Open Journal of Animal Sciences
Vol. 2 No. 4 (2012) , Article ID: 23961 , 9 pages DOI:10.4236/ojas.2012.24034
Murine embryo development following cytoplasmic injection of linear and condensed DNA
![]()
1Department of Dairy Science, Virginia Polytechnic Institute, State University, Blacksburg, USA; *Corresponding Author: guaz@vt.edu
2Department of Chemical Engineering, University of Nebraska, Lincoln, USA
Received 25 May 2012; revised 27 June 2012; accepted 5 July 2012
Keywords: Linear DNA; Condensed DNA; Mice; Cytoplasmic Injection
ABSTRACT
In 1985 Brinster et al. [1] observed that linearized DNA injected in the cytoplasm of mouse zygotes underwent spontaneous supercoiling within 24 h. This finding suggests that DNA prefers and functions best in tertiary structure. In an effort to improve the efficiency of transgenesis by cytoplasmic injection, DNA was condensed with MgCl2 to form a three dimensional rod-shaped DNA prior to injection in pronuclear stage murine zygotes. The DNA used was enhanced green fluorescent protein on a cytomegalovirus promoter (CMV-EGFP) and served as a marker for gene integration and protein expression in culture conditions. The condensed CMV-EGFP construct was injected in the cytoplasm at 3 concentrations (100, n = 816; 425, n = 464; and 625 µg/ml, n = 708). For comparison linear CMV-EGFP construct was injected into the pronucleus (5 µg/ml, n = 196) and into the cytoplasm (625 µg/ml, n = 628). In all treatment groups the control and buffer injected embryos developed similarly. Among DNA treatment groups, the highest development of fluorescing embryos was observed in zygotes injected in the cytoplasm with linear CMV-EGFP (625 µg/ml); however, zygotes injected in the cytoplasm with condensed CMV-EGFP (625 µg/ml) had the highest percentage (44%) of fluorescing embryos, the highest percentage (16.7%) of fluorescing morula and blastocysts, and the lowest percentage of fluorescence mosaicism at every stage of embryo development after 4 d in culture; thereby making it the best method for generating transgenic animals.
1. INTRODUCTION
Page et al. [2], attempted to improve the rate of transgenesis by cytoplasmic injection by condensation of DNA with polylysine prior to injection into pronuclear stage mouse zygotes. Their efforts resulted in a transgenesis rate of 12.8% of the live animals tested. This certainly was an improvement over the 3.4% rate of transgenesis reported by Brinster et al. [1]. However, the new technique was demanding. Polylysine is a very large molecule and the large DNA aggregates frequently obstructed the microinjection needle pore (standard < 1 µm). Thus, a larger needle pore (>1 µm) became necessary to accommodate the transfer of the condensed DNA. The larger needle pore made the tip of the needle blunt by normal pore size comparison and microinjecting DNA with the larger needle reduced the survival of the embryos compared to microinjection of non-condensed DNA [2].
Complexing DNA with a smaller cation prior to cytoplasmic injection might greatly improve embryo survival because a smaller molecule would allow a smaller pore on the tip of injection needle. Monovalent cations do not condense DNA. Divalent cations require the addition of alcohol to facilitate greater cation-DNA interaction in an aqueous environment [3]. An added bonus to this process is that unlike condensation with poly-cations, aggregation is typically not observed when condensing with divalent cations [4]; fewer and smaller aggregates pass more easily through the injection needle into the cytoplasm. Greater embryo survival following cytoplasmic injection may result in an overall higher rate of transgenesis.
The objective of this study was to quantify the developmental effects of cytoplasmic injection of CMV-EGFP condensed with MgCl2.
2. MATERIALS & METHODS
2.1. DNA Preparation
The cytomegalovirus-enhanced green fluorescent protein (CMV-EGFP) construct was purchased already cloned into pIRES plasmid (Clonetech Laboratories, Palo Alto, CA, USA). The plasmid construct contained the CMVIE (immediate early) promoter (700 bp), followed by the multiple cloning site (MCS, 65 bp), the internal ribosomal entry site (IRES, 702 bp), the intervening sequence (IVS, 211 bp), and the EGFP complimentary DNA (cDNA, 1022 bp, including the polyadenylation sequence). Isolation and purification of the gene construct followed the general guidelines described in Chauhan et al. [5]. Isolation began with digestion of the plasmid overnight at 37.5˚C in 1× Universal Buffer (Stratagene, La Jolla, CA, USA) with endonuclease BamHI (Stratagene) to remove the IRES, IVS and a portion of the MCS. The restriction products were separated on a 0.8% agarose gel. The vector containing fragment was purified by Ultra Clean15 Kit, re-ligated with T4 DNA ligase (Stratagene) per manufacturer’s instructions, and used to transform E. coli XL-Blu competent cells (Stratagene) per manufacturer’s instructions which were then streaked on agar plates with Luria Broth and ampicillin (50 mg/L). Plasmid containing colonies were selected and cultivated in Terrific Broth (Gibco BRL, Carlsbad, CA, USA) with amplicillin (50 mg/L) over several days and several steps. The plasmid was then isolated and purified by column absorption (Qiagen Plasmid Maxi Kit; Qiagen Inc., Valencia, CA, USA). Next the plasmid was digested overnight at 37.5˚C in 1× Universal Buffer with NruI (Promega, Madison, WI, USA) and XhoI (Promega). The final construct which contained CMVIE promoter, followed by the truncated MCS (30 bp), and the intact EGFP cDNA. The total construct size was 1.752 kbp and had one sticky overhanging end left by the XhoI cut and one blunt end left by the NruI cut. The gene construct was isolated from a nondenaturing 1% agarose gel and purified by the UltraCleanTM15 DNA Purification Kit (Agarose Gels and Solutions, MoBio Laboratories Inc., Carlsbad, CA, USA). The purified construct was reconstituted in sterile deionized water. The DNA concentration was determined by photospectroscopy at 260 nm. The DNA was diluted to 5 and 625 µg/ml in intra-physiological saline (IPS) buffer comprised of 10 mM Tris HCl and 0.25 mM EDTA. Both diluted and undiluted DNA was stored at –20˚C until use.
