World Journal of Nuclear Science and Technology
Vol.4 No.2(2014), Article ID:44754,8 pages DOI:10.4236/wjnst.2014.42014
Transference Kinetics of Radionuclide 95Nb in the Aquatic Ecosystem
Xiyue Zhao1*, Lei Huang2, Zhiqiang Cai1, Shouxiang Wang2
1School of Pharmaceutical Engineering and Life Science, Changzhou University, Changzhou, China
2Institute of Nuclear Agricultural Sciences, Zhejiang University, Key Laboratory of Nuclear Agricultural Science, Ministry of Agricultural, Hangzhou, China
Email: *xyzhao@cczu.edu.cn
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 22 December 2013; revised 27 January 2014; accepted 14 February 2014
ABSTRACT
The dynamics of transportation, accumulation, diminishment and distribution of 95Nb in a simulated aquatic ecosystem was studied using the isotope-tracer technique, and a fitting equation was established by application of a closed, five-compartment model. The results showed that when 95Nb was introduced into an aquatic system, it was transported and transformed via deposition in combination with other ions, and adsorption and absorption by aquatic organisms, resulting in redistribution and accumulation in different parts of the organisms. Following addition, the specific activity of 95Nb in water decreased sharply within a short time, and then after reaching a certain value, it decreased more slowly. Sediment accumulated large amounts of 95Nb through the exchange of ions. Hyacinth (Eichhornia crassipes) also adsorbed a large amount of 95Nb in a short period of time. Snails (Bellamya purificata) and fish (Carassius auratus) were found to have a poor adsorption capacity of 95Nb. The amount of 95Nb found in the snail flesh was greater than that in the shell, and the 95Nb found in the fish was mainly distributed in the viscera. The amount of 95Nb in each individual component of the experimental system was affected over time.
Keywords
95Nb, Transference, Enrichment, Distribution, Water, Sediment, Hyacinth, Snail, Fish

1. Introduction
The study of the effects of nuclear fission products on the environment is an active field and is needed for maintaining harmonization between the sustainable development of nuclear power and high environmental quality. 95Nb is one of the primary fission products in a nuclear reactor. A primary radionuclide in the radioactive liquid effluents from a pressurized water reactor, its half-life is 35.1 days [1] . In safety assessment of French long-lived nuclear waste disposal, data concerning the mobility and the bioavailability of Nb in soils are needed as well as general trends of its fate in the specific environment around the site of French underground research laboratory [2] . Relevant reports on 95Nb focus on environmental monitoring and evaluation after nuclear accidents, as well as the absorption, distribution and accumulation in natural ecosystems [3] -[7] .
In this study, the isotopic tracer technique is used to study the dynamics of 95Nb in a simulated aquatic ecosystem, and its dynamics are described mathematically using a compartment model with a non-linear fitting method.
2. Materials and Methods
2.1. Isotope
95Nb2O5, in the form of a black powder, was supplied by the Academy of Atomic Energy of China. Its specific radioactivity was 1.14 × 108 Bq∙g−1 and its radiochemical purity is greater than 95%. Prior to use, it was chemically transformed with HF into 95NbF5, at a concentration of 6.17 × 106 Bq∙mL−1 [8] . Then the 95NbF5 solution was diluted to a concentration of 6.17 × 104 Bq∙mL−1 for this experiment.
2.2. Soil
Sediment was paddy soil on powdery loam from an experimental farm in the Huajiachi campus of Zhejiang University. It was air-dried, pulverized, and sieved to remove stones and plant debris prior to use. The physicalchemical properties of the soil are summarized in Table 1.
2.3. Aquatic Ecosystem
The ecosystem was composed of water, sediment, aquatic organisms: hyacinth (Eichhornia crassipes), snails (Bellamya purificata), and fish (Carassius auratus).
