World Journal of Nuclear Science and Technology
Vol.3 No.2(2013), Article ID:30807,6 pages DOI:10.4236/wjnst.2013.32012
Production and Quality Control of 64Cu from High Current Ni Target
Atomic Energy Commission of Syria, Chemistry Department, Cyclotron Division, Damascus, Syria
Email: *cscientific@aec.org.sy
Copyright © 2013 A. H. Al Rayyes, Y. Ailouti. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 17, 2012; revised February 9, 2013; accepted February 21, 2013
Keywords: 64Cu; Targetry; Radioisotope Production; Ion Exchange Chromatography
ABSTRACT
A new production method of no-carrier-added 64Cu was tested using a new target prepared by electroplating of Ni on a silver layer (thickness 35 µm) previously electroplated on a pure copper target support. This method meets cost effective production and quality of the produced 64Cu criteria. The quality of the electroplated layers has been tested under the bombardment by more than 200 µA of proton beam using water cooled target system. A separation and purification setup was elaborated to produce high quantity and high specific activity of 64CuCl2 suitable for labeling different ligands in order to be used in therapy and diagnosis. A semi-automated target dissolution and separation system has been developed and achieved for 64Cu production. The separation chemistry is based on a chromatographic column system.
1. Introduction
The radionuclide 64Cu (T1/2 = 12.7 h) emits β− 39% and β+ 17.4% (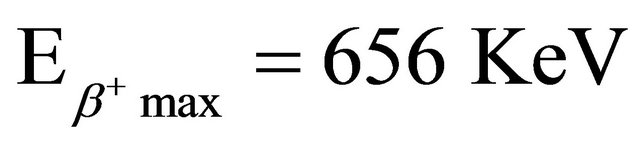 ;
;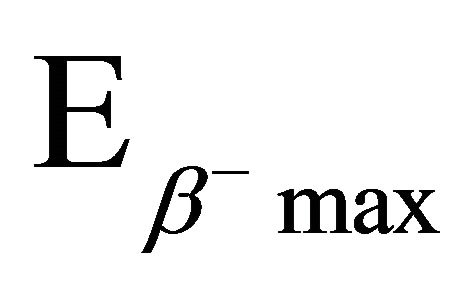 = 573 KeV). These characteristics make it useful for both high resolution PET imaging and targeted endoradiotherapy. In addition, its electron capture decay associated with Auger emission gives more efficient cell killing when this radioisotope is deposited in the cell [1].
= 573 KeV). These characteristics make it useful for both high resolution PET imaging and targeted endoradiotherapy. In addition, its electron capture decay associated with Auger emission gives more efficient cell killing when this radioisotope is deposited in the cell [1].
Another advantage of this important radionuclide is the stability of its complexes with bifunctional chelators consisting of the metal complex ligand and the functional group for attachment to the targeting molecule [2].
Until now 64Cu-ATSM is the most investigated radiolabeled compound and it seems to be a promising agent for endoradiotherapy [3-5].
Many methods for the production of 64Cu have been investigated; 1) based on low energy cyclotron via the nuclear reaction 64Ni (p, n) 64Cu [6-9], 2) proton irradiation of enriched 68Zn using the nuclear reaction 68Zn (p, αn) 64Cu as a side reaction in the production of 67Ga [10,11]. In the first method Ni is electroplated on gold disk. Due to the fact that target support (gold disk) can be used for one time and irradiation beam intensity used for this target support is very low resulting low quantity of the produced 64Cu radioactivity. In this study, we would like to prove that high quality and quantity of 64Cu can be produced using a new target prepared by the electroplating of Ni on silver layer previously electroplated on a pure copper target support. Then we describe an effective set up for the separation and purification of 64Cu from the irradiated target. Also a quality control of the produced radioactive copper is carried out.
2. Materials and Methods
The nickel nitrate Ni(NO3)2∙6H2O (purity 99%, GR) was purchased from Merck. AgNO3 (purity 99.9%, GR) was purchased from BDH. Hydrochloric acid (GR) was purchased from Merck. Ion exchange resin (Dowex 1 × 8) was bought from Sigma-Aldrich.
Radioisotopes were identified by gamma spectrometry using a high purity germanium (HPGe) detector with 25% efficiency, where the amplifier output of the detector was processed by a 4096 channels multi-channel analyzer (MCA) system. The fitting program (INTER WINER) was used for spectral data processing.
