World Journal of Cardiovascular Diseases
Vol. 2 No. 1 (2012) , Article ID: 16600 , 9 pages DOI:10.4236/wjcd.2012.21006
Paraoxonase 1 gene (Gln192-Arg) polymorphism and the risk of coronary artery disease in type 2 diabetes mellitus
![]()
Faculty of Medicine, Menoufyia University, Menoufyia, Egypt
Email: mnoamany@hotmail.com
Received 25 October 2011; revised 7 December 2011; accepted 18 December 2011
Keywords: Paraoxonase 1; Coronary Artery Disease; Diabetes Mellitus
ABSTRACT
Background: Paraoxonase 1 (PON1) is reported to have an antioxidant and cardioprotective properties. Recently, an association of glutamine (Gln) or (type A)/arginine (Arg) or (type B) polymorphism at position 192 of PON1 gene has been suggested with coronary artery disease (CAD) among patients with diabetes mellitus (DM). However, conflicting results have also been reported. Objectives: To investigate the relationship between PON1 gene (Gln192-Arg) polymorphism and the presence, extent and severity of CAD in type 2 DM. Methods: The study comprised 180 patients recruited from those undergoing coronary angiography for suspected CAD, who were divided according to the presence or absence of CAD and DM into 4 groups; Group I (n = 40 patients) nondiabetic subjects without CAD, Group II (n = 45 patients) diabetic patients without CAD, Group III (n = 47 patients) non diabetic patients with CAD and Group IV (n = 48 patients) diabetic patients with CAD. PON1 (Gln192-Arg) genotype was assessed using polymerase chain reaction (PCR) followed by AlwI digestion. Results: The frequency of Gln allele (Type A) was significantly higher in group I and group II compared to group III and group IV (62.5%, 60% vs 38.3%, 31.25% respectively, p < 0.001) while the frequency of Arg allele (Type B + Type AB) was significantly higher in ischemic groups (III, IV) compared to non ischemic groups (I), (II) (61.7%, 68.75% vs 37.5%, 40% respectively, p < 0.001). Patients with CAD and DM (group IV) have significantly higher severity score and vessel score than those with CAD only (group III) (9.7 ± 2.97, 2.44 ± 0.56 vs 6.99 ± 3.71, 1.67 ± 0.89 respectively, p < 0.001) Patients with vessel score 3 had significantly higher severity score and higher Arg allele frequency than patients with vessel score 2, the latter group had also significantly higher severity score and Arg allele frequency than patients with vessel score 1 (8.9 ± 2.79 vs 5.21 ± 2.13 and 80.49% vs 67.86%), (5.21 ± 2.13 vs 3.11 ± 0.89 and 67.86% vs 53.85%), p < 0.001 for all. In multivariate logistic regression analysis of different variables for prediction of CAD, age [OR 2.99, CI (1.11 - 10.5), P < 0.01], smoking [OR 4.13, CI (1.37 - 11.7), P < 0.001], low density lipoprotein (LDL) cholesterol > 100 mg/dL [OR 4.31, CI (1.25 - 12.5), P < 0.001], high density lipoprotein (HDL) cholesterol < 40 mg/dL [OR 5.11, CI (1.79 - 16.33), P < 0.001] and PON1 192 Arg allele [OR 4.62, CI (1.67 - 13.57), P < 0.001] were significantly independent predictors of CAD. Conclusion: Arg allele of PON1 192 gene polymorphism is an independent risk factor for CAD and it is associated not only with the presence of CAD but also with its extent and severity and its impact is clearly more pronounced in diabetic patients.
1. INTRODUCTION
Diabetes mellitus (DM) is a major risk factor for the development of coronary artery disease (CAD), which is one of the leading causes of morbidity and mortality in developed countries [1,2]. In addition to the traditional cardiovascular risk factors, many previous studies suggested that, there may be a genetic predisposition [3,4]. Many genes are likely to be involved in the pathogenesis of CAD, including those involved in lipoprotein metabolism [5].
Human paraoxonase 1/arylesterase (PON1) is an HDLassociated Ca2+ dependent glycoprotein (lactonase) which posses antioxidant and anti-atherogenic properties through its ability to hydrolyze paraoxon, the active toxic metabolite of the organophosphate parathion and exclusively bound to HDL in human plasma and has been shown to reduce the accumulation of lipid oxidation products on LDL [6], thus preventing the transformation of LDL into atherogenic particles [7]. The human serum paraoxonase is a 43-kD to 45-kD protein. Its gene is located at q21 to q22 on the long arm of chromosome 7 [8]. The amino acid sequence of paraoxonase is highly conserved among animal species, suggesting an important metabolic role for this enzyme [9]. Human serum paraoxonase activity toward paraoxon shows large interindividual variation and underlies tight genetic control. The molecular basis of this variation and decreased PON1 activity is a polymorphism in the coding region of the gene, resulting in an amino acid substitution glutamine (Gln)/ arginine (Arg) in position 192 [10,11]. Recently, the presence of the (Gln192-Arg) polymorphism in the PON1 gene was reported to be an independent risk factor for CHD in a French Caucasian population and Japanese [11] patients with type 2 diabetes and in a North American general population [12] but could not be confirmed in Finnish [13], Japanese [14], and Chinese [15] CAD patients, mostly without diabetes.
2. AIM OF THE WORK
To investigate the relationship between PON1 gene polymorphism and the presence, extent and severity of CAD in type 2 DM.
3. PATIENTS AND METHODS
3.1. Study Population
The study comprised 180 patients recruited from those undergoing coronary angiography for suspected CAD, who were divided according to the presence or absence of CAD and DM into 4 groups; Group I (n = 40 patients) non diabetic subjects without CAD, Group II (n = 45 patients) diabetic patients without CAD, Group III (n = 47 patients) non diabetic patients with CAD and Group IV (n = 48 patients) diabetic patients with CAD.
The study was performed prospectively in the period from September 2009 to June 2011.
The study was approved by the appropriate Institutional Review Board and Institutional Ethical Committee for Human Research. All study subjects provided written informed consent regarding the study procedures.
Diabetes mellitus (DM) was diagnosed according to World Health Organization (WHO) and the American Diabetes Association (ADA) [16-18] as fasting blood glucose ≥ 126 mg/dL (≥7.0 mmol/L) or 2 hour post-load plasma glucose ≥ 200 mg/dL (≥11.1 mmol/L) or random plasma glucose ≥ 200 mg/dL.
Inclusion criteria: patients included in the study are those undergoing diagnostic coronary angiography for suspected ischemic heart disease with one or more of the following:
-Typical chest pain.