2.2. DNA Condensation
The EGFP construct was condensed with MgCl2 according to a protocol developed by S. Butler (personal communication) to form rod shaped condensates [6] for cytoplasmic injection. The condensing medium contained 8 mM MgCl2, 10 mM Tris buffer, 40% deionized H2O, and 60% isopropyl alcohol. Condensation occurred during centrifugation at 15,000 × g for 45 min at room temperature. The DNA condensates were re-suspended overnight at 4˚C in injection medium which was intra-physiological saline (IPS) buffer containing 160 mM KCl, 20 mM MgCl2, and 5 mM NaCl at pH 7 (Butler, personal communication). Condensates were identified by dynamic light-scattering technology. The concentration was identified by photospectroscopy at 260 nm and then diluted with injection buffer to three concentrations for cytoplasmic injection: 100, 425, and 625 µg/ml. Condensates were then stored in injection buffer at 4˚C up to 7 d prior to use, or at –80˚C for later use.
2.3. Embryo Collection
Embryos were generated by superovulation, harvested, and cultured according to guidelines set forth by Hogan et al. [7]. Weaned female mice (strain CD-1) between 25 and 35 d of age, and weighing 19.5 to 22.5 g were administered 10 IU of equine chorionic gonadotropin (eCG, Diosynth, Chicago, IL, USA) intraperotineally (i.p.). These females were administered 5 IU human chorionic gonadotropin (hCG, Sigma Aldrich Chemical Company, St. Louis, MO, USA) i.p. 46 to 48 h later. The females were individually placed in cages containing a single male and 21 to 24 h later were examined for vaginal plugs, euthanized by cervical dislocation, and had their ovaries and oviducts removed for embryo harvesting. Embryos were collected at the cumulus stage and placed into 37.5˚C M2 medium (Specialty Media, Phillipsburg, NJ, USA) at pH of 7.4 [7]. The cumulus cells were dissociated from the embryos in M2 medium containing 1 mg hyaluronidase (320 IU/mg; Sigma Aldrich Chemical Company). All procedures were approved by the Animal Care & Use Committee.
2.4. Pronuclear Injection
Pronuclear microinjection was performed according to guidelines described by Canseco et al. [8]. Embryos were loaded into the injection chamber, a 100 mm Becton Dickinson Petri dish (Falcon, Franklin Lakes, NJ, USA) containing a narrow, centered droplet of M2 medium at 37.5˚C that was gently layered with mineral oil (Specialty Media) to reduce embryo exposure by media evaporation and temperature flux. Embryos were visualized and the male pronucleus was injected with 1 to 4 pl of DNA at 5 µg/ml. Following manipulation, the surviving embryos were cultured 4 d at 37.5˚C in 5% CO2 equilibrated CZB medium [9].
2.5. Cytoplasmic Injection
The injection chamber used for cytoplasmic injection was the same design as the one used for pronuclear injections. For cytoplasmic injection, the mouse embryos were loaded into the injection chamber, visualized, and injected in the cytoplasm, avoiding the pronuclear area. The volume of DNA injected was 10 to 15 pl [2] and the copy number injected ranged between 3750 and 5652 (Butler, personal communication). The murine embryos received one of six treatments: no injection, IPS injection buffer, 625 µg/ml linear CMV-EGFP in IPS buffer, or condensed CMV-EGFP in IPS with 160 mM KCl, 20 mM MgCl2, and 5 mM NaCl at 100, 425 or 625 µg/ml condensed CMV-EGFP. Following manipulation, surviving embryos were cultured 4 d at 37.5˚C in 5% CO2 equilibrated CZB medium [9].
2.6. In Vitro Embryo Development
Embryo development was assessed on d 4 and given a numerical score between one and four. Degenerated and lysed embryos were assigned the numerical score 1; one-cell and two-cell embryos were assigned the numerical score 2; three-cell to ten-cell embryos were assigned the numerical score 3; and embryos that progressed to the morula through blastocyst stages were assigned the numerical score 4. Data on embryos that received no injection were used to establish optimum development in the culture system. Embryos injected with IPS buffer were analyzed to establish the mechanical effects of the injection technique on embryo development.
2.7. Embryo Fluorescence
Embryos in the pronuclear injection study were evaluated for protein expression on d 4 as a percentage at each stage of development. Embryos in the cytoplasmic injection study were evaluated for protein expression as evidenced by fluorescence 4 d post cytoplasmic injection of CMV-EGFP. Fluorescing embryos were assigned a numerical score based on their stage of development, the same score design used to assess in vitro embryo development. Differences in embryo fluorescence between the treatment groups were then assessed as the percentage of embryos that showed protein activity in each treatment group and in the average development of the embryos that exhibited fluorescence across the treatment groups. To induce fluorescence, all embryos were subjected to several seconds of blue light at 488 nm, the excitation maximum for EGFP. Green fluorescent emission was examined in darkness at a wavelength of 508 nm (Nikon Filter set #EF-4 FITC HYQ, 480/(40) and 535/(50); Nikon Corporation, Tokyo, Japan).
