrand, A. Tabor and M. Worn, “Agreement of Efficacy Assessment for Five Grass—Pollen Sublingual Tablet in Immunotherapy,” Allergy, Vol. 64, No. 1, 2008, pp. 166- 171.
- J. M. Portnoy, “Immunotherapy for Inhalant Allergies: Guidelines for Why, When & How to Use This Treatment,” Postgraduate Medical Journal, Vol. 100, 2001, pp. 89-106.
- J. Susmitha, V. Vijayalaxmi, G. Sumanlatha and K. J. R. Murthy, “Combination of Allergens in Specific Immunotherapy for IgE Mediated Allergies,” Lung India, Vol. 24, No. 1, 2007, pp. 3-5.
- G. W. Canonica, C. E Baena-Cagnani., J. Bousquet, P. J. Bousquet, R. F. Lockey, H. T. Mailing, et al., “Recommendations for Standardizations for Clinical Trials with Allergen Specific Immunotherapy for Respiratory Allergy. A Statement of WHO Task Force,” Allergy, Vol. 62, No. 3, 2007, pp. 317-324.
- J. Clark and R. Schall, “Assessment of Combined Symptom & Medication Scores for Rhino Conjunctivitis Immunotherapy Clinical Trials,” Allergy, Vol. 62, No. 9, 2007, pp. 1023-1028.
- G. Passalacqua and S. R. Durham, “Allergic Rhinitis and Its Impact on Asthma Update & Allergen Immunotherapy,” The Journal of Allergy and Clinical Immunology, Vol. 119, No. 4, 2007, pp. 881-891. doi:10.1016/j.jaci.2007.01.045
- H. J. Malling, “Immunotherapy as an Effective Tool in Allergy Treatment,” Allergy, Vol. 53, No. 5, 1998, pp. 461-472.
- H. J. Malling, “New ideas in Allergen Specific Immunotherapy,” American Journal of Rhinology, Vol. 4, No. 4, 1990, pp. 155-158. doi:10.2500/105065890782018136