-Electrocardiographic (ECG) changes suggesting CAD.
-Previous admission to hospital by CAD.
-Previous cardiac interventions e.g. percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG).
-Other investigations suggesting CAD such as, positive exercise stress test, positive myocardial perfusion scanning or echocardiographic regional wall motion abnormality suggesting CAD.
3.2. For Each Patient the Following Was Done
1) Complete history taking.
2) Thorough clinical evaluation.
3) 12-lead resting ECG.
4) Conventional echocardiographic examination: echocardiography was performed with the patient in the left lateral decubitus position. The equipment used was Vivid S 5 system. Measurements were performed according to the recommendations of the American Society of Echocardiography [19]. With the use of the long axis parasternal, apical 4, 5 and 2-chamber views. All patients underwent conventional M mode and 2-D echocardiographic examination. Visual assessment of regional wall motion was performed. Endsystolic volume, end diastolic volume (by manual tracing of endocardial borders) and ejection fraction of each ventricle was calculated using Simpson’s rule [20]. Convensional (continuous-wave and color) Doppler valvular flow was determined.
5) Angiographic analysis: diagnostic coronary angiography (CA) was carried out in all patients using Judkins technique. Quantitative analysis of coronary arteries was performed with the computer-assisted coronary angiography analysis system. End-diastolic frames from each arteriogram were selected for analysis [21]. The percentage diameter stenosis (DS) was assessed in different projections and the highest value of each lesion was chosen. Images of the coronary tree were obtained with the digital Philips set system and reviewed by an experienced cardiologist who had no knowledge of the patients’ biochemical results to assess the extent and severity of CAD and morphology of all coronary artery stenoses.
The coronary tree was divided into 16 segments as follows: [22].
The left main coronary artery (LMCA) as one segment.
The left anterior descending (LAD) artery was divided into: proximal, mid, distal segments beside two diagonals Dl and D2.
The left circumflex (LCX) artery was divided into: proximal, mid, distal segments beside two marginal branches OMl and OM2.
The right coronary (RCA) artery was divided into: proximal, mid, distal segments beside posterior descending artery (PDA) and postrolateral (PL) branch.
Coronary angiograms were scored according to:
Vessel score [23]: this was the number of vessels with a significant stenosis (50% or greater reduction in lumen diameter). Degree of stenosis was defined as the greatest percentage reduction of luminal diameter in any view compared with the nearest normal segment and was determined visually. Scores ranged from 0 to 3, depending on the vessels involved. Left main artery stenosis was scored as single vessel disease [23].
Severity score [24]: the coronary circulation was divided into eight proximal segments. Disease in the distal segments was not considered because of difficulty in quantitating the severity of lesions in these areas. The eight proximal segments scored included the left main coronary artery, the left anterior descending artery (LAD) up to the junction of the middle and distal third of the vessel, the proximal third of the major septal branch of the LAD, the proximal third of the major diagonal branch of the LAD, the left circumflex coronary artery (LCX) up to the junction of the middle and distal thirds of the vessel, the proximal third of the major obtuse marginal branch of the LCX, the right coronary artery (RCA) up to and including the origin of the posterior descending coronary artery (PDA), and the proximal third of the PDA. In cases in which the PDA was supplied by the LCX vessel (LCX dominance), lesions in the LCX up to the origin of the PDA were included, as were lesions of the RCA up to the origin of the middle and distal thirds of the vessel. The PDA was scored identically for RCA and LCX dominant circulations. The percentage by which each lesion in the proximal coronary circulation narrowed the artery was assessed according to the maximal narrowing of the diameter of the artery in all projections. The severity of the proximal coronary disease was assessed by assigning points to each lesion as follows: less than 50% stenosis of the luminal diameter, 1 point; 50% to 74% stenosis, 2 points; 75% to 99% stenosis, 3 points; total obstruction, 4 points. The points for each lesion in the proximal coronary circulation were summed and a score for severity of coronary atherosclerosis was obtained. In previous study, the coefficient of variation between two angiograms analyzed several months apart without knowledge of the previous score was 4.9% [24].
6) Laboratory examination Blood sampling: ten ml of fasting (12 hour - 14 hour) venous blood were withdrawn from the cubital vein of every patient within one hour before coronary angiography. Four ml was transferred slowly into vacunated EDTA tube for isolation of white blood cells for genotyping. Two ml was transferred slowly into vacunated EDTA tube for measuring Glycated hemogobin (HbA1c). Two ml was transferred slowly into vacunated EDTA tube and centrifuged for 5 min at 4000 rpm. The plasma obtained for determination of plasma glucose frozen at –20˚C till analysis. Two ml was transferred slowly into a plain tube for determination of serum total cholesterol, triglycerides, HDL cholesterol left for 30 min for clotting and centrifuged for 10 min at 4000 rpm. The serum obtained frozen at –20˚C till analysis. Blood glucose, HbA1c, serum cholesterol, triglycerides and HDL cholesterol were determined by enzymatic colorimetric test. LDL cholesterol was estimated by Friedewald’s formula [25].
Leukocyte isolation: leukocytes were isolated from the whole blood by removing erythrocytes by suspending cells in 8 ml erythrocyte lysing buffer (ELB), then centrifuged for 5 min at 1000 rpm. Centrifugation was repeated twice more till a white pellet appeared. Carefully, the blood platelets were removed and the white pellet washed twice with 1 ml phosphate buffer solution (PBS) and then transferred to eppendorf tubes for next step DNA extraction [26].
DNA Extraction: by using QIAamp® DNA Blood Mini Kits (QIAGEN HILDEN, Germany).
Paraoxinase 192 Genotyping: the amplification reaction for Gln192 Arg was performed in 25 µl volumes (10 µl DNA template + {15 µl Master Mix containing 2.5 µl of 10× PCR buffer, 0.25 µl MgCl 25 mM, 1.0 µl dNTPs 10 mM, 1.0 µl Forward primer (F5’-TAT TGT TGC TGT GGG ACC TGA G-3’), 1.0 µl reverse primer (R5’- CAC GCT AAA CCC AAA TAC ATC TC-3’), 0.5 µl Taq polymerase 5 µ/µl and 8.75 µl distilled water}. Using an initial denaturation (5 min at 95˚C), followed by 35 cycles each consisting of {denaturation (1 min at 95˚C), annealing (1 min at 61˚C), extension (1 min at 72˚C)} and final extension (10 min at 72˚C) using Perkin Elmer thermal cycler 2400 (USA) [27].
