Advances in Sexual Medicine
Vol.3 No.1(2013), Article ID:27144,7 pages DOI:10.4236/asm.2013.31006
Aphrodisiac Activity of Aqueous Extract of Phoenix dactylifera Pollen in Male Rats
Physiology Department, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Email: *Sadeghipour@sina.tums.ac.ir
Received June 13, 2012; revised November 15, 2012; accepted December 11, 2012
Keywords: Aphrodisiac Activity; Microdialysis; Dopamine; Testostrone; Estradiol; Male Rat; Phoenix dactylifera Pollen Grain; Pre-Ejaculation; Sexual Behavior
ABSTRACT
Aim of study: Ancient literature alluded to the use of a number of plants/preparations as sex enhancer. One of such botanicals is Phoenix dactylifera in which the pollen grain has been acclaimed to be used as an aphrodisiac. However, the validity has not been scientifically tested. Dopamine is known to facilitate male sexual function. Therefore, the present study was undertaken to evaluate the effect of aqueous extract of Phoenix dactylifera pollen on the sexual behavior of male rats and to measure of serum Estradiol and Testostrone. Also, dopamine transmission in the nucleus accumbence (NAc) was studied in male rats using in vivo microdialysis. Methods and Materials: sixty male rats were randomized into 6 groups (A-F). Group A received 0.2 ml of Normal Saline mixed with Dimethyl Sulphate (DMSO), while groups B-F were injected same volume containing 35 mg/kg, 70 mg/kg, 105 mg/kg, 140 mg/kg and 350 mg/kg of DPP extract, respectively. Sexual behavioral parameters including mounting, intromission and ejaculation frequencies and latencies were recorded in male rats one hour after injection of extract by mating with a receptive female (1:1). The male serum testosterone and estradiol concentrations were also determined. Results: All doses stimulated male sexual behavior. Extract significantly increased mount, ejaculation, intromission frequencies and ejaculation latency in comparison to controlled ones (p < 0.001). Mount and intromission latencies significantly reduced (p < 0.001). Maximum effect was observed in dose 140 mg/kg. This extract was found to enhance Testestrone, Estradiol and the orientation of males toward female ones by increasing mounting and ano-genital investigatory behavior. Conclusions: Data from this study identified that the aqueous extract of Phoenix dactylifera pollen grain enhanced sexual behaviour in male rats. The improved sexual appetitive behaviour in male rats may be attributed, to the alkaloids, saponins, and or flavonoids since these phytochemicals has engorgement, androgen enhancing. Also, our findings support the traditional use of this plant as acclaimed aphrodisiac and for the treatment of pre-ejaculation and impotency.
1. Introduction
Male sexual behavior comprises a complex pattern of genital and somatomotor responses, elicited, directed, and maintained by external and internal signals. It includes copulation as well as precopulatory behaviors that allow the male to detect and locate a mate, assess her potential mating appropriateness, and stimulate a receptive response [1,2]. For a normal sexual intercourse in males, the sexual organs and factors relating to erection of the copulatory organ must function as normally [3]. Dopamine (DA), releases in several major integrative areas before and/or during copulation, facilitates sexual motivation, motor performance, and genital reflexes. Dopamine has long been known to facilitate male sexual function. L-Dopa (the precursor of DA) administered to parkinsonian patients increases libido and sexual potency, and the nonspecific DA agonist apomorphine has been used to treat sexual dysfunction.
Male reproductive capacity was found to be deficient in no less than 50% of infertile couples [4]. The incidence of sexual inadequacy in human males has led to the development of a number of available treatment options. These options are expensive with serious side effects. This problem, has necessitated the need for less side effect drugs [5,6]. Medicinal plants continue to provide valuable therapeutic agents, both in modern and in traditional medicine [7].
Traditional medicines are gaining importance and nowadays are being studied to find the scientific basis of their therapeutic actions [8,9]. Plant-derived chemicals are used to relieve sexual dysfunction and they have sex enhancing potentials. These phytochemicals increase libido, sexual potency and sexual pleasure [5,6]. The use of herbal medicine has become increasingly popular worldwide especially in the Asian and African countries [10].