2.4. Methods
Three glass tanks with dimensions of 60 cm × 40 cm × 50 cm were constructed. 8 kg of paddy soil on powdery loam as sediment was filled into each pool. The sediment thickness was about 5 cm. The tanks were flooded with 60 L of water, and it was buried 12 plastic sediment sampler with Ф 1 cm × 5 cm. 10 mL of 95NbF5 with a specific activity of 4.32 × 105 Bq∙mL−1 was introduced into the water after one week. The water was stirred gently to get a homogeneous distribution of radionuclide, instantly taking 20 mL water samples. The specific activity of the radionuclide in water was 72 Bq∙mL−1. Fifteen water hyacinth, and 30 snails and 15 fish were put into each tank. Each fish weighed between 130 - 220 g. During the period of the experiment, a filling oxygen pump was used to keep the dissolved oxygen in the water constant throughout day and night. Water was added at intervals of 3 days in order to maintain a constant volume of water.
2.5. Sampling
Samples were collected at time intervals of 1, 2, 4, 6, 9, 15, 22, 29 and 34 days, from each pool by randomly taking four 5 mL aliquots of water and depositing them in disposable plastic cups for activity measurements. Each sample from each pool was thus 20 mL. A hyacinth, and 3 snails and a fish were randomly taken from each tank. The hyacinth was divided into root, gourd, leaf, and snails were divided into flesh and shell. The fish was washed in water, and then dried with absorbent paper. The fish was dissected after weighing, and divided into fin, scale, viscera, skin, gill, flesh, skeleton and head. In total, there were eight parts in all (or nine parts
Table 1. Physico-chemical properties of the soil studied.
including the fish eggs). Each part was weighed individually and then cut into smaller pieces. Twenty gram samples from each part were put into the disposable plastic cups for measurements.
In the meantime, three sediment columns were collected from each tank using a sediment sampler. Each sediment column was sectioned into two equal parts, and each part was smashed and mixed thoroughly. Afterwards, 20 g of sediment samples were put into plastic cups for measurement. This was repeated 3 times for each sample.
2.6. Measurements and Statistical Analysis
95Nb emits β and γ particles when it decays. Those were measured with a multi-channel γ spectrometer (model BH-1224, Beijing Nuclear Instrumentation Factory) equipped with a NaI scintillation detector (70 mm in diameter). The spectrometer was installed in a lead-shielded chamber. Disposable plastic cups of 75 mm diameter and 110 mm height were placed on the top of the scintillation detector during measurements. A homemade location device was used for fixing the counting position, to ensure homogeneity in the geometrical position of all samples. The counting error was controlled to be lower than 5%. All samples were measured on the sampling day to avoid errors due to water evaporation. The counts were calibrated with counting efficiency, diminished time, disintegration and other factors [9] .
A non-linear regression analysis method was used to describe the variation dynamics of 95Nb in the individual compartment of the system [10] .
3. Results and Discussion
3.1. Increase and Decrease of 95Nb in the Pool Water and Sediment
The 95Nb specific activity in the pool water and sediment on different days are shown in Table 2. The specific activity of 95Nb in the pool water decreased rapidly in the first few hours due to the deposition, complexation, and adsorption and absorption by the sediment and aquatic living organisms. After 1 day, the specific activity of 95Nb in the pool water reduced to 19.52 Bq∙mL−1, only 27.11% of the initial specific activity (72.0 Bq∙mL−1). From then on, it decreased gradually, and 29 days later, the exchanging of 95Nb in the pool water with ions in the ecosystem reached dynamic equilibrium.
As shown in Table 2, due to the deposition of 95Nb in water and exchange with ions in the soil, the specific activity of 95Nb in the sediment increased rapidly in the first four days to a maximum of 156.55 Bq∙g−1 on Day 4, and decreased to an average of 118.93 Bq∙g−1 in the following days. This is because the 95Nb was mainly located within the upper sediment and the Nb ions that were not combined closely with sediment began desorbing. As time went on, 95Nb moved into and combined stably with the deep sediment through the processes of complexation and the adsorption of iron and manganese oxides [11] -[14] .
Table 2. Change of 95Nb specific activity in water and sediment with time.