Identification of the Gln192 Arg polymorphism: the PON1 192 (99 bp) PCR product was digested with AlwI at 37˚C for 3 h (2 µl 10× buffer, 1 µl AlwI, 7 µl distilled water and 10 µl PCR product). The AlwI digestive products were run by 5% polyacrylamide gel electrophoresis for 30 minutes and stained with ethidium bromide, and the bands were visualized under ultraviolet light. Digested PCR products yielded 99 bp bands in Gln/Gln homozygotes, 66 and 33 bp bands in Arg/Arg homozygotes, and all three bands in Arg/Gln heterozygotes [27] (Figures 1 and 2).
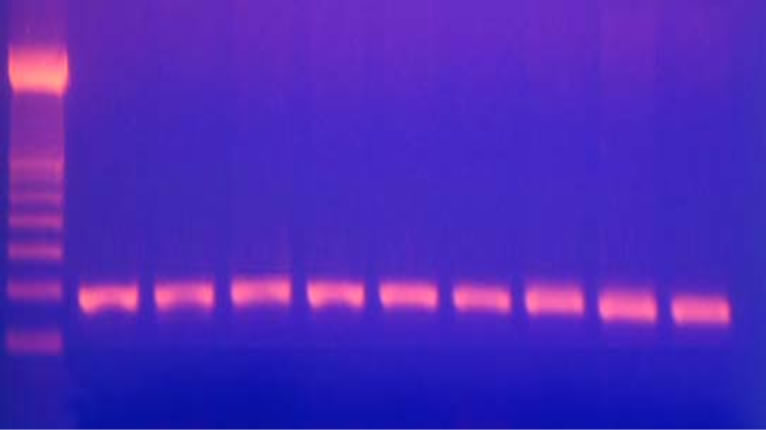
Figure 1. PCR products yielded 99 bp bands.
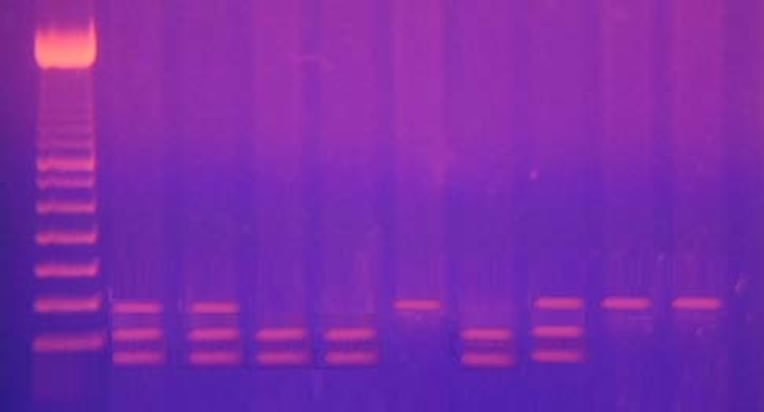
Figure 2. Arg/Arg: 66 and 33 bp. Gln/Gln: 99bp. Arg/Gln: 99 bp, 66 bp and 33 bp. Lane (1) DNA ladder 50 bp. Lane (2, 3 and 8) all three bands in Arg/Gln heterozygotes. Lane (4, 5 and 7) Arg/Arg homozygotes 33 bp and 66 bp bands Lane (6, 9 and 10) Gln/Gln homozygotes 99 bp bands.
4. STATISTICAL ANALYSIS
Data were analyzed by SPSS statistical package version 11.0 (SPSS Inc, Chicago, IL, USA). Quantitative data expressed as mean and standard deviation (SD). Qualitative data expressed as number and percentage and analyzed by Chi-square (X2) or Fisher exact test when appropriate.
Comparisons between means were evaluated by unpaired t-test or ANOVA (F) test (with post hoc test) for continuous variables with determination of the least significant difference (LSD) by pair wise comparison between group means, and by chi-square test for proportions.
Multivariate logistic regression was used to assess independent predicators of coronary artery disease among patients using, demographic, clinical, laboratory and echocardiographic variables. Level of significance was set as P value < 0.05 [28].
5. RESULTS
The study comprised 180 patients recruited from those undergoing coronary angiography for suspected CAD, who were divided according to the presence or absence of CAD and DM into 4 groups; Group I (n = 40 patients, 30 males and 10 females with mean age 50.19 years ± 5.5 years) non diabetic subjects without CAD, Group II (n = 45 patients, 33 males and 12 females with mean age 51.11 years ± 6.7 years) diabetic patients without CAD, Group III (n = 47 patients, 34 males and 13 females with mean age 53.33 years ± 4.9 years) non diabetic patients with CAD and Group IV (n = 48 patients, 35 males and 13 females with mean age 54.79 years ± 6.5 years) diabetic patients with CAD.
There was no significant difference between the studied groups as regard age, gender, body mass index (BMI), systolic or diastolic blood pressure, left ventricular ejection fraction or left ventricular mass index.
The number of smokers was significantly higher in group III and IV compared to each of group I and group II. Glycated hemogobin (HbA1c) was significantly higher in diabetic groups (group II and group IV) in comparison to non diabetic groups (group I and group III), P < 0.05 (Table 1).