The various parts of Phoenix dactylifera widely are used in traditional medicine for the treatment of various disorders which include memory disturbances, fever, inflammation, paralysis, loss of consciousness and nervous disorders [11,12]. Suspension of Phoenix dactylifera date palm pollen (DPP) is an herbal mixture that is widely used as a folk remedy for curing male infertility in traditional medicine. Date palm fruit suspensions improve the sperm count, motility, morphology, and DNA quality with a concomitant increase in the weights of testis and epididymis [10]. However, there is no scientific research about the effect of DPP on sexual behavior in literature. Therefore, the present study was undertaken to determine the aphrodisiac activity of aqueous extract of Phoenix dactylifera pollen grain on the sexual behavior in male rats.
2. Materials and Methods
2.1. Plant Material
Samples of the plant were collected from botanical garden at Bushehr city (South of Iran), Iran.
2.2. Preparation of Aqueous Extract of Phoenix dactylifera Pollen Grain
Phoenix dactylifera pollen grain is a small and oval shape gametocyte with a fine bark. After removing the bark, pollen grain washed with distilled water and then dried. Dried pollen grain was pulverized with a small electric blender (blender model/Pars khazar, Iran). 100 gram of powder was extracted in 0.5 liter of warm distilled water (30˚C) with constant shaking (magnetic shaker model). The solution was passed through a filter paper and lyophilized. After vaporizing of water, the resultant yield was reconstituted in normal saline to give the required doses of 35, 70, 105, 144 and 350 mg/kg used in our study. Solutions were stored in refrigerator (2˚C - 8˚C). The doses were mixed with Dimethyl Sulphate (DMSO) and IP injection was done 1 hour before experimentations with volume of 0.2 ml/rat.
2.3. Animals
The study adheres to the principles of laboratory care established by Ethic Committee of Tehran University of Medical Sciences. A total of 120 animals made up of equal number of male and female rats were used for this study in a complete randomized design. Healthy sexually experienced male rats after sex screening test (wistar albino), 4 months old, weighting between 280 - 320 grams and females, 3 months old weighting between 220 - 250 grams were selected from our department (physiology department of Tehran university of medial sciences). Male and female rats were housed separately 4 to a glass cage placed in a well-ventilated animal room. Room temperature was 22˚C ± 2˚C with humidity of 60%. Animals were housed in a reversed light-dark cycle (light on: 7 p.m to 7 a.m). Animals were acclimated for about 4 weeks before the experimentation. They were allowed free access to food pellets and water ad libitum. Before experiments, sex screening test was done. In this test, male rats must exhibit consistent parameters of sexual behavior, including intromission within 30 sec of the presentation of female, ejaculation within 15 min of the first intromission. Experiments were done in dark period in a dim red florescent lamp.
2.4. Estrous Females
Sixty female rats were used as mating stimulus. The females were bilaterally ovariectomized via lumbar incisions under ketamine (100 µl/0.1 kg of rat) and xylazin (10 µl/0.1 kg of rat) anesthesia at least two weeks before the experiments began. The females were rendered sexually receptive by single subcutaneous injections of estradiol benzoate (10 µg) 52 h and progesterone (1 mg) 4 h, prior to pairing. Estrous females displayed a high degree of lordosis responding and proceptivity.
2.5. Animal Grouping and Extract Administration
Sixty male rats were randomly grouped into 6 (A-F) consisting of 10 animals each. Animals in group A, which served as the control, received 0.2 ml of saline mixed with DMSO (IP injection). Animals in groups B, C, D, E and F were treated with doses of 35, 70, 105, 140 and 350 mg/kg of the extract, respectively, with volume of 0.2 ml/rat. All administrations were done daily at the time of during 8:00 a.m and 9:00 a.m. The experimental rats allowed free access to rat pellets and water.