Developed anodic stripping voltammetry system purchased from Metrohm (VA processor 693 with VA stand 694) was used for trace metals analysis in the produced Cu bulk. This system uses a working electrode (Hanging Mercury Dropped Electrode (HMDE)), a reference electrode Ag/AgCl/KCl (3 mol) and an auxiliary electrode from platinum.
The electroplating is carried out on copper target support, (2 × 10 cm), designed by IBA. The blank cooper target is back water cooled and designed to be irradiated with more than 300 µA at 30 MeV proton beam.
3. Results and Discussion
The production process of 64Cu was performed according to the block diagram presented in Figure 1.
3.1. Target Support Electroplating
The surface of the copper target support, (2 × 10 cm), was cleaned by fine abrasive wool and acetone. This surface was electroplated by silver using (AgNO3, NaCN and Na2CO3) bath having pH ≥ 12 at room temperature. The voltage and current density used for silver electroplating were 4 V and 4 mA/cm2 respectively. The thickness of the electroplated silver layer was calculated to be 35 µm.
The function of the electroplated silver layer is to prevent the dissolution of copper target support in order to produce non-carrier added 64Cu.
On the silver layer, natural nickel was electroplated on a smaller surface (1 × 10 cm) using [Ni(NO3)2, Na2SO4, NH4Cl and H3BO4] bath having pH ≈ 9 at room temperature. The voltage and current density for Ni electroplating was 6 V and 50 mA/cm2 and the electroplated thickness was about 12 µm. Figures 2-4 show the electroplated target and target surface.
3.2. Target Irradiation
The electroplated target was irradiated by a 15 MeV proton beam of 200 μA intensity using Cyclone-30 cyclotron (IBA, Belgium) during 3 h. The back side of the target was cooled with a very high speed stream of deionized water flowing through IBA irradiation station. No any damage on electroplated target has been noticed. Which prove that the two electroplated layers stick very well on the copper substrate (Figure 5).
Different isotopes can be produced after the proton irradiation of our target.
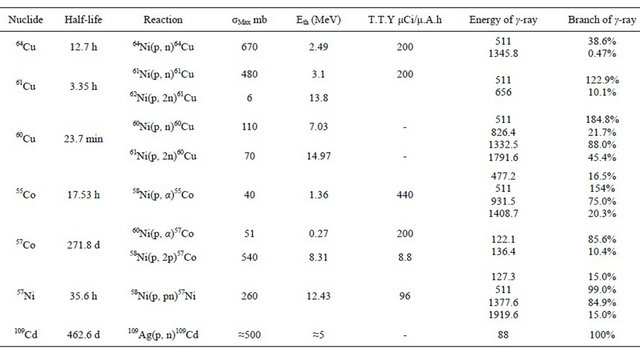
Table 1 summarizes production conditions and the characteristics of the resulting isotopes in our target.

Figure 1. Block diagram of 64Cu production process.

Figure 2. Target support: On the right, without electroplating; In the middle, silver electroplated; On the left, nickel electroplated on silver layer.
 (a)
(a)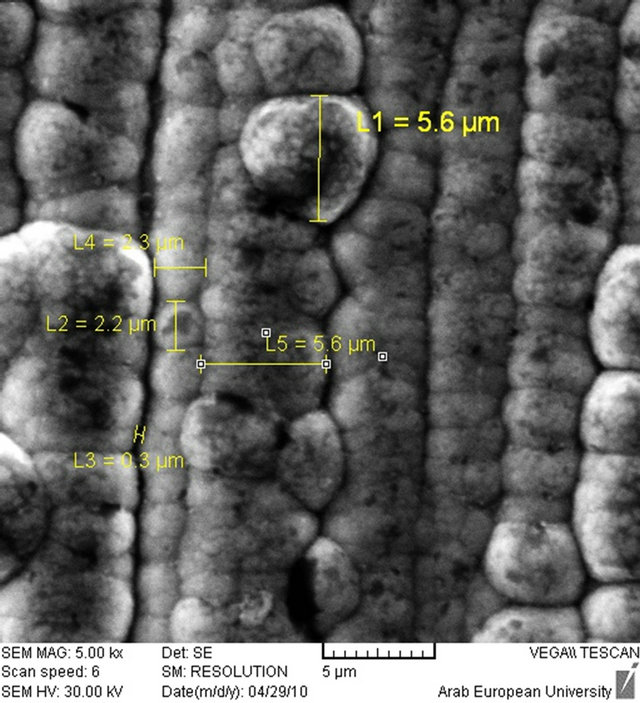 (b)
(b)
Figure 3. Nickel electroplated surface. (a) Nickel surface by microscope ×50 time; (b) Nickel surface by Scanning electron microscopy (SEM).