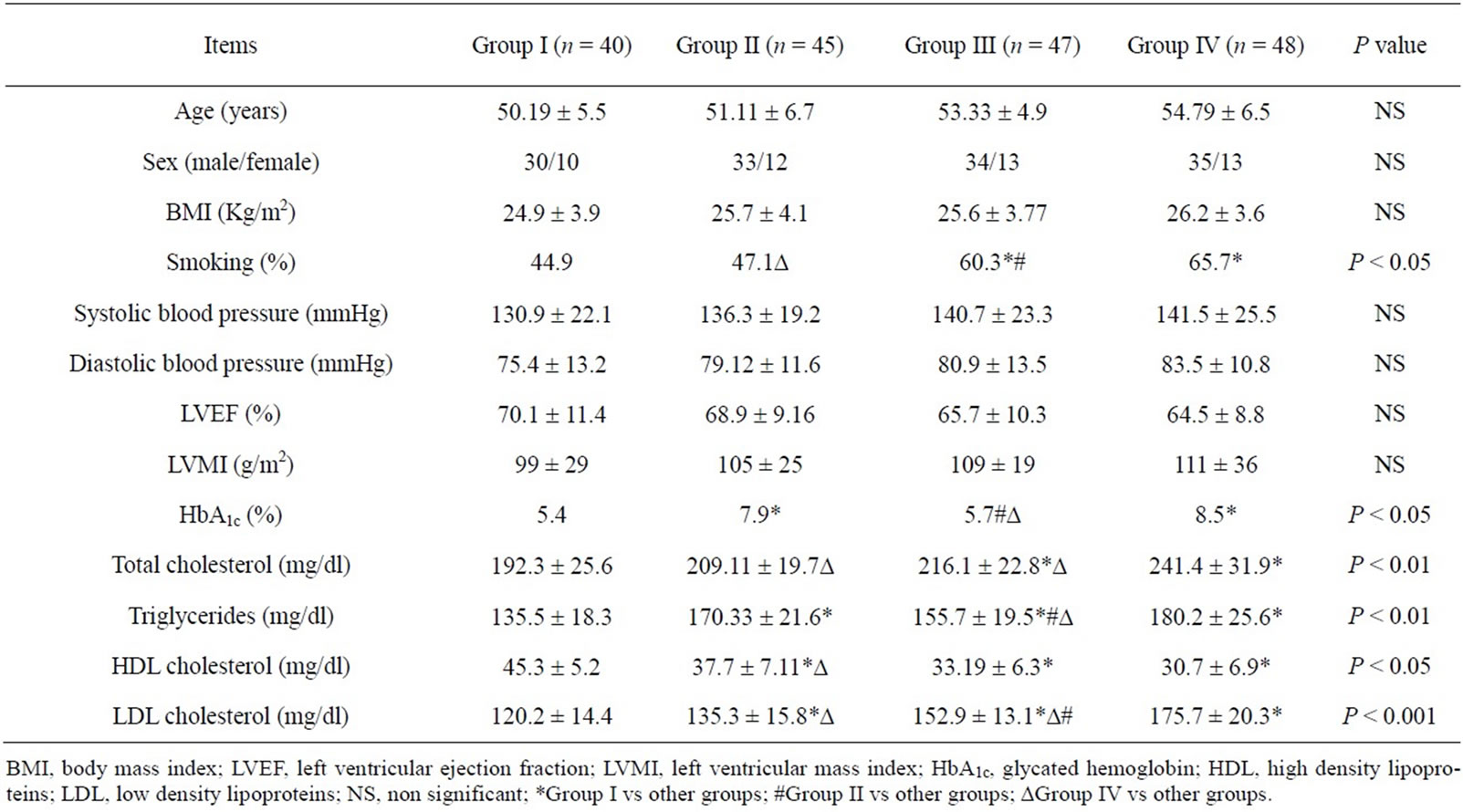
Table 1. Baseline demographic, clinical and laboratory characteristics of studied groups.
Total cholesterol was significantly higher in group IV compared to other groups and in group III in comparison to group I, while triglycerides were significantly higher in all groups compared to group I and each of group II and group IV in comparison to group III, P < 0.01. HDL cholesterol was significantly higher in group I in comparison to other groups and in group II compared to group IV, P < 0.05. LDL cholesterol was significantly higher in all groups compared to group I, while it was significantly higher in group IV in comparison to group II and group III and in group III compared to group II, p < 0.001 (Table 1).
As regard paraoxonase 1 genotype, the prevalence of Gln/Gln (Type A) genotype was significantly higher in group I and group II compared to group III and group IV (62.5%, 60% vs 38.3%, 31.25% respectively, p < 0.001). On the other hand, the percentage of patients having Arg/ Arg (Type B) genotype was significantly higher in group III and group IV in comparison to group I and group II (29.79%, 39.58% vs 5%, 8.89% respectively, p < 0.001). While the percentage of patients having Gln/Arg (Type AB) genotype did not differ significantly among all studied groups (Table 2).
Collectively, the frequency of Gln allele (Type A) was significantly higher in group I and group II compared to group III and group IV while the frequency of Arg allele (Type B + Type AB) was significantly higher in ischemic groups (III, IV) compared to non ischemic groups (I, II) (61.7%, 68.75% vs 37.5%, 40% respectively, p < 0.001). Patients with CAD and DM (group IV) have significantly higher severity score and vessel score than those with CAD only (group III) (9.7 ± 2.97, 2.44 ± 0.56 vs. 6.99 ± 3.71, 1.67 ± 0.89 respectively, p < 0.001) (Table 2).
There were no significant differences between carriers of the Arg allele (Type B + Type AB) and those homozygous for Gln allele (Type A) with respect to age, gender, smoking, body mass index, systolic or diastolic blood pressure, plasma cholesterol, and triglycerides.
Patients with vessel score 3 had significantly higher severity score and higher Arg allele frequency than patients with vessel score 2, the latter group had also significantly higher severity score and Arg allele frequency than patients with vessel score 1. (8.9 ± 2.79 vs 5.21 ± 2.13 and 80.49% vs 67.86%), (5.21 ± 2.13 vs 3.11 ± 0.89 and 67.86% vs 53.85%), p < 0.001 for all (Table 3).
In multivariate logistic regression analysis of different variables for prediction of CAD, age [OR 2.99, CI (1.11 - 10.5), P < 0.01], smoking [OR 4.13, CI (1.37 - 11.7), P < 0.001], LDL cholesterol > 100 mg/dL [OR 4.31, CI (1.25 - 12.5), P < 0.001], HDL cholesterol < 40 mg/dL [OR 5.11, CI (1.79 - 16.33), P < 0.001] and paraoxonase 192 Arg allele [OR 4.62, CI (1.67 - 13.57), P < 0.001] were significantly independent predictors of CAD (Table 4).
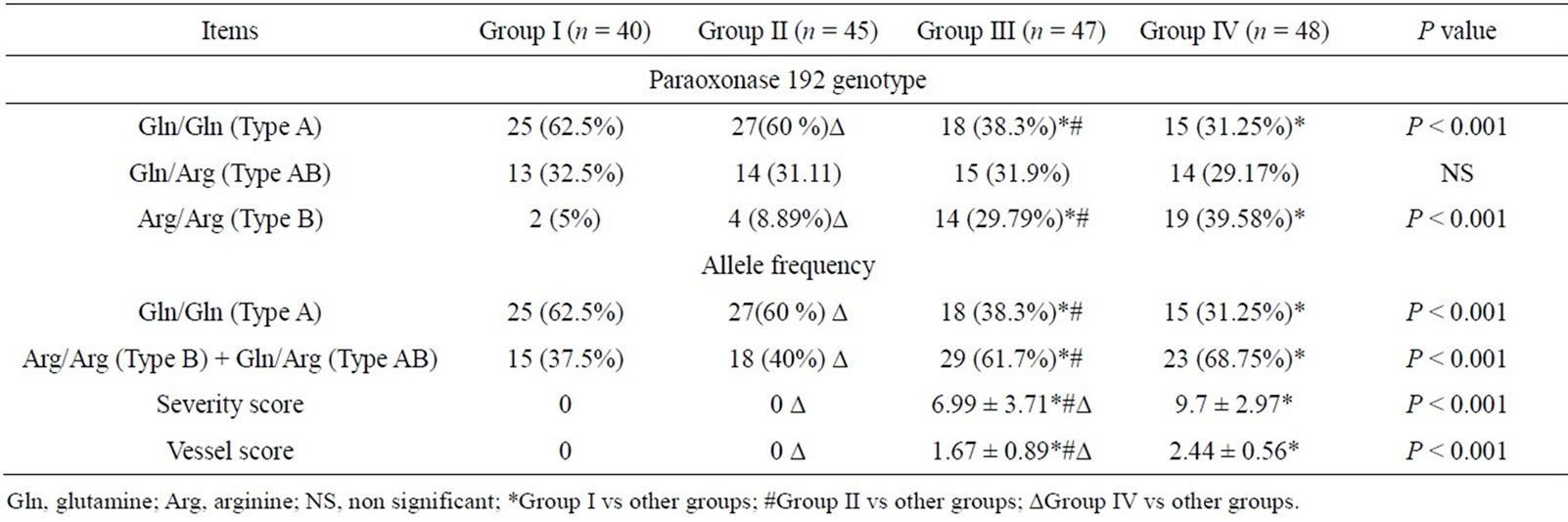
Table 2. Paraoxonase1 genotypes, allele frequencies and angiographic scores in the studied group.