3.2. Increase and Decrease of 95Nb in Hyacinth and Snail
The 95Nb specific activity in hyacinth and snail with time were given in Table 3. On Day 1, the 95Nb specific activity in hyacinth increased rapidly to a maximum of 247.25 Bq∙g−1, and then in the following days, the 95Nb specific activity in hyacinth decreased due to being deabsorbed. Only a little 95Nb was absorbed and steadily combined by hyacinth due to the fact that 95Nb was not an essential element of the organisms. With a water exchange in vitro and in vivo of the snails, a large amount 95Nb went into the snail body, and the 95Nb specific activity of the snails increased sharply and started to decrease after reaching a maximum. Subsequently, because the specific activity of 95Nb in the water sharply decreased, the 95Nb in the snails began desorbing and the specific activity was essentially at equilibrium 15 days later. The specific activity of 95Nb in the snail flesh was obviously higher than that in the snail shell [15] [16] .
3.3. Increase and Decrease of 95Nb in Fish
The dynamic migration and distribution of 95Nb in different parts of the fish in the last sample are given in Table 4. The specific activity data of 95Nb in the whole fish is equaled from weighing each part of the body. The
Table 3. Relationship between specific activity and time of 95Nb in aquatic organisms in hyacinth and snail.
Table 4. Relationship between specific activity and time of 95Nb in aquatic organisms in fish/Bq∙g−1.
results showed that the organs taking up and adsorbing 95Nb were mainly the intestines and stomach (viscera). 95Nb was absorbed by the gill and fin through direct contact with water. The specific activity of 95Nb in these three parts reached a maximum in 2 days, after which 95Nb rapidly reduced in water and then gradually decreased tendency with time. 4 days later, the specific activity of 95Nb in these three parts showed a decreased tendency to balance, and the general trend was similar to the increase and decrease found in the snails. The specific activity of 95Nb in flesh, bone, liver and eggs was relatively low, and were only a little higher than the background level. It was indicated that 95Nb remained in the intestines, stomach, gill and fin, and could not readily transport into the inner organs such as the flesh, bone, liver and eggs. The absorption capacity of the fish skin and the scale were lower than that of the fin the maximums respectively being 0.99 Bq∙g−1 and 0.71 Bq∙g−1 after 2 days, then gradually reducing as the specific activity of 95Nb in the water reduced. The structure of the head was more complicated, as it comprised bone, flesh, and other organs, as well as the epidermis and mouth organs, which were exposed outside the water surface. 95Nb was mainly found in the external organs which had direct contact with the water, and they were relatively low compared with other parts of the specific activity, their dynamics were similar to the fish skin and scale.
In summary, the specific activities of 95Nb in various parts of the fish descend in the following order: viscera > gill > fin > skin, fish scale > bone, head, eggs > flesh.
3.4. Transportation Model of 95Nb in Simulated Aquatic Ecosystem
In this experiment, the aquatic ecosystem is composed of water, sediment, hyacinths, snails and fish. Due to there being a lack of exchange of water and ions between the system and outside, it could be regarded as a closed five-compartment system. According to the specific situation of this experiment, a model was established by ignoring some minor processes in Figure 1 [17] -[20] .
k12, k13, k14, k15 are the transfer rate constants from water to sediment, fish, snails, and hyacinths, respectively. k21, k24 are the transfer rate constants from sediment to water and snails, respectively. k31 is the transfer rate constant from fish to water. k42 is the transfer rate constant from snails to sediment. k51 is transfer rate constant from hyacinths to water. The rates of change of quantity (q1, q2, q3, q4, q5) of 95Nb in different compartments with time are as follows:
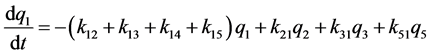
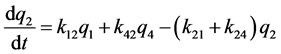
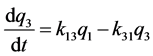
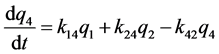
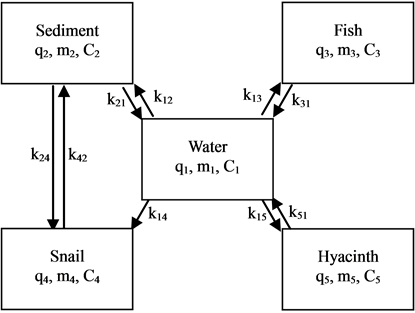
Figure 1. The closed five-compartment model of aquatic ecology.