2.6. Male Rat Sexual Behaviors
A Plexiglas observation cage (0.3 m × 0.5 m × 0.3 m) with a mesh plate in the middle was put on a metal box that has an oblique mirror within. One hour after the treatment, the male rat was placed in this cage. After 10 minutes, a sexually receptive female rat was introduced on the other side of the cage (Anticipatory phase) and after 10 minutes, the mesh plate was removed for 30 minutes (Consummatory phase). Sexual behaviors of a male with female were observed from the cage side for proceptive and precopulatory behaviors. Sexual behavior parameters were monitored for 30 min observatory period by camera (Panasonic model with O-Lux) and direct observations.
If male rat couldn’t have an intromission within the first 15 minutes, that rat was removed and replaced with a new one According to the standard and basic procedures, the following male sexual parameters were recorded or calculated for the observatory period. 1—Mount Latency (ML), time from introduction of the female until the first mount. 2—Intromission Latency (IL), time from introduction of the female until the first intromission. 3—Ejaculation Latency (EL), time from the first intromission until ejaculation. 4—Mount Frequency (MF), the number of mounts in a series. 5—Intromission Frequency (IF), the number of intromissions in a series. 6—Ejaculation frequency (EF), the number of times there was expulsion of semen by males after vaginal penetration-characterized by rhythmic contraction of the posterior abdomen. 7— Post-Ejaculatory Interval (PEI), the time from the occurrence of ejaculation until the initiation of a new series, as indicated by the next intromission. Other computed male sexual behavioural parameters include: % index of libido = (number mated/number paired) × 100; % mounted = (number mounted/number paired) × 100; % intromitted = (number of rats that intromitted/number paired) × 100; % ejaculated = (number of rats that ejaculated/number paired) × 100; copulatory efficiency = (number of intromissions/ number of mounts) × 100; intercopulatory efficiency = average time between intromissions [13-16].
2.7. Assay Kits
The testosterone. Estradiol and Dopamine enzyme immunoassay test kits were used according to their manufacture’s instruction (ABO Switzerland Co.), while estradiol benzoate and progesterone were products of Sigma Chemical (Germany).
After finding the effective dose (140 mg/kg), we measured the Dopamine concentration of nucleus accumbence in male rats using in vivo microdialysis in aperiod of 70 minutes.
2.8. Determination of Serum Testestrone and Estradiol Concentration.
After recording of the sexual behavior parameters and at the end of experiments, all male rats were deeply anaesthetized With Ketamine and Xylazine. Blood samples were collected from the aorta, centrifuged and the serum separated, Samples stored at −20˚C for the measurement of testosterone and estradiol.
2.9 Adverse Effects
All treated rats were observed any signs of toxicity (Salivation, lachrymation, ptosis, squinted eyes, writhing, convulsions, tremors) stress (erection of fur), diarrhea and changes in behaviour (such as spontaneous movements in the cage, climbing, cleaning of face, nongenital self grooming). In addition, food and water intake were noted.
2.10. Statistical Analysis
After recording the sexual behavioral parameters, Data was expressed as mean ±SD and analyzed. Statistical analysis was done by Kruskal Wallis test (ANOVA). The comparison of means between control and each experimental group was done by the Wilcoxon-test. P < 0.05 was regarded as significant. SPSS software version 11 and Excel 2007 were used for analyzing.
3. Results
The effects of various doses of DPP on sexual behavior are summarized in Tables 1 and 2. Intrapritonaly (IP) injection of various concentration of DPP modified all parameters. Maximum effect was observed in rats treated with dose 140 mg/kg of DDP. The aqueous extract of DPP produced a significant increase in MF, IF, EJF, EJL, PEI and decrease in ML, IL (p < 0.05). Treated male rats displayed vigorous ano-genital sniffing and mounting on females and restricted them to one side of cage (Table 1). Computed index in treated rats showed that all index increased especially in doses 105 mg/kg, 140 mg/kg and 350 mg/kg (Table 2).