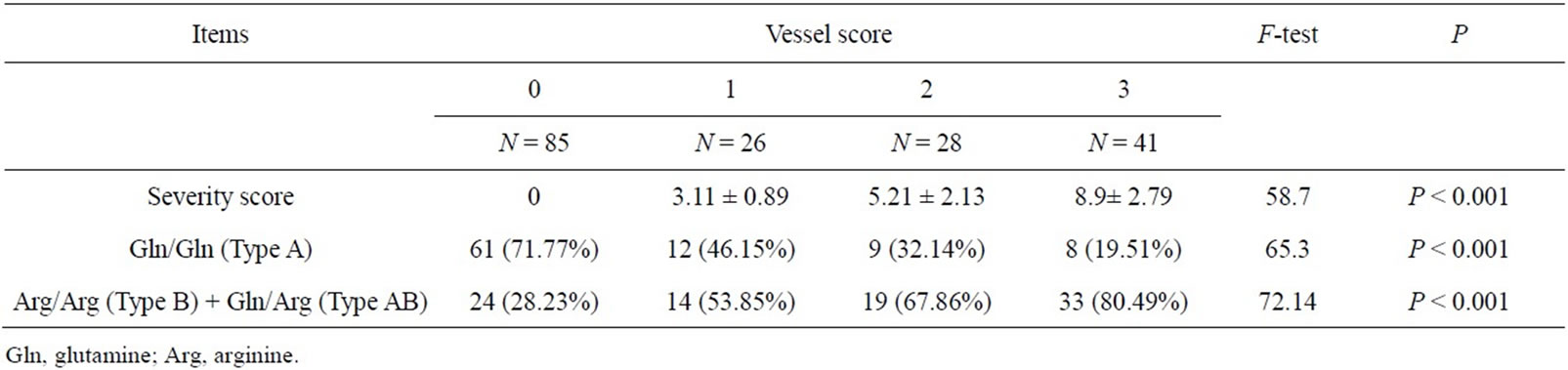
Table 3. Paraoxonase 1 allele frequencies in different vessel scores and severity scores.
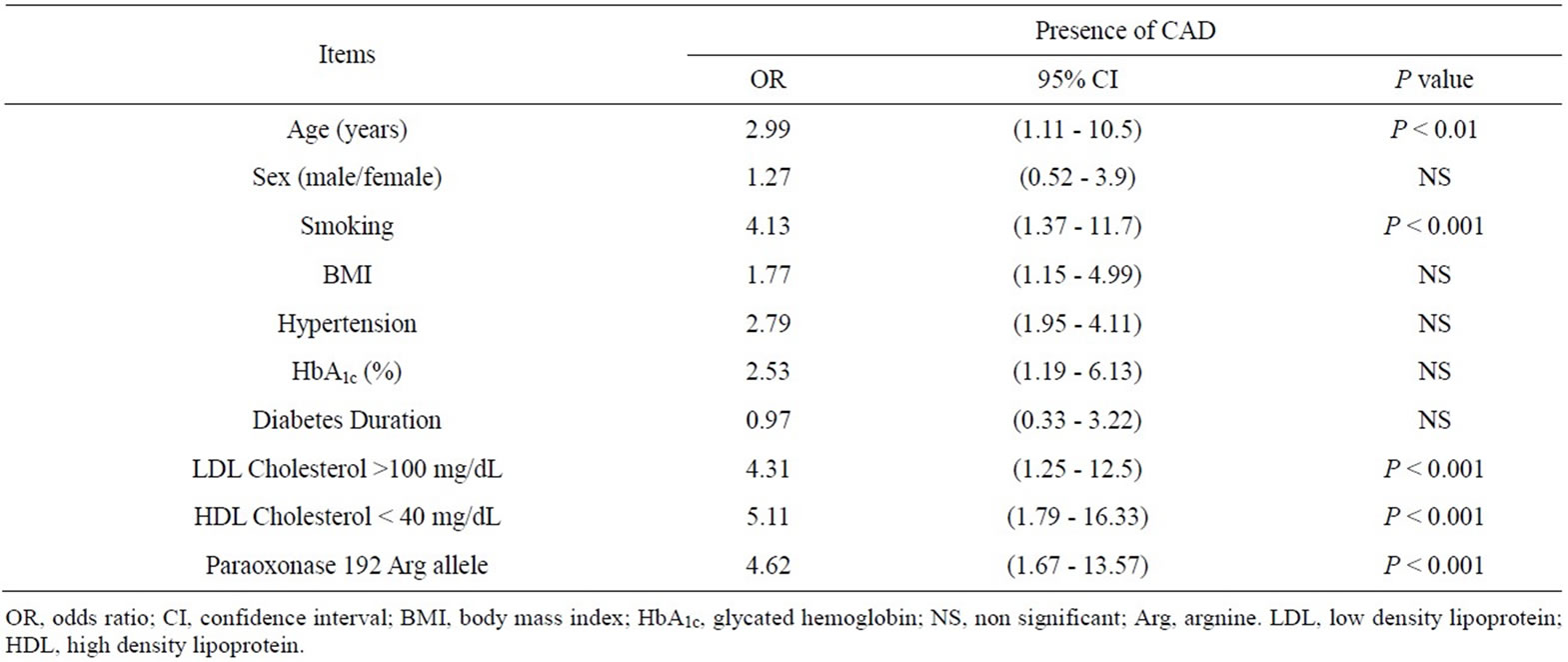
Table 4. Multivariate regression analysis of predictors of coronary artery disease in studied populations.