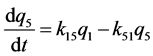
According to the experimental data and initial conditions, the differential equations were solved by fitting a code and mathematic modes for the dynamic behavior of 95Nb in water (C1), sediment (C2), fish (C3), snails (C4), hyacinths (C5) of the aquatic ecosystem are obtained as follows:
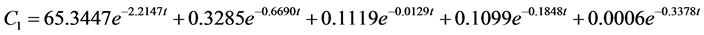
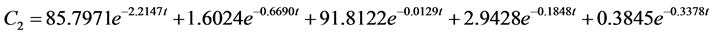
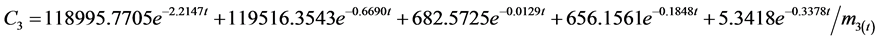
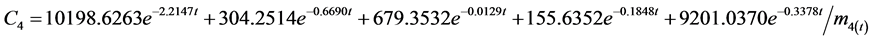

The correlation coefficient for water is r = 0.92, for sediment r = 0.91, for fish r = 0.69, for snail r = 0.97, for water hyacinth r = 0.96. Where mx(t) is the mass at moment t. Transfer coefficients are shown below with the unit∙d−1:

3.5. CF Value of 95Nb by Aquatic Organisms
Aquatic organisms for radionuclide concentration factor in water is defined as the specific activity of the radionuclide in a certain component of aquatic ecosystem divided by the specific activity of the same radionuclide in the same time and space, and is usually called the concentration-factor (CF). The CF values of 95Nb by aquatic organisms are shown in Table 5.
The 95Nb enriching abilities of water hyacinth in water are the highest, followed by snail, and then fish. Their CF value increased gradually over time. At the end of the experiment (34 days), the CF value of 95Nb for water hyacinth and snail reached 396.57 and 15.83. The CF values of 95Nb for the main part of the fish (fish flesh) remained at almost zero, which shows the 95Nb in water is not enriched by fish. Niobium is not an essential element for use by organisms, it is of universal insolubility and has a very low organism absorption, as show the date for 95Nb.
Table 5. CF value of 95Nb by aquatic organisms as a function of time.
4. Conclusions
When 95Nb was introduced into an aquatic system, it was transported and transformed via deposition and absorption, and was adsorbed by aquatic organisms, leading to the distribution and accumulation in individual parts of the system. Over time, 95Nb was desorbed and released, leading to 95Nb redistribution in individual parts of the aquatic ecosystem. After 1 day, the specific activity of 95Nb in water was 27.11% of the initial specific activity.
The 95Nb enriching abilities of the various organisms in the aquatic ecosystem are very different. The 95Nb enriching ability of hyacinth is far greater than for snail and fish, and the 95Nb enriching ability of snail was greater than for fish. The 95Nb in water by fish is not enrichment.
The 95Nb enriching abilities of the different parts of the same organism are very different.
Dynamics of 95Nb concentrations in different components in the aquatic ecosystem can be described by using a tracer kinetic compartment model. Under these study conditions, quantitative expressions reach notable levels.
Acknowledgements
The project was financially supported by the National Natural Science Foundation of China under the approval number 21247003. The critical reviewing of manuscript and comments by anonymous reviewers are greatly acknowledged.
References
- Whicker, F.W. and Schultz, V. (1982) Radioecology: Nuclear Energy and the Environment. CRC Press, Boca Raton.