2.8. Fluorescence Mosaicism
On d 4 embryos were also evaluated for mosaicism. Mosaic embryos did not exhibit fluorescence in every cell. Embryos with expression in every cell were identified as non-mosaic and were assigned the numerical score 0. Embryos with fluorescence in only some cells were assigned the numerical score 1. Mosaicism was evaluated by treatment and stage of development per treatment for both the pronuclear and cytoplasmic injection studies, and for stage of development across treatments for the cytoplasmic injection study. Data are expressed as least squares means.
2.9. Statistics
On d 4 embryos were evaluated and data collected for development, presence of fluorescence, and prevalence of fluorescence among blastomeres. Data were analyzed using one-way analysis of variance with SAS [10] or GraphPad Prism version 4.00 for Windows with Tukey's Multiple Comparison post test (GraphPad Prism, GraphPad Software, San Diego, CA, USA). Statistical models included treatment and residual effects. Bartlett’s statistic was used to test for equal variances.
3. RESULTS
3.1. Embryo Development in Vitro
Thirty-seven percent of pronuclear injected embryos lysed prior to culture. The process of pronuclear injection had a significant negative impact on embryo development (Table 1). Embryos that received no pronuclear injection had the highest (P < 0.05) mean development. Embryos injected into the pronucleus with either buffer or 5 µg/ml CMV-EGFP had similar (P > 0.05) development. Specific notation in numbers and percentages of embryos at each stage of development are in Table 2.
The in vitro evaluation of cytoplasmic injection as a means of producing transgenic mice included 3358 test zygotes. Three thousand and fifty three (90.9%) embryos were injected with either buffer or CMV-EGFP (condensed or linear) and the rest received the minimal manipulation and culture for 4 d. Of those injected, 62.8% lysed immediately (Table 2) and were discarded.
Control embryos totaled 305, of which 100% went into culture (Table 2). On d 4, the control group had the lowest percentage of degenerated and lysed embryos (Table 3) and the highest percentage to maximally develop to the morula to hatched blastocyst stages. The least squares mean development score of the d 4 control embryos was not different (P > 0.05) from the mean development score of the buffer treated embryos, which also had approximately 60% embryo development to the morula and hatched blastocyst stage.
Embryos in the group of zygotes injected in the cytoplasm with the transgene in linear form (625 µg/ml) had a low rate of degeneration and lysing (10.1%) and one of
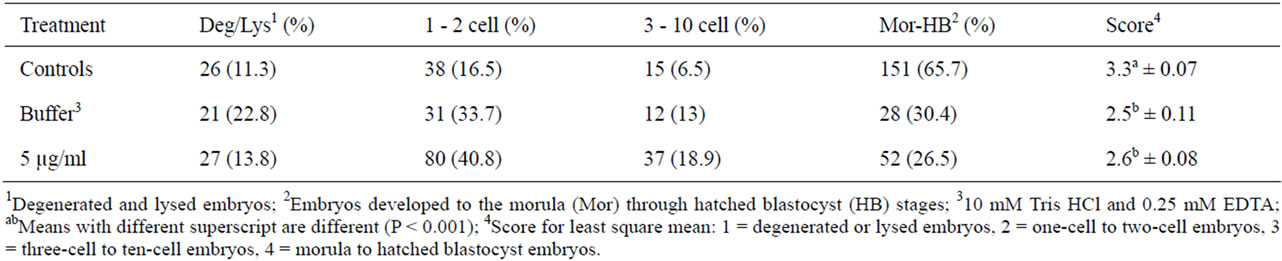
Table 1. Distribution of embryo development 4 d following manipulation or pronuclear injection.
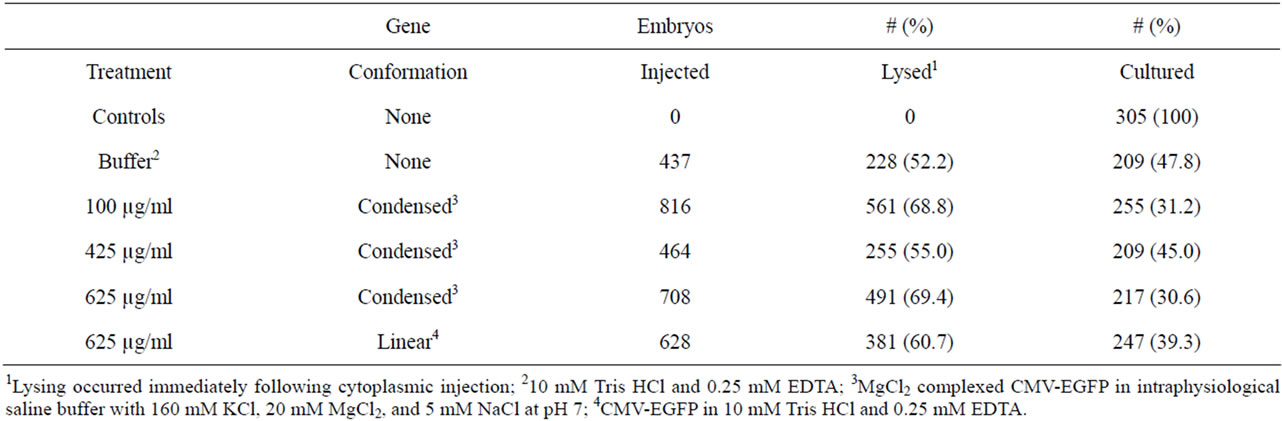
Table 2. Embryo lysing following cytoplasmic injections of CMV-EGFP.