 (a)
(a)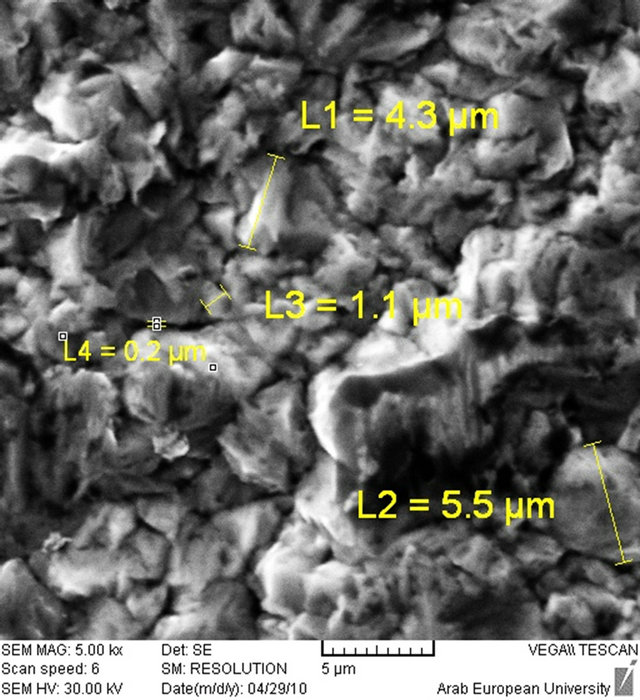 (b)
(b)
Figure 4. Silver electroplated surface. (a) Silver surface by microscope ×50 time; (b) Silver surface by scanning electron microscopy (SEM).

Figure 5. Irradiated target (200 µA, 3 h).
Table 1. Reaction cross sections, threshold energies thick target yields and radiation characteristics of the reaction products.
The irradiated target has been dissolved in concentrated hydrochloric acid under heating to 90˚C. Figure 6 shows the target dissolution unit used in this study. The dissolution speed is increased by adding 500 µl of hydrogen peroxide if necessary.
The resulting solution contains *Cu, *Ni, *Co, *Cd and traces of silver. A column (150 × 10 mm) containing Dowex 1 × 8 was conditioned by 30 ml of HCl 9 N and used for the separation and purification of 64Cu.
The resulting solution was passed through the separation column using flow rate 2.5 ml/min. All nickel and trace silver are removed from the column by 30 ml HCl 9 N, flow rate 2.5 ml/min. Figure 7 shows the separation and purification unit.
Then Cu-64 is eluted from the column by 20 ml of deionized water. The elution profile of Ni and 64Cu are shown in Figures 8 and 9.
Radioactive cobalt will remain in the resin; to be eluted it would need larger amount of water.
The produced 109Cd formed from the nuclear reaction 109Ag(p, n)109Cd and all cobalt radioisotopes will be fixed in the column at the end of the two successive elutions (HCl 9 N and deionized water). For removing these isotopes from the resin, HCl of 12 N is used.