6. DISCUSSION
Peroxidation of low density lipoproteins (LDL) plays a central pivotal role in atherogenesis. [29] Enzymes associated with HDL particles, including paraoxonase 1, platelet-activating factor acyltransferase and lecitin-cholesterol acyltransferase (LCAT), can cleave oxidized lipids from LDL. High-density lipoproteins (HDL) diminish the accumulation of lipid peroxides in LDL mainly due to paraoxonase activity [30].
The present study was designed to investigate the relationship between PON1 gene polymorphism and the presence, extent and severity of CAD in type 2 DM. In our study, the frequency of Gln allele (Type A) was significantly higher in group I and group II compared to group III and group IV while the frequency of Arg allele (Type B + Type AB) was significantly higher in ischemic groups (III and IV) compared to non ischemic groups (I and II). To confirm the causative role of PON1 gene polymorphism in CAD and to exclude the possibility that Arg allele carriers (Type B + Type AB) have other risk factors related to CAD, we investigated these risk factors between the 2 groups (type A vs type B + type AB). There were no significant differences between carriers of the Arg allele (Type B + Type AB) and those homozygous for Gln allele (Type A) with respect to age, gender, smoking, body mass index, systolic or diastolic blood pressure, plasma cholesterol, and triglycerides.
This observation related to the causative role of PON1 gene polymorphism in CAD was first reported by Ruiz et al. [31] who found the same association between the paraoxonase 192 Arg allele and CAD in 434 French type 2 diabetic, and has recently been confirmed by Odawara et al. [11] who reported a highly significant increase in AB + B genotypes in diabetic patients with CAD compared with those without CAD. They also reported a trend toward an increased frequency of the Arg allele in patients with CAD, although this did not reach statistical significance, presumably because of the relatively limited number of patients they analyzed.
In agreement with our findings, Pfohl et al. [10] reported that, paraoxonase 192 Arg allele was significantly associated with both the presence and the extent of CAD, comparing the patients with angiographically proven CAD and those without angiographic, clinical, or ECG evidence of CAD.
In accord with our results, Jalilian et al. [32] reported that, Arg allele frequency was significantly higher in patients with CAD than those with normal coronaries and more interestingly they reported significantly higher Arg allele frequency with increased CAD severity.
Furthermore, James et al. [33] found that, the PON1 Arg genotype was significantly higher in diabetic patients with CAD than diabetic patients without CAD.
Consistent with the same observations, Lavi et al. [34] postulated that, coronary endothelial dysfunction in humans with early coronary atherosclerosis is associated with the paraoxonase-1 allelic variant 192Arg > Gln.
Interestingly, in our study, PON 1 gene polymorphism was associated not only with the presence of CAD but also with the extent and severity of CAD. Patients with CAD (group III and group IV) have significantly higher Arg allele frequency (type B + type AB) compared to non ischemic groups (group I and group II). Moreover, in subgroup analysis of patients with CAD, we found that, patients with higher vessel score had significantly higher Arg allele frequency and significantly higher severity score compared to patients with lower vessel score and these findings are more pronounced in patients with CAD and diabetes mellitus than patients with CAD alone.
In type 2 diabetic patients the functional role of paraoxonase is more important than in nondiabetic subjects. Chronic hyperglycemia causes considerable modification of protein structure and function due to nonenzymatic glycation of amino acid residues [35], and LDL cholesterol containing glycated apolipoprotein B100 interacts with vascular endothelium [36] and platelets, thereby increasing thromboxane production and decreasing thrombolytic prostaglandins [37]. In addition, glycated LDL cholesterol is more readily oxidized [38], resulting in acelerated macrophage uptake by the scavenger receptor pathway. It can therefore be speculated that, the protective effects of paraoxonase against peroxidation of LDL particles are more important in diabetic patients. The difference could explain the much clearer effect of the paraoxonase 192 genotype on CAD in type 2 diabetic patients than in general populations. This hypothesis is further supported by the results of Pfohl et al. [10] concerning subgroup analysis in current and former smokers, which supposed an even stronger association between the paraoxonase 192 Arg allele and CAD among smokers. In accordance with diabetes, smoking is an established risk factor for CAD in which oxidative mechanisms play an important role. Cigarette smoke contains large amounts of free radicals [39] and plasma antioxidative capacity is lower in smokers [40,41] so oxidized LDL particles from smokers generate more lipid peroxidation products than LDL from nonsmokers [42]. Thus it seems conceivable that, the paraoxonase activity has a more important role in smokers in protecting from the lipid peroxidation process in vivo. Plasma paraoxonase activity has recently been shown to be inhibited by cigarette smoke extract [43].
In the current study, and in multivariate logistic regression analysis of different variables for prediction of CAD, we found that, age, smoking, LDL cholesterol > 100 mg/dL, HDL cholesterol < 40 mg/dL and paraoxonase 192 Arg allele were significantly independent predictors of CAD. In accordance to our findings, similar data reported by Pfohl et al. [10] who postulated that, age, paraoxonase 192 Arg allele, history of smoking, hypertension, and hyperlipidemia were significantly associated with CAD. Furthermore they found that, factors significantly associated with three-vessel disease were age, paraoxonase 192 Arg allele, BMI, and hypertension. On the other hand, Odawara et al. [11] reported that, only PON1 genotype contributed to the pathogenesis of CAD in Japanese type 2 diabetic patients, independently of other known risk factors for CAD. Nevertheless, Lavi et al. [34] found that, in the multivariable model, only paraoxonase allelic variants remained a predictor of endothelial dysfunction.
PON1 protects against macrophage-mediated LDL oxidation, and increases HDL binding to macrophages which, in turn, stimulates HDL’s ability to promote cholesterol efflux [44]. Macrophage cholesterol accumulation and foam cell formation is the hallmark of early atherogenesis. In addition to macrophages, at least three more major players regulate atherosclerosis development; PON1, antioxidants, and HDL. These major anti-atherogenic properties of HDL (and of PON1) require, at least in part, macrophage binding sites for HDL-associated PON1 [44].