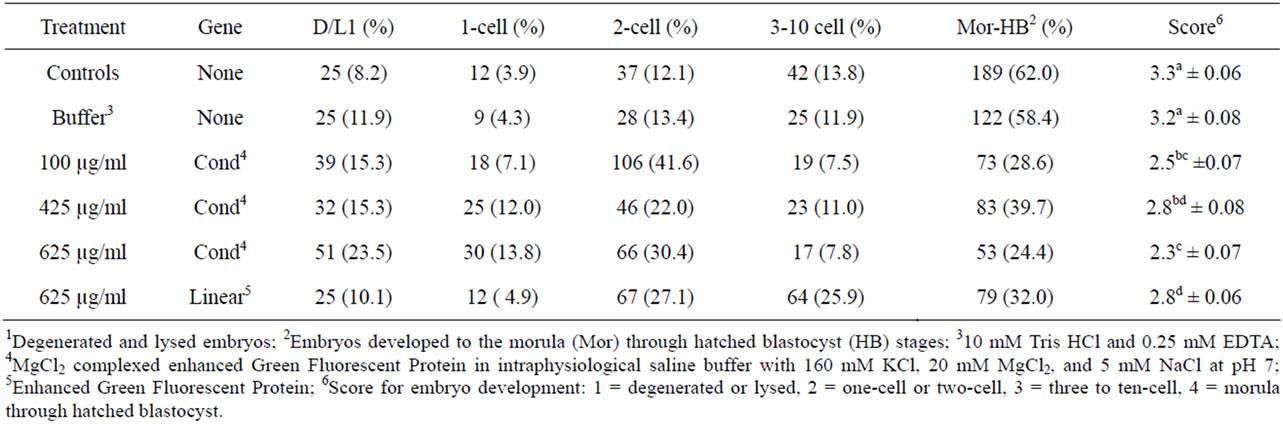
Table 3. Distribution of embryo development 4 d following manipulation or cytoplasmic injection.
the highest percentages to develop to the morula to hatched blastocyst stage (32.0%, Table 3). The development score for this group was 2.8 ± 0.06, the highest of all the groups injected cytoplasmically. Similar mean embryo development was observed in the zygotes injected in the cytoplasm with 425 µg/ml condensed CMV-EGFP (2.8 ± 0.08). The embryos cytoplasmically injected with 625 µg/ml condensed CMV-EGFP had the highest rate of degeneration and lysing (23.5%) and the lowest percentage of embryos develop to the morula to hatched blastocyst stage (24.4%). The development score for this group was 2.3 ± 0.07, the lowest of all the groups injected. The groups of embryos injected with 100 and 425 µg/ml condensed CMV-EGFP had similar (P > 0.05) development scores (2.5 ± 0.07 and 2.8 ± 0.08, respectively) between the two-cell and three-cell to ten-cell stages.
3.2. Embryo Fluorescence
Forty percent of the pronuclear injected zygotes (79/196) exhibited fluorescence at 4 d in culture (Table 4). As the embryos in this treatment group developed, protein expression became more variable ending with 38%, 19%, and 25% non-mosaicism across the latter three stages of growth and statistically there was no difference between them (P > 0.05).
The zygotes injected in the cytoplasm with CMVEGFP, whether linear or condensed, exhibited the highest percentages of fluorescence in the one-cell embryos (56.5%). The lowest fluorescence was exhibited in morula and blastocysts (11.5%, Table 5). A calculation (total number of fluorescing embryos/total number of cultured embryos) revealed that only 26.9% of all zygotes injected in the cytoplasm with the DNA exhibited fluorescence, of those only 13.2% reached the highest stage of maturity.
Conversely, examination between the treatment groups and across all five stages of development, revealed the highest percentage of fluorescing embryos were in the zygotes injected in the cytoplasm with 625 µg/ml condensed CMV-EGFP (44.2%, Table 6), of which 16.7%, the highest among cytoplasmic injected zygotes, were morula and blastocysts (Table 7). The percentage of fluorescing embryos from cytoplasmic injection of 425 µg/ml condensed CMV-EGFP was similar (15.2%, Table 7) to that of zygotes injected in the cytoplasm with 625 µg/ml condensed CMV-EGFP; however, that number is skewed because the overall percentage of fluorescing embryos in that group was only 15.8% (Table 6).

Table 4. Embryo fluorescence and degree of mosaicism at five stages of development 4 d following pronuclear injection with CMV-EGFP (5 µg/ml).