4. Quality Control of the Produced Radioactive Copper
As mentioned, we used natural Ni for the production of radioactive copper. Since natural nickel contains different Ni isotopes the proton irradiation will lead to the formation of 60Cu, 64Cu, 61Cu, 56Ni, 57Ni, 55Co, 56Co, 57Co
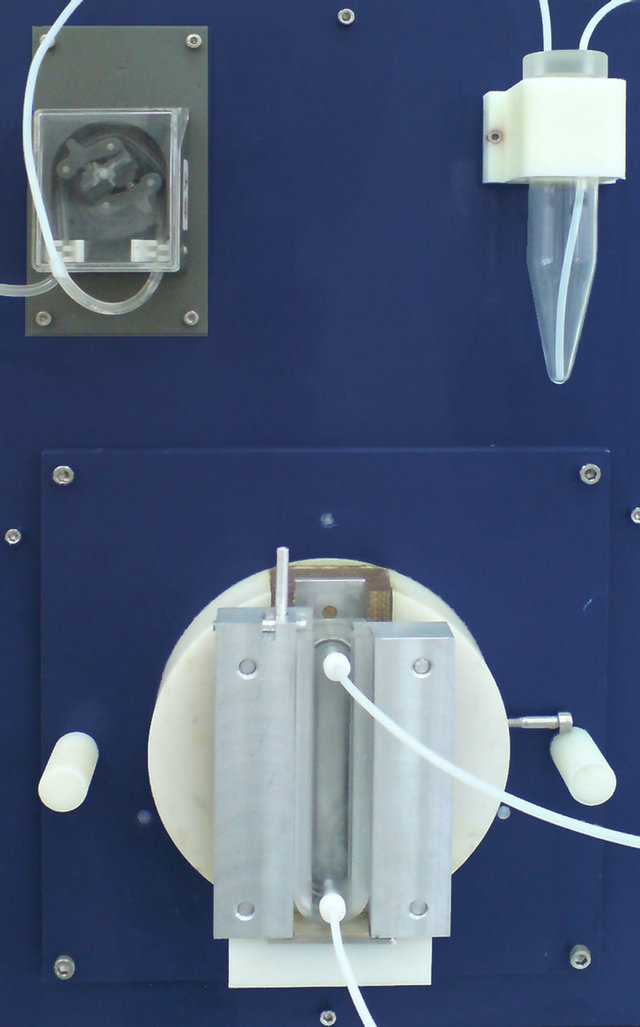
Figure 6. Target dissolution unit.
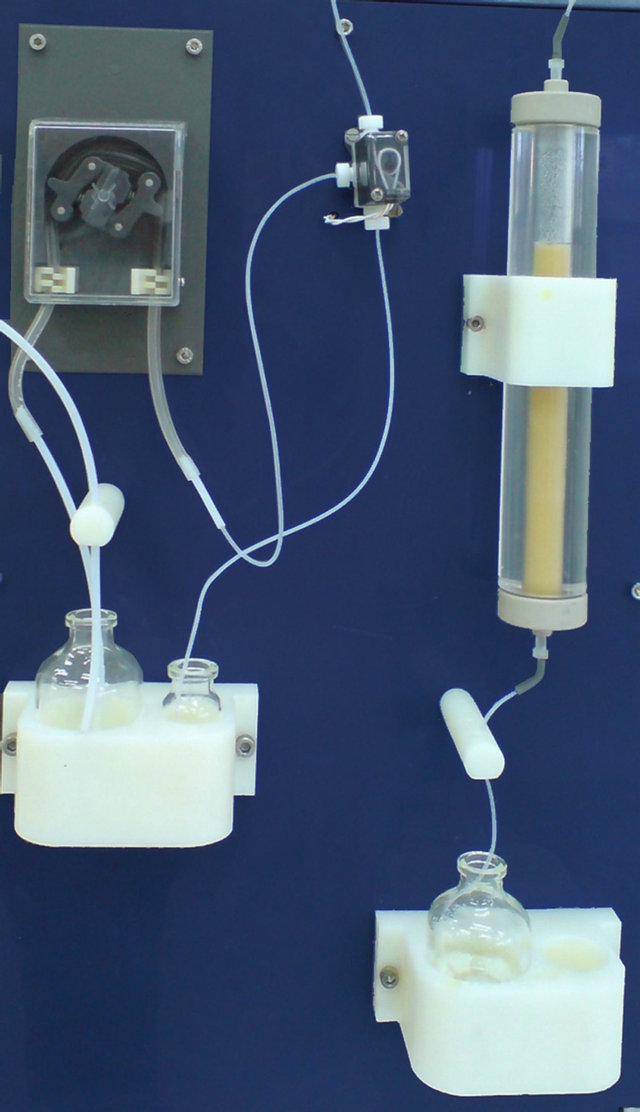
Figure 7. Separation and purification unit.
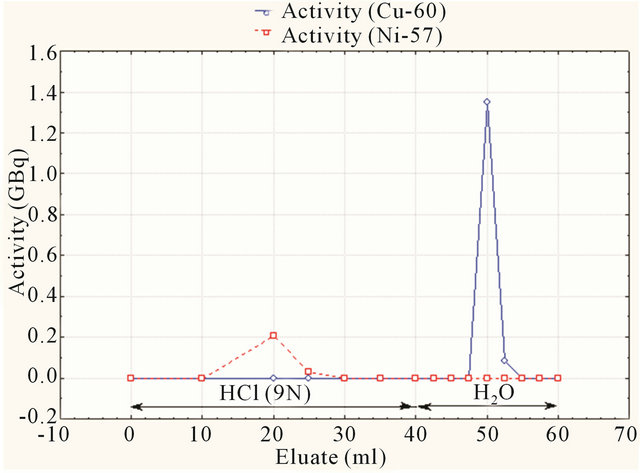
Figure 8. Elution profile of Cu-60 and Ni-57.