The extract produced a significant increase in the penile erection index as compared to control value. There were homosexual mountings in the treated animals after experimentations. Our studies on the orientation of male towards the female rats showed that males treated with the extract displayed more frequent and vigorous anogenital sniffing and mounting on females, as compared to controlled cases. The orientation towards environment (climbing on cage wall, raring and exploration) was found to be inconsistently reduced whereas towards self (genital grooming) was increased. The rats treated with the extract tended to show confinement to a particular area of the cage (around female) showing restriction in movement.
There was an increase in the blood level of Estradiol and Testosterone (Figures 1 and 2). As shown in Figures, the effective dose for estradiol and testosterone was 140 mg/kg.
Releasing of Dopamine increased significantly in the nucleus accumbence when a receptive female was introduced behind a screen (P < 0.001). During copulation, Dopamine increased markedly in control and extracted rats. Improving of sexual behavior and Dopamine release was higher in treated rats with extract in comparison with control (P < 0.001).
There were no defects and clinical signs of toxicity, stress or changes in behaviour and appearance evident.
Table 1. Effect of various doses of aqueous extract of DPP on sexual behavior in male rats.
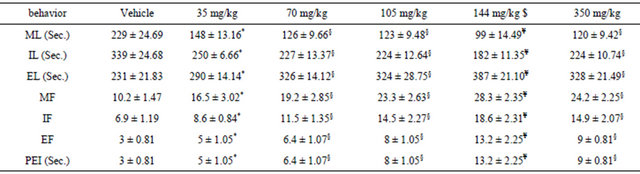
Values are presented as mean ± SD. All latencies and interval are expressed in seconds. Significant from control: *p < 0.05, §p < 0.01, ¥p < 0.001; N/group = 10 rats; $= Max. Effect.
Table 2. Effect of various doses of aqueous extract of DPP on computed male rat sexual behavior parameters.
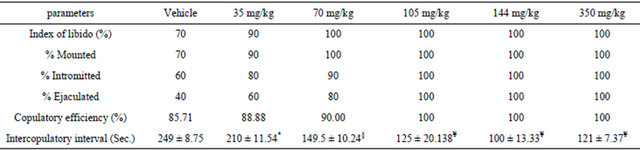
Values are presented as mean ± SD. Significant from control. *p < 0.05, §p < 0.01, ¥p < 0.001; N/group = 10.
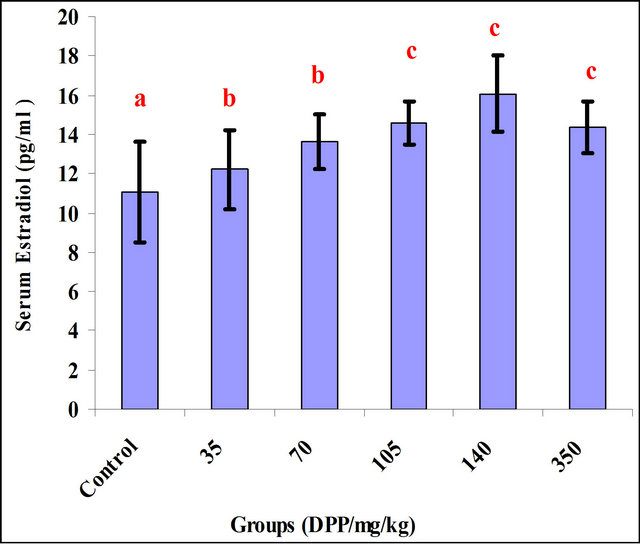
Figure 1. Effect of DPP extract on the srum Estradiol. Values are Means ± SD. N = 10 for each group. Different from control. a: non-significant; b: significant. P < 0.01.
Diarrhea was observed in animal treated with 350 mg/kg. The food and water intake of all treated rats was similar to those of controls.
4. Discussion
Many plants with medicinal properties are effective as aphrodisiac through mechanisms such as vasodilation, generation of nitric oxide, elevation of androgens and gonadotropins [17]. Treatments that alter the concentration of circulating sex hormones may also modify sexual behaviour [18].