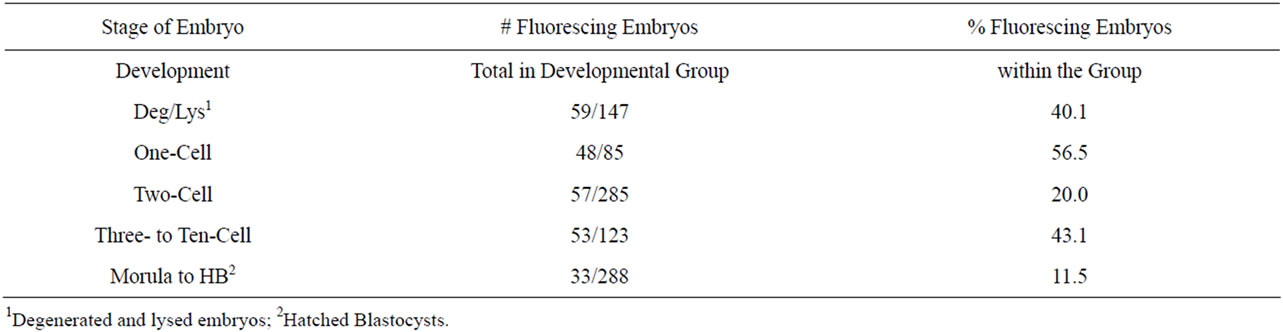
Table 5. Fluorescence in embryos across treatments at all five stages of development 4 d following cytoplasmic injection with CMV-EGFP, both linear and condensed.

Table 6. Fluorescence at each stage of embryo development within treatment groups.
Zygotes injected into the cytoplasm with 625 µg/ml linear construct and 100 µg/ml condensed construct had the two the lowest total percentages of fluorescing embryos (24.3% and 23.6%, respectively, Table 6). Zygotes injected into the cytoplasm with 625 µg/ml linear construct also produced the lowest percentage of fluorescing morula and blastocysts (6.7%, Table 7). Similarly this treatment group had the lowest percentage of fluorescing degenerated and lysed embryos (11.7%), and the highest development score of fluorescing embryos across treatments (2.6 ± 0.10, Table 6) with 53.3% development to the three-cell to ten-cell stage (Table 7).
All three groups of zygotes injected in the cytoplasm with condensed CMV-EGFP had low mean development among fluorescing embryos which was lower (P < 0.05) than development among fluorescing embryos injected with linear DNA (Table 6). That means the biggest differences between the groups are observed in the highest overall percentage of fluorescing embryos, and in the highest percentage of maximally developed fluorescing embryos, both of which are seen in the group of zygotes injected into the cytoplasm with 625 µg/ml condensed CMV-EGFP.
Figure 1 is visual depiction of the differences and similarities between treatment groups of fluorescing embryos. Note the zygotes injected into the pronucleus with linear CMV-EGFP (5 µg/ml) had the lowest average development of fluorescing embryos.
3.3. Fluorescence Mosaicism
As a group, fluorescing embryos injected at the highest concentration of condensed EGFP had the lowest rate (14%) of mosaicism, whereas fluorescing embryos in the other three injection groups had 25% to 62% mosaic expression of the transgene (Table 8). By breaking the fluorescent embryos into developmental groups in Table 9, it became clear that mosaicism was more prevalent in the higher developed embryos. Summarizing the other data findings in Table 9: the highest concentration DNA injected in linear form had no mosaicism in degenerated and lysed embryos; the 425 µg/ml concentration of DNA injected cytoplasmically had absolute mosaicism beyond the two-cell stage and high mosaicism throughout; and pronuclear microinjection as a technique for delivering DNA is not the best choice for obtaining homogenous cell populations within embryos. The best choice for obtaining low mosaicism in embryos is cytoplasmic injection of 625 µg/ml of CMV-EGFP condensed with MgCl2.
4. DISCUSSION
Across cytoplasmic injection treatments there was 61.5% lysis of zygotes, and 38.8% for pronuclear injections. In both events the immediacy of embryo demise following cytoplasmic and pronuclear injection indicated lysis was a direct result of the mechanics of the injection
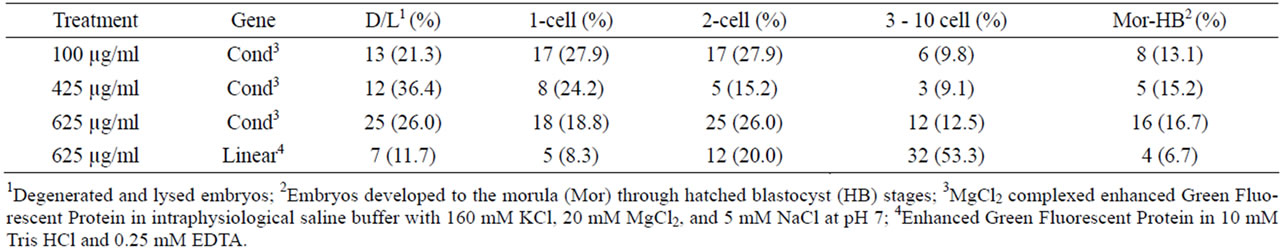
Table 7. Fluorescence within treatment groups at each stage of embryo development.

Figure 1. Range of fluorescing embryo development for each injection treatment. M to HB = Morula to hatched blastocyst; 3 to 10 cell embryos; 2-cell embryos; 1-cell embryos; Deg/Lys = Degenerated or lysed; PNM = Pronuclear microinjection; CI = Cytoplasmic injection; 100, 425, 625 = concentration DNA in µg/ml; − = linear, + = condensed DNA.