High-density lipoprotein (HDL) interrupts the process of atherogenesis at several key stages. HDL opposes atherosclerosis directly, by removing cholesterol from foam cells, by inhibiting the oxidation of LDLs, and by limiting the inflammatory processes that underlie atherosclerosis. Endogenous, purified, or reconstituted HDLs or HDL-associated lysosphingolipids (sphingosylphosphorylcholine or lysosulfatide), have been shown to inhibit the expression of E-selectin, ICAM-1, and VCAM-1 or other adhesion molecules by vascular endothelial cells exposed to cytokines. Furthermore, low HDL-cholesterol levels has been shown to correlate with elevated PAI-1, increased levels of fibrinogen and high index of platelet agreeability in humans. Accordingly HDL also has antithrombotic properties [45]. Indeed, PON1, as well as HDL-associated PON1, specifically binds to macrophages, leading to anti-atherogenic effects. Macrophage PON1 binding sites may thus be a target for future cardioprotection therapy. Studying the interactions among PON1, antioxidants, and macrophages can thus assist in achieving appropriate treatment and prevention of atherosclerosis [44].
Study Limitations: Although the number of patients analyzed was not large in our study, the highly significant results suggest an important role of the PON1 gene Gln192-Arg polymorphism in the pathogenesis of CAD but large scale studies are strongly recommended for more validation of the results. Another limitation we did not measure paraoxonase activity. However, doing so might not clarify the complex causal relationship between paraoxonase activity and early atherosclerosis. Furthermore, paraoxonase levels in serum might not correlate with lipoprotein-bound paraoxonase. Genetic associations are more likely to clarify the causal relationship.
7. CONCLUSION
Arg allele of PON1 192 gene polymorphism is an independent risk factor for CAD and it is associated not only with the presence of CAD but also with its extent and severity and its impact is clearly more pronounced in diabetic patients.
REFERENCES
- Navab, M., Berliner, J.A., Watson, A.D., Hama, S.Y., Territo, M.C., Lusis, A.J., Shih, D.M, Van Lenten, B.J., Frank, J.S., Demer, L.L., Edwards, P.A. and Fogelman, A.M. (1996) The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thrombosis and Vascular Biology, 16, 831-842. doi:10.1161/01.ATV.16.7.831
- Stamler, J., Vaccaro, O., Neaton, J.D. and Wentworth, D. (1993) The Multiple Risk Factor Intervention Trial Research Group. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care, 16, 434- 444. doi:10.2337/diacare.16.2.434
- Tas, S. (1991) Genetic predisposition to coronary heart disease and gene for apolipoprotein-CIII. Lancet, 337, 113-114. doi:10.1016/0140-6736(91)90770-P
- Ukkola, O., Savolainen, M.J., Salmela, P.I., von-Dickhoff, K. and Kesaniemi, Y.A. (1995) DNA poly-morphisms at the lipoprotein lipase gene are as-sociated with macroangiopathy in type 2 (non-insulin-dependent) diabetes mellitus. Atherosclerosis, 115, 99-105. doi:10.1016/0021-9150(94)05504-C
- Steinberg, D. and Witzum, J.L. (1990) Lipoproteins and atherogenesis. The Journal of American Medical Association, 264, 3047-3052. doi:10.1001/jama.1990.03450230083034
- Mackness, M.I., Arrol, S., Abbott, C. and Durrington, P.N. (1993) Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis, 104, 129-135. doi:10.1016/0021-9150(93)90183-U
- Mackness, M.I., Mackness, B., Durrington, P.N., Connelly, P.W. and Hegele, R.A. (1996) Paraoxonase: Biochemistry, genetics and relationship to plasma lipoproteins. Current Opinion in Lipidology, 7, 69-76. doi:10.1097/00041433-199604000-00004
- Humbert, R, Adler, D.A., Disteche, C.M., Hassett, C., Omiecinski, C.J. and Furlong, C.E. (1993) The molecular basis of the human serum paraoxonase activity polymorphism. Nature Genetics, 3, 73-76. doi:10.1038/ng0193-73
- Hassett, C., Richter, R.J., Humbert, R., Chapline, C., Crabb, J.W., Omiecinski, C.J. and Furlong, C.E. (1991) Characterization of cDNA clones encoding rabbit and human serum paraoxonase: The mature protein retains its signal sequence. Biochemistry, 30, 10141-10149. doi:10.1021/bi00106a010
- Pfohl, M., Koch, M., Enderle, M., Kühn, R., Füllhase, J., Karsch, K. and Häring, H. (1999) Paraoxonase 192 Gln/ Arg gene polymorphism, coronary artery disease, and myocardial infarction in type 2 diabetes. Diabetes, 48, 623- 627. doi:10.2337/diabetes.48.3.623
- Odawara, M., Tachi, Y. and Yamashita, K. (1997) Paraoxonase polymorphism (Gln192-Arg) is associated with coronary heart disease in Japanese noninsul-independent diabetes mellitus. Journal of Clinical Endocrinology Metabolism, 82, 2257-2260. doi:10.1210/jc.82.7.2257
- Serrato, M. and Marian, A.J. (1995) A variant of human paraoxonase/arylesterase (HUMPONA) gene is a risk factor for coronary artery disease. Journal of Clinical Investion, 96, 3005-3008. doi:10.1172/JCI118373
- Antikainen, M., Murtomaki, S., Syvanne, M., Pahlman, R., Tahvanainen, E., Jauhiainen, M., Frick, M.H., Ehnholm, C. (1996) The Gln-Arg191 polymorphism of the human paraoxonase gene (HUMPONA) is not associated with the risk of coronary artery disease in Finns. Journal of Clinical Investigation, 98, 883-885. doi:10.1172/JCI118869
- Suehiro, T., Nakauchi, Y., Yamamoto, M., Arii, K., Itoh, H., Hamashige, N. and Hashimoto, K. (1996) Paraoxonase gene polymorphism in Japanese subjects with coronary heart disease. International Journal of Cardiovascular, 57, 69-73. doi:10.1016/S0167-5273(96)02779-9
- Sanghera, D.K., Saha, N., Aston, C.E. and Kamboh, M.I. (1997) Genetic polymorphism of paraoxonase and the risk of coronary heart disease. Arterioscler Thrombosis and Vascular Biology, 17, 1067-1073. doi:10.1161/01.ATV.17.6.1067
- WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Report No 99.2. World Health Organization, Geneva, 1999.
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997). Diabetes Care, 20, 1183-1197.
- American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care, 28, S37- S42. doi:10.2337/diacare.28.suppl_1.S37
- Schiller, N.B., Shah, P.M., Crawford, M., DeMaria, A., Devereux, R., Feigenbaum, H., Gutgesell, H., Reichek, N., Sahn, D. and Schnittger, I. (1989) Recommendations for quantitation of the left ventricle by 2-dimensional echocardiography. Journal of American Society Echocardiography, 2, 358-367.