Administration of the Aqueous extract at all dose levels modified rat copulatory behavior as well as orientation activities, the main determinant for measuring male sexual behavior [19]. The results of the present study revealed a significant increase in penile erection and genital grooming. The increase of penile erection induced by Pheonix dactylifera could be due to their androgenic effects [10]. Also, our study showed that DPP can increase plasma level of testosterone and estradiol, It has been documented that sexual behavior and erection are dependent on androgen which may act through central and peripheral mechanisms. Androgens regulate the magnitude of penile erectile response, partly by regulating the venous outflow from cavernous spaces [20,21]. Therefore, the increase in serum testosterone concentration by extract might be responsible for improved sexual behavior in males and are essential for libido [10]. In fact, it has been shown that copulatory behaviour is maintained in castrated rats by treatment with testosterone [3,21]. Clinical data on testosterone also suggest that a slight increase in the levels of the hormone in adult males results in a moderate but significant increase in sexual desire and libido [22]. Increasing of testosterone of serum

Figure 2. Effect of DPP extract on the serum Testestrone. Values are Means ± SD. Different from control. All doses (b) are significant. P < 0.005.

Figure 3. Changes in dialysis concentration of dopamine (pg/microliter) taken from nucleus accumbence of male rats in 3 groups in a period of 70 min. Values are means ± SD; bars carrying letters, b and c, different from their controls, a, significantly different at P < 0.5.
by the aqueous extract might be responsible for the enhanced sexual behaviour in the animals [23].
Extract prolonged the ejaculatory latency in male rats, indicating an increase of ejaculatory threshold [24]. These findings support the use of these medicinal plant for the treatment of premature ejaculation in traditional medicine.
Increased MF and IF in treated rats, indicating the sexual motivation and efficiency of erection and penile orientation. Increasing of the libido might be the result of increasing several hormones that are secreted from pituitary. The main components of DPP are stroids, flavonoids, saponins, and lipid. These agents can increase sexual behavior. They stimulate endogenous testosterone levels probably by raising the level of luteinizing hormones (LH) [24-26]. Following adequate erection and activity of muscles, intromission is possible and increasing of IF by the extract show the activation of penile erection mechanism. Alkaloids of DPP have estrogenic properties [27,28] that induce vasodilation of blood vessels of penis which results in erection [29]. Saponin of PDD acts as nitric oxid and cuases smoth muscle relaxation of corpus cavernosum. DPP has gonadotrphin effects [6]. Steroidal saponins increase LH and FSH levels that in turn increase testosterone. Our data showed that using DPP extract increases the plasma levels of estradiol and testosterone. These hormones are found at high concentrations in rat testis and seminal fluids. Estrogen is synthesized in male reproductive system by at least three different cell types, Sertoli, Leydig and germ cells. Estrogen regulates the reabsorption of luminal fluid in the head of the epididymis [6]. Testosterone enhances sexual desire, index of libido, motivation and sexual performance. Another component of DPP is alkaloid that elevates testicular cholesterol in male testis [30,31].
Steroidogenesis produces steroid hormones that they increase dehydroepiandrosterone. The last agent enhances sexual behavior and prolongs EF and reduces EL, PEI and intercopulatory intervals. All of these mechanisms are central. Peripheral acting of alkaloids can relax smooth muscles of corpus [32]. Our study showed that extract increases dopamine from accumbance nucleus (Figure 3). Enhanced dopamine efflux causes facilitation of sexual behavior and has effects on sexual motivation, copulatory proficiency and genital reflexes. Dopamine influences motor activity in mesolimbic tract and activates numerous behavior and genital reflexes [18,32]. Also, dopamine increases IF and reduces IL and ML [6, 33]. Pollen grain carry the male genetic material, by variety of means, for gametogenesis in the plant kingdom and it contains many active materials such as polyunsaturated fatty acids and sterols which have mutual benefits for example they act as hypolipidemic and hypocholesterolemic factors [34]. No side effects weren’t observed in our study except diarrhea for rats that received 350 mg/kg of extract. Probably, hypotension caused by diarrhea resulted in decreased sexual behavior parameters.