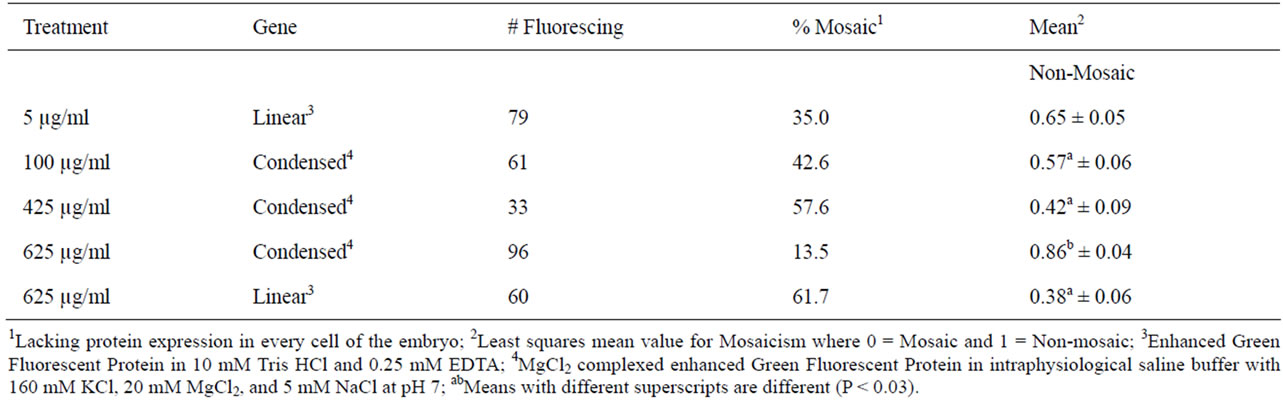
Table 8. Mosaicism of CMV-EGFP expression 4 d following microinjection.
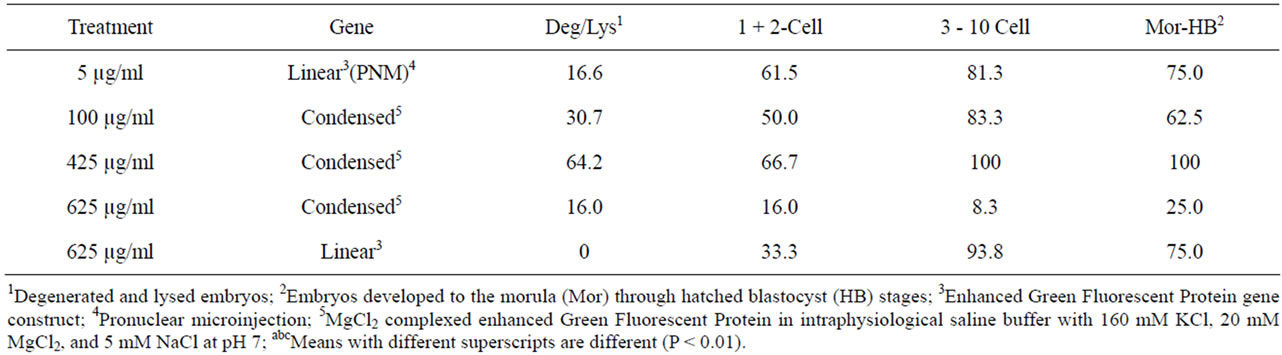
Table 9. Percent mosaic expression of EGFP at each stage of embryo development within treatments.
process. Demise of zygotes from the damage inflicted during microinjection is a common occurrence across species; including murine [11,12] and cattle [5,11] embryos.
Among the surviving zygotes, there was a similarity in development of the control and buffer injected embryos 4 d following pronuclear and cytoplasmic injection, indicating the injection buffer and injection volume did not hinder post-injection embryo growth, a finding that supports the literature [1,2,12]. The contrasting embryo development 4 d following cytoplasmic injection with DNA may result from the varying concentrations and conformations of the CMV-EGFP gene construct. Embryo loss occurs at various developmental stages following pronuclear and cytoplasmic injections of DNA [13]. Brinster et al. [1] reported it was the pronuclear microinjection of DNA that hindered maturation of embryos, not the injection process. Furthermore, loss of injected zygotes occurs during earliest embryo development [14]. Schmotzer et al. [12] reported similar findings for cytoplasmic injection of DNA. These results were repeated for intra-cytoplasmic sperm injection mediated transgenesis [15-17]. In particular, Szczygiel et al. [16] reported the incubation of spermatozoa with 5 µg/ml GFP lead to chromosomal damage in the fertilizing sperm that ultimately arrested pre-implantation murine embryo development.
All groups of zygotes injected with DNA had d 4 embryo development greater than two-cell, but less than 10-cell. The zygotes injected in the cytoplasm with 625 µg/ml of CMV-EGFP complexed with MgCl2 had the lowest development with only 24.4% development to morula and blastocysts. The group with the highest percentage of morula and blastocysts was injected into the cytoplasm with 425 µg/ml condensed CMV-EGFP, an indication that this treatment group may produce more fetuses. Previous research suggested the lethality of DNA is concentration dependent; the higher the concentration of DNA, the higher the rate of murine embryonic death [2,18].
Volgina et al. [19] reported the presence of Ca, Mgdependent endonucleases in cell nuclei of organs and tissues of many species. Szczygiel et al. [16] reported that most endonucleases are dependent on Ca and/or Mg ions. Increasing Mg2+ in the cytoplasm and nucleus of zygotes may increase chromosomal breakage. An increase in chromosomal breakage would facilitate transgene integration, particularly in the presence of high exogenous DNA copy numbers. Embryos injected with only IPS buffer developed similarly to control embryos following cytoplasmic injections supporting these theories (Table 3).