- Echevarria, G., Morel, J.L. and Leclerc-Cessac, E. (2005) Retention and Phytoavailability of Radioniobium in Soils. Journal of Environmental Radioactivity, 78, 343-352. http://dx.doi.org/10.1016/j.jenvrad.2004.05.010
- Szabolcs, O., Nora, V. andZsuzsa, M. (2008) Determination of Long-Lived Nb Isotopes in Nuclear Power Plant Wastes. Applied Radiation and Isotopes, 66, 24-27. http://dx.doi.org/10.1016/j.apradiso.2007.07.007
- Arvic, H., Lena, J. and Desmond, M. (2009) Decay Correction of 95Nb. Applied Radiation and Isotopes, 67, 641-642. http://dx.doi.org/10.1016/j.apradiso.2008.12.002
- Yirchenko, Y.P. and Agapkina, G.I. (1993) Organic Radionuclide Compounds in Soils Surrounding the Chernobyl Nuclear Power Plant. Eurasian Soil Science, 25, 51-59.
- Kruglov, S.V., Vasil’yeva, N.A., Kurinov, A.D., et al. (1996) Distribution of Radionuclides from Chernobyl Fallout with Regard to Fractions of the Soil-Particle Distribution of Sod-Podzolic Soils. Eurasian Soil Science, 28, 26-35.
- Zhu, N.K., Wang, C.L. and Teng, W.F. (2004) Status of Radiation Sterilization of Healthcare Products in China. Radiation Physics and Chemistry, 71, 591-595. http://dx.doi.org/10.1016/j.radphyschem.2004.03.036
- Chen, S.C. (1994) Important Inorganic Chemical Reactions. 3rd Edition, Shanghai Science & Technology Press, Shanghai.
- Shi, J.J. and Guo, J.F. (2006) Distribution and Migration of 95Zr in a Tea Plant/Soil System. Journal of Environmental Radioactivity, 87, 170-174. http://dx.doi.org/10.1016/j.jenvrad.2005.11.007
- Zhang, X.D. (1995) Applied Regression Analysis. Zhejiang University Press, Hangzhou.
- Xiong, Y. (1990) Soil Colloid-Volume 3: The Property of the Soil Colloid. Science Press, Beijing.
- Wang, Y. (1995) Soil Environment Element Chemistry. Chinese Environment Science Press, Beijing.
- Echevarria, G., Morel, J.L. and Leclerc-Cessac, E. (2005) Retention and Phytoavailability of Radioniobium in Soils. Journal of Environmental Radioactivity, 78, 343-352. http://dx.doi.org/10.1016/j.jenvrad.2004.05.010
- Shi, J.J., Guo, J.F. and Chen, H. (2003) Distribution and Migration of 95Zr in Marine Ecosystem. Water, Air and Soil Pollution, 149, 177-187. http://dx.doi.org/10.1023/A:1025679921911
- Mosulishvili, L.M., Shoniya, N.I., Katamadze, N.M., et al. (1994) Environmental Radionuclide Distribution in the Republic of Georgia after the Chernobyl Catastrophe. Zhurnal Analiticheskoi Khimii, 49, 135-139.
- Liu, L.L., Shi, J.J., Zhao, X.Y., et al. (2005) Dynamics of Transfer and Distribution of 95Zr in the Broadbean-Soil Ecosystem. Journal of Environmental Radioactivity, 80, 217-223. http://dx.doi.org/10.1016/j.jenvrad.2004.09.001
- Shi, J.J., Guo, J.F. and Chen, H. (2002) Dynamics of 95Zr in the Rice/Water/Soil System. Applied Radiation and Isotopes, 56, 735-740. http://dx.doi.org/10.1016/S0969-8043(01)00257-3
- Zhao, X.Y., Cai, Z.Q., Gong, F.H., et al. (2008) Transference Kinetics of 60Co in an Aquatic-Terrestrial Ecosystem. Nuclear Science and Techniques, 19, 213-217. http://dx.doi.org/10.1016/S1001-8042(08)60052-4
- Michael, A.S., Ingvar, L.L. and Audeen, W.F. (2008) Fate of 60Co at a Sludge Land Application Site. Journal of Environmental Radioactivity, 99, 1611-1616. http://dx.doi.org/10.1016/j.jenvrad.2008.06.006
NOTES

*Corresponding author.