5. Conclusion
Finally, our study showed that DPP possess facilitatory effects that increase sexual arousal, the state of sexual excitement or desire during sexual interaction. Dose 140 mg/kg had greater activity in stimulating of erection, genital grooming and orientation activities of male rats. In addition of improving the sperm quality [10], unpublished data, DPP can enhance penile erection and other sexual behaviors. our results showed that DA concentration of nucleus accumbence increased in male rats following seeing and during their first encounter with a female in various phases of sexual behavior and this increase was higher in extract treated rats in comparison with control. copulation stimulates mesolimbic DA function in male rats and mounts with intromission are associated with increasing of DA. Increasing of DA following administration of DPP indicates that, DPP can be used as stimulator of sexual behavior and curing of male impotency (i.e. sexual arousal/erection disorders) and pre-mature ejaculation (37). The present Data suggest potential utility in managing sexual dysfunction. Its action may be due to influence on sexual arousal and releasing of DA from nucleus accumbence. Effects of DPP may be due to the presence of steroids, alkaloids and flavonoids through a multitude of central and peripheral means Therefore, it may be useful to solve the sexual problems such as pre-ejaculation and impotency. It can influence the sexual arousal and performance. The aphrodisiac effect of the DPP extract may be due to the presence of alkaloids, saponins and flavonoids through a central and peripheral pathway.
6. Acknowledgements
This study was supported by Vice Chancellor for Research of Tehran University of Medical Sciences, Tehran, Iran (No. 90). Authors wish to thank Mr. Ghasemi and Mr. Sohanaki (instructors of medical physiology) and also Mr. Fanaee and Mr. Bakhshesh (Ph.D. students of medical physiology) for their technical assistances.
REFERENCES
- J. H. Jung, S. C. Kam, S. M. Choi, S. U. Jae, S. H. Lee, et al., “Sexual Dysfunction in Male Stroke Patients: Correlation between Brain Lesions and Sexual Function,” Urology, Vol. 71, No. 1, 2008, pp. 99-103. doi:10.1016/j.urology.2007.08.045
- M. N. Anil Kumar, N. B. Pai, T. S. Rao and N. Goyal, “Biolgy of Sexual Dysfunction,” Health and Allied Sciences, Vol. 8, No. 1, 2009, pp. 1-7.
- R. L. Meisel and B. D. Sachs, “The Physiology of Male Sexual Behavior,” In: E. Knobil and J. Neill, Physiology of Reproduction, 2nd Edition, Raven Press, New York, 1994, pp. 3-105.
- WHO, “WHO Manual for Standardized Investigation, Diagnosis and Management of the Infertile Male,” Cambridge University Press, Cambridge, 2000, pp. 10-50.
- A. F. G. Cicero, E. Bandieri and R. Arletti, “Lepidium meyenii Improves Sexual Behaviour in Male Rats Independently from Its Action on Spontaneous Locomotor Activity,” Journal of Ethnopharmacology, Vol. 75, No. 2-3, 2001, pp. 225-229. doi:10.1016/S0378-8741(01)00195-7
- A. Adimoelja, “Phytochemicals and the Breakthrough of Traditional Herbs in the Management of Sexual Dysfunctions,” International Journal of Andrology, Vol. 23, No. 2, 2000, pp. 82-84. doi:10.1046/j.1365-2605.2000.00020.x
- A. J. Krentz and C. J. Bailey, “Oral Antidiabetic Agents: Current Role in Type 2 Diabetes Mellitus,” Drugs, Vol. 65, No. 3, 2005, pp. 385-411. doi:10.2165/00003495-200565030-00005
- Y. K. Gupta and S. Briyal, “Animal Models of Cerebral Ischemia for Evaluation of Drugs,” Indian Journal of Physiology and Pharmacology, Vol. 48, No. 4, 2004, pp. 379-394.