The PCR of embryos reveals a higher detection of transgenes than does tissue from offspring [20,21]. The opposite is also true. When PCR is applied to embryo biopsies for the purpose of screening positive embryos for transplant, as in the case of mosaic embryos, it is possible to miss a positive embryo if it expresses the transgene in a mosaic fashion [14]. Furthermore, they noted that PCR of embryos is not sensitive enough to distinguish integrated from non-integrated DNA. For these reasons, and because visual screening is easier and less intrusive of embryos than blastomere biopsying and PCR, this study used EGFP expression as the screening method for detecting transgenesis in injected embryos; the expression of which in cultured embryos correlates to incidence of GFP transgenics in mice [22,23].
Although DNA microinjection may inhibit embryo development, it does not inhibit DNA transcription, mRNA translation, and protein expression [24]. The zygotes injected into the cytoplasm with 625 µg/ml condensed CMV-EGFP had 44.2% of embryos exhibit fluorescence on d 4 in culture, followed closely by the zygotes injected in the pronucleus with 40%. Across treatment groups, the largest percentage of fluorescing embryos were one-cell and two-cell and the smallest percentage were morula and blastocysts. Iqbal et al. [11] injected covalently closed circular plasmids with a cDNA encoding EGFP (10 µg/ml) into the cytoplasm of murine zygotes evoking expression in one-cell embryos at approximately 12 hour post-injection. All of this is feasible because in the mouse embryo, transcriptional activity begins during S/G2 phase of the first cell cycle, prior to the joining of the male and female pronucleus; and it is the male pronucleus that exhibits greater transcriptional activity [25].
Thirty-five percent of pronuclear injected zygotes grew to exhibit mosaic CMV-EGFP expression in embryos after 4 d in culture. Among the cytoplasmic injection treatment groups, mosaicism was highest (61.7%) in the group that received 625 µg/ml linear CMV-EGFP and lowest (14.6%) in the group that received 625 µg/ml condensed CMV-EGFP. Low levels of mosaicism were seen at every stage of development for these embryos, ranging from 8.3% to 25%. Transgenic mosaicism was observed initially [26] when the offspring of transgenic mice were born non-transgenic. It was reported many times since then [20,21,27]. Schmotzer [12] reported 71% and Whitelaw et al. [28] reported 76% mosaicism in pronuclear injected murine embryos. Mosaic expression of GFP in bovine embryos was both low [5] and quite high at 79% [29]. Mosaic integration of transgenes from slow, post cleavage gene integration [20] or from proposed gene silencing [5] is common. Mosaicism is not necessarily a problem. A mosaic embryo can still form a transgenic animal with appropriate protein expression that is heritable, even though the animal does not carry the transgene in every cell. However, because a portion of the blastomeres develop into placenta, it is possible to have a normal fetus and a transgenic placenta [8].
In conclusion, among DNA treatment groups, the highest development of fluorescing embryos was observed in zygotes injected into the cytoplasm with linear CMV-EGFP (625 µg/ml); however, zygotes injected in the cytoplasm with condensed CMV-EGFP (625 µg/ml) had the highest percentage (44%) of fluorescing embryos, the highest percentage (16.7%) of fluorescing morula and blastocysts, and the lowest percentage of fluorescence mosaicism; thereby making it the best method for generating transgenic animals.
REFERENCES
- Brinster, R.L., Howard, Y.C., Trumbauer, M.E., Yagle, M.K. and Palmiter, R.D. (1985) Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proceedings of the National Academy of Sciences, 82, 4438-4442. doi:10.1073/pnas.82.13.4438
- Page, R.L., Butler, S.P., Subramanian, A., Gwazdauskas, F.C., Johnson, J.L. and Velander, W.H. (1995) Transgenesis in mice by cytoplasmic injection of polylysine/DNA mixtures. Transgenic Research, 4, 353-360. doi:10.1007/BF01973753
- Widom, J. and Baldwin, R.L. (1980) Cation-induced toroidal condensation of DNA. Journal of Molecular Biology, 144, 431-453. doi:10.1016/0022-2836(80)90330-7
- Ma, C. and Bloomfield, V.A. (1994) Condensation of supercoiled DNA induced by MnCl2. Biophysical Journal, 67, 1678-1681. doi:10.1016/S0006-3495(94)80641-1
- Chauhan, M.S., Nadir, S., Bailey, T.L., Pryor, A.W., Butler, S.P., Notter, D.R., Velander, W. H. and Gwazdauskas, F.C. (1999) Bovine follicular dynamics, oocyte recovery, and development of oocytes microinjected with a green fluorescent protein construct. Journal of Dairy Science, 82, 918-926. doi:10.3168/jds.S0022-0302(99)75310-5
- Bloomfield, V.A. (1991) Condensation of DNA by multivalent cations: Considerations on mechanism. Biopolymers, 31, 1471-1481. doi:10.1002/bip.360311305
- Hogan, B., Costantini, F. and Lacy, E. (1986). Manipulating the mouse embryo. A laboratory manual. Cold Spring Harbor Laboratory Press, New York.
- Canseco, R.S., Sparks, A.E.T., Page, R.L., Russell, C.G., Johnson, J.L., Velander, W.H., Pearson, R.E., Drohan, W.N. and Gwazdauskas, F.C. (1994) Gene transfer efficiency during gestation and the influence of co-transfer of non-manipulated embryos on production of transgenic mice. Transgenic Research, 3, 20-25. doi:10.1007/BF01976023
- Chatot, C.L., Ziomek, C.A., Bavister, B.D., Lewis, J.L. and Torres, I. (1989) An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. Journal of Reproduction and Fertilty, 86, 679-688. doi:10.1530/jrf.0.0860679
- SAS Institute, Inc. (1985) SAS user’s guide: Statistics. Cary, NC, USA.