- Yvorchuk, K.J., Davies, R.A. and Chang, K.L. (1994) Measurement of left ventricular ejection fraction by acoustic quantification and comparison with radionuclide angiography. American Journal of Cardiology, 74, 1052- 1056. doi:10.1016/0002-9149(94)90858-3
- Reiber, J.H., Serruys, P.W., Kooijman, C.J., Wijns, W., Slager, C.J., Gerbrands, J.J., Schuurbiers, J.C., den Boer, A. and Hugenholtz, P.G. (1985) Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation, 71, 280-288. doi:10.1161/01.CIR.71.2.280
- Murk, M. (2007) Levinson: Leaning center for coronary angiography. The heart surgery forum. Cardio-Thoracic Multimedia Journal, 54, 534-539. doi:10.2337/diabetes.54.2.534
- Schulze, M.B., Shai, I. and Rimm, E.B. (2005) Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes, 54, 534-539.
- Wolk, R., Berger, P., Lennon, R.J., Brilakis, E.S. and Somers, V.K. (2003) Body mass index: A risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation, 108, 2206-2211.
- Friedewald, W.T., Levy, R.I. and Fredrickson, D.S. (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemical, 18, 499-502.
- Miller, S.A., Dykes, D.D. and Polesky, H.F. (1989) A simple salt-out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research, 16, 1215. doi:10.1093/nar/16.3.1215
- Adkin, S., Gan, K.N., Mody, M. and La Du, B.N. (1993) Molecular basis for the polymorphic forms of human serum paraoinase/arylesterase: Glutamine or arginin at position 192, for the respective A or B allozymes. American Journal of Human Genetics, 52, 598-608.
- Saunders, B.D. and Trapp, R.G. (1994) Basic and clinical biostatistics. 2nd Edition, Appleton & Lange, Norwalk.
- Maritim, A.C., Sanders, R.A. and Watkins, J.B. (2003) Diabetes, oxidative stress, and antioxidants: A review. Journal of Biochemical Toxicology, 17, 24-38. doi:10.1002/jbt.10058
- Flekac, M., Škrha, J., Zidkova, K., Lacinova, Z., Hilgertova, J. (2008) Paraoxonase 1 Gene polymor-phisms and enzyme activities in diabetes mellitus. Physiological Research, 57, 717-726.
- Ruiz, J., Blanche, H., James, R.W., Garin, M.C., Vaisse, C., Charpentier, G., Cohen, N., Morabia, A., Passa, P. and Froguel, P. (1995) Gln-Arg192 polymorphism of paraoxonase and coronary heart disease in type 2 diabetes. Lancet, 346, 869-872. doi:10.1016/S0140-6736(95)92709-3
- Jalilian, A., Javadi, E., Doosti, M., Amiri, P., Mohaghegh, A. and Shariati, B. (2008) Association between the severity of angiographic coronary artery disease and paraoxonase 1 promoter gene polymorphism T(–107) in iranian population. Acta Medica Iranica, 46, 197-202.
- James, R.W., Leviev, I., Ruiz, J., Passa, P., Froguel, P. and Garin, M.C. (2000) Promoter polymorphism T(–107)C of the paraoxonase PON1 gene is a risk factor for coronary heart disease in type 2 diabetic patients. Diabetes, 49, 1390- 1393. doi:10.2337/diabetes.49.8.1390
- Lavi, S., Mcconnell, J., Lavi, R., Barsness, G., Rihal, C., Novak, G., Lerman, L. and Lerman, A. (2008) Association between the paraoxonase-1 192Q > R allelic variant and coronary endothelial dysfunction in patients with early coronary artery disease. Mayo Clinical Proceeding, 83, 158-164. doi:10.4065/83.2.158
- Schwartz, C.J., Valente, A.J., Sprague, E.A., Kelley, J.L., Cayatte, A.J. and Rozek, M.M. (1992) Pathogenesis of the atherosclerotic lesion: Implications for diabetes mellitus. Circulation, 15, 1156-1167.
- Lyons, T.J., Li, W., Wells-Knecht, M.C. and Jokl, R. (1994) Toxicity of mildly modified low density lipoproteins to cultured retinal capillary endothelial cells and pericytes. Diabetes, 43, 1090-1095. doi:10.2337/diabetes.43.9.1090
- Watanabe, J., Wohltmann, H.J., Klein, R.L., Colwell, J.A. and Lopes-Virella, M.F. (1988) Enhancement of platelet aggregation by low-density lipoproteins from IDDM patients. Diabetes, 37, 1652-1657. doi:10.2337/diabetes.37.12.1652
- Kobayashi, K., Watanabe, J., Umeda, F. and Nawata, H. (1995) Glycation accelerates the oxidation of low density lipoprotein by copper ions. Endocrine Journal, 42, 461- 465. doi:10.1507/endocrj.42.461
- Church, D.F. and Pryor, W.A. (1985) The free radical chemistry of cigarette smoke and toxicological implications. Environmental Health Perspectives, 64, 111-126.
- Chow, C.K., Thacker, R.R., Changcit, C., Bridges, R.B., Rehm, S.R., Humble, J. and Turbe, K.J. (1986) Lower levels of vitamin C and carotenes in plasma of cigarette smokers. Journal of the American College Nutrition, 5, 305-312.
- Princen, H.M.G., van Poppel, G., Vogelezang, C., Buytenhek, R. and Kok, F.J. (1992) Supplementation with vitamin E but not b-carotene in vivo protects low density lipoprotein from lipid peroxidation in vitro: Effect of cigarette smoking. Arteriosclerosis Thrombosis, 12, 554-562. doi:10.1161/01.ATV.12.5.554
- Scheffler, E., Wiest, E., Woehrle, J., Otto, I., Schulz, I., Huber, L., Ziegler, R. and Dresel, H.A. (1992) Smoking influences the atherogenic potential of low-density lipoprotein. Clinical & Investigative Medicine, 70, 263-268.
- Nishio, E. and Watanabe, Y. (1997) Cigarette smoke extract inhibits plasma paraoxonase activity by modification of the enzyme’s free thiols. Biochemical and Biophysical Research Communications, 236, 289-293.
- Efrat, M. and Aviram, M. (2010) Paraoxonase 1 interactions with HDL, antioxidants and macrophages regulate atherogenesis—A protective role for HDL phospholipids. Advances in Experimental Medicine and Biology, 660, 153-166. doi:10.1007/978-1-60761-350-3_14
- Barter, P. (2005) The role of HDL-cholesterol in preventing atherosclerotic disease. European Heart Journal, 7, F4-F8. doi:10.1093/eurheartj/sui036