- M. W. Islam, M. Tariq, A. M. Ageel, M. S. Al-said and A. M. Al-Yaya, “Effect of Salvia hematodes on Sexual Behavior of Male Rats,” Journal of Ethnopharmacology, Vol. 33, 1991, pp. 67-72. doi:10.1016/0378-8741(91)90163-8
- S. Bahmanpour, T. Talaei, Z. Vojdani, M. R. Panjehshahin, A. Poostpasand, S. Zareei and M. Ghaeminia, “Effect of Phoenix dactylifera Pollen on Sperm Parameters and Reproductive System of Adult Male Rats,” International Journal of Molecular Sciences, Vol. 31, No. 4, 2006, pp. 208-212.
- F. Biglari, A. F. M. AlKarkhi and M. E. Azhar, “Antioxidant Activity and Phenolic Content of Various Date Palm (Phoenix dactylifera) Fruits from Iran,” Food Chemistry, Vol. 107, No. 4, 2008, pp. 1636-1641. doi:10.1016/j.foodchem.2007.10.033
- A. A. Al-Qarawi, H. M. Mousa, B. E. H. Ali, H. Abdel-Rahman and S. A. El-Mougy, “Protective Effect of Extracts from Dates (Phoenix dactylifera) on Carbon Tetrachloride-Induced Hepatotoxicity in Rats,” The Journal of Applied Research in Veterinary Medicine, Vol. 2, No. 3, 2004, pp. 176-180.
- A. Agmo, “Male Rat Sexual Behaviour,” Brain Research Protocols, Vol. 1, No. 2, 1997, pp. 203-209. doi:10.1016/S1385-299X(96)00036-0
- R. L. Meisel, J. K. O’Hanlon and B. D. Sachs, “Differential Maintenance of Penile Responses and Copulatory Behaviour by Gonadal Hormones in Castrated Male Rats,” Hormones and Behavior, Vol. 18, No. 1, 1984, pp. 54-56. doi:10.1016/0018-506X(84)90050-3
- M. A. Ageel, M. W. Islam, O. T. Ginawi and M. A. Al-Yahya, “Evaluation of the Aphrodisiac Activity of Litsea chinenesis and Orchis masculata Extract in Rats,” Phytotherapy Research, Vol. 8, No. 2, 1994, pp. 103-105. doi:10.1002/ptr.2650080211
- R. C. Schiavi and R. T. Segraves, “The Biology of Sexual Function,” Annals of Clinical Psychiatry, Vol. 7, No. 4, 1995, pp. 189-201.
- H. J. Kim, D. S. Woo and J. J. Lee Kim, “The Relaxation Effects of Ginseng Saponin in Rabbit Corporal Smooth Muscle, Is It a Nitric Oxide Donor?” British Journal of Urology, Vol. 82, No. 5, 1998, pp. 744-748. doi:10.1046/j.1464-410X.1998.00811.x
- S. A. Padashetty and S. H. Mishra, “Aphrodisiac Studies of Tricholepis glaberrima with Supportive Action from Antioxidant Enzymes,” Pharmaceutical Biology, Vol. 45, No. 7, 2007, pp. 580-586. doi:10.1080/13880200701501326
- A. Morales, D. H. C. Surridge, P. G. Marshall and J. Fenemote, “Nonhormonal Pharmacology Treatment of Organic Impotence,” Journal of Urology, Vol. 128, 1982, pp. 45-47.
- F. Giuliano, O. Rampin, A. Schiar, A. Jardin and J. P. Rousseau, “Autonomic Control of Penile Erection: Modulation by Testosterone in the Rat,” Journal of Neuroendocrinology, Vol. 9, 1993, pp. 141-150.