- Iqbal, K., Barg-Kues, B., Broll, S., Bode, J., Niemann, H. and Hues, W.A. (2009) Cytoplasmic injection of circular plasmids allows targeted expression in mammalian embryos. BioTechniques, 47, 959-968. doi:10.2144/000113270
- Schmotzer, C.A., Butler, S.P., Pearson, R.E., Velander, W.H. and Gwazdauskas, F.C. (2003) Assessment of murine embryo development following cytoplasmic microinjection of condensed DNA into murine embryos using electropulsation. Transgenics, 4, 55-63.
- Yamauchi, Y., Doe, B., Ajduk, A. and Ward, M.A. (2007) Genomic DNA damage in mouse transgenesis. Biology of Reproduction, 77, 803-812. doi:10.1095/biolreprod.107.063040
- Page, R.L., Canseco, R.S., Russell, C.G., Johnson, J.L., Velander, W.H. and Gwazdauskas, F.C. (1995) Transgene detection during early embryonic development after pronuclear microinjection. Transgenic Research, 4, 12-17. doi:10.1007/BF01976496
- Perry, A.C., Wakayama, T., Kishikawa, H., Kasai, T., Okabe, M., Toyoda, Y. and Yanag-imachi, R. (1999) Mammalian transgenesis by intracytoplasmic sperm injection. Science, 284, 1180-1183. doi:10.1126/science.284.5417.1180
- Szczygiel, M.A., Kusakabe, H., Yanagimachi, R. and Whittingham, D.G. (2002) Intracytoplasmic sperm injection is more efficient than in vitro fertilization for generating mouse embryos from cryopreserved spermatozoa. Biology of Reproduction, 67, 1278-1284. doi:10.1095/biolreprod67.4.1278
- Szczygiel, M.A., Moisyadi, S. and Ward, W.S. (2003) Expression of foreign DNA is associated with paternal chromosome degradation in intracytoplasmic sperm injection-mediated transgenesis in the mouse. Biology of Reproduction, 68, 1902-1910.
- Covarrubias, L., Nishida, Y. and Mintz, B. (1986) Early postimplantation embryo lethality due to DNA rearrangements in transgenic mouse strain. Proceedings of the National Academy of Sciences USA, 83, 6020-6024. doi:10.1073/pnas.83.16.6020
- Volgina, V.V., Khodarew, N.N., Volgin, A.Y., Votrin, I.I. and Pevnitskii, L.A. (1988) Comparative study of Ca, Mg-dependent endonuclease in cell nuclei by the use of monoclonal antibodies. Bulletin of Experimental Biology and Medicine, 105, 498-500. doi:10.1007/BF00841185
- Burdon, T.G. and Wall, R.J. (1992) Fate of microinjected genes in preimplantation mouse embryos. Molecular Reproduction and Development, 33, 436-442. doi:10.1002/mrd.1080330410
- Cousens, C., Carver, A.S., Wilmut, I., Colman, A., Garner, I. and O’Neil, G.T. (1994) Use of PCR-based methods for selection of integrated transgenes in preimplantation embryos. Molecular Reproduction and Development, 39, 384-391. doi:10.1002/mrd.1080390406
- Ikawa, M., Kominami, K., Yoshimura, Y., Tanaka, K., Nishimune, Y. and Okabe, M. (1995) A rapid and noninvasive selection of transgenic embryos before implantation using green fluorescent protein (GFP). FEBS Letters, 375, 125-128. doi:10.1016/0014-5793(95)01162-8
- Krisher, R.L., Gibbons, J. R., Canseco, R.S., Johnson, J.L., Russell, C.G., Notter, D.R., Velander, W.H. and Gwazdauskas, F.C. (1994) Influence of time of gene microinjection on development and DNA detection frequency in bovine embryos. Transgenic Research, 3, 226-231. doi:10.1007/BF02336775
- Adenot, P.G., Mercier, Y., Renard, J. and Thompson, E.M. (1997) Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development, 124, 4615-4625.
- Wu, G.Y. and Wu, C.H. (1987) Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. Journal of Biological Chemistry, 262, 4429-4432.
- Palmiter, R.D., Wilkie, T.M., Chen, H.Y. and Brinster, R.L. (1984) Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell, 36, 869-877. doi:10.1016/0092-8674(84)90036-9
- Wall, R.J., Kerr, D.E. and Bondioli, K.R. (1997) Transgenic dairy cattle: Genetic engineering on a large scale. Journal of Dairy Science, 80, 2213-2224. doi:10.3168/jds.S0022-0302(97)76170-8
- Whitelaw, C.B.A., Springbett, A.J., Webster, J. and Clark, J. (1993) The majority of G0 transgenic mice are derived from mosaic embryos. Transgenic Research, 2, 29-32. doi:10.1007/BF01977678
- Chan, A.W.S., Kukolj, G., Skalka, A.M. and Bremel, R.D. (1999) Timing of DNA integration, transgenic mosaicism, and pronuclear microinjection. Molecular Reproduction and Development, 52, 406-413. doi:10.1002/(SICI)1098-2795(199904)52:4<406::AID-MRD9>3.0.CO;2-P