- T. M. Mills, V. S. Stopper and V. T. Wiedmeier, “Effects of Castration and Androgen Replacement on the Hemodynamics of Penile Erection in the Rat,” Biology of Reproduction, Vol. 51, No. 2, 1994, pp. 234-238. doi:10.1095/biolreprod51.2.234
- M. Thakur and V. K. Dixit, “Aphrodisiac Activity of Dactylorhiza hatagirea (D. Don) Soo in Male Albino Rats,” Evidence-Based Complementary and Alternative Medicine, Vol. 4, No. 1, 2007, pp. 29-31. doi:10.1093/ecam/nem111
- M. D. Majewska, F. L. Bellino, R. A. Davies, P. J. Hornsby, D. H. Lavrin and J. E. Nestler, “Neuronal Activities of Dehydroepiandrosterone in Dehydroepiandrosterone (DHEA) and Aging,” The New York Academy of Sciences, Vol. 774, 1995, pp. 111-120 . doi:10.1111/j.1749-6632.1995.tb17375.x
- M. T. Yakubu, M. A. Akanji, A. T. Oladiji and A. A. Adesokan, “Androgenic Potentials of Aqueous Extract of Massularia acuminata (G. Don) Bullock ex Hoyl. Stem in Male Wistar Rats,” Journal of Ethnopharmacology, Vol. 118, No. 3, 2008, pp. 508-513. doi:10.1016/j.jep.2008.05.020
- P. K. Suresh Kumar, A. Subramoniam and P. Pushpangadan, “Aphrodisiac Activity of Vanda tessellata (Roxb.) Hook. ex Don Extract in Male Mice,” Indian Journal of Pharmacology, Vol. 32, No. 5, 2000, pp. 300-304.
- M. T. Yakubu, M. A. Akanji and A. T. Oladiji, “Aphrodisiac Potentials of the Aqueous Extract of Fadogia agrestis (Schweinf ex Hiern) Stem in Male Albino Rats,” Asian Journal of Andrology, Vol. 7, No. 4, 2005, pp. 399-404. doi:10.1111/j.1745-7262.2005.00052.x
- G. H. Mahran, S. M. Abdul-Wahab and A. M. Attia, “A Phytochemical Study of Date Palm Pollen,” Planta Medica, Vol. 29, No. 2, 1976, pp. 171-175. doi:10.1055/s-0028-1097648
- E. Amine, O. Awad, El-Samad and M. N. Iskander, “Pharmacological Studies on Pollen Grains of Dates (Pheonix dactylefera),” Phytochemistry, Vol. 8, 1996, pp. 295-298.
- M. R. Zarrindast, K. Nojoomi, M. Sharifzadeh and A. Mokri, “Niric Oxide Agents and Apomorphine-Induced Rat Behaviors,” Pharmacology, Vol. 71, No. 4, 2003, pp. 169-173 doi:10.1159/000078082
- S. K. Putnam, J. Du and E. M. Hull, “Testosterone Restoration of Copulation and Medial Preoptic Dopamine Release in Castrated Male Rats: 2-, 5-, and 10-Day Treatments,” Hormones and Behavior, Vol. 39, No. 3, 2001, pp. 216-224. doi:10.1006/hbeh.2001.1648
- C. L. Ballard and R. I. Wood, “Partner Preference in Male Hamsters: Steroids, Sexual Experience and Chemosensory Cues,” Physiology and Behavior, Vol. 91, No. 1, 2007, pp. 1-8. doi:10.1016/j.physbeh.2007.01.005
- K. Gauthaman and P. G. Adaikan, “The Hormonal Effects of Tribulus terrestris and Its Role in the Management of Male Erectile Dysfunction—An Evaluation Using Primates, Rabbit and Rat,” Phytomedicine, Vol. 15, No. 1, 2008, pp. 44-54. doi:10.1016/j.phymed.2007.11.011
- P. Sandroni, “Aphrodisiacs Past and Present, a Historical Review,” Clinical Autonomic Research, Vol. 11, No. 5, 2001, pp. 303-307. doi:10.1007/BF02332975
- A. Resho and Al-Shagrawi, “Enzyme Activities, Lipid Fractions, and Fatty Acids Composition in Male Rats Fed Palm Pollen Grains (Phoenix dactylifera),” Research Bulletin, Vol. 79, 1998, pp. 5-18.
NOTES
*Corresponding author.