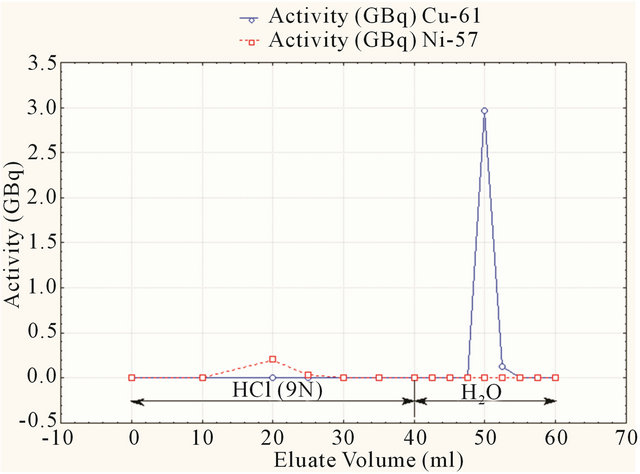
Figure 9. Elution profile of 61Cu and 57Ni.
and 58Co.
The efficiency of the proposed separation and purification method is demonstrated by gamma spectrum shown in Figure 10. This figure shows that no other radioactive tracers formed in the target are present in separated solution of 64Cu. This method of separation is valuable even when none highly enriched 64Ni is used for the production of high quality 64Cu.
5. Recovery and Recycling of Ni
In case of the use of enriched Ni, recovery process should be used. In our proposed method Ni collected in HCl 9 N contains trace amount of silver. The presence of trace amount of silver induces no effect on the purity of 64Cu due to the fact that the 109Cd generated from the reaction 109Ag (p, n) 109Cd will be fixed on the 64Cu separation column. The separation of Ni from silver is carried out using chromatographic column (150 × 20 mm) filled with the cation exchanger Dowex 50WX4. This column is preconditioned by 30 ml of HCl 2 N. The Ni and silver cation solution is adjusted to HCl 2 N.
Ag cation is removed from the column by HCl 2 N and then Ni cation can be recovered in a small volume by HCl 0.05 N.
Gamma spectrum of the separated Ni to be used for another Ni electroplating of a new target is shown in Figure 11. This figure shows no presence of radio-cobalt or radio-copper in the recovered Ni solution.
All radio-cobalt and radio-cadmium is fixed in the resin at the end of separation and purification. Gamma spectrum of the resin is shown in Figure 12.
6. Conclusion
Good quantity and high quality of 64 Cu can be produced using electroplated enriched 64Ni on silver layer previously electroplated on a pure copper target support. Chromatographic separation techniques have been used in order to separate high purity no carrier added 64CuCl2 suitable for labeling different ligands in order to be used in therapy and diagnosis. A semi-automated target dissolution and separation system has been developed and achieved for Cu-64 production. An efficient method to recover high costly enriched 64Ni is also performed.
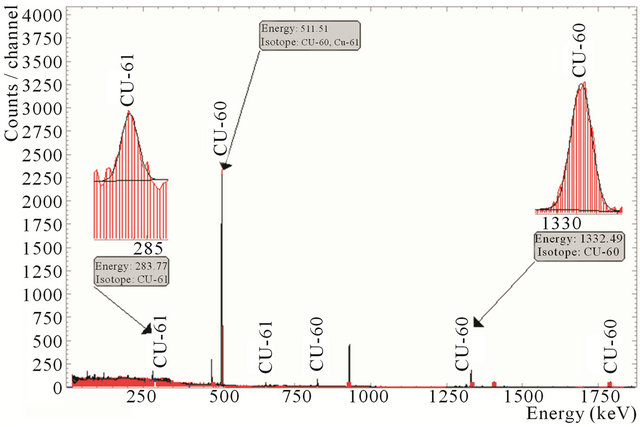
Figure 10. Gamma spectra of the produced radioactive copper.
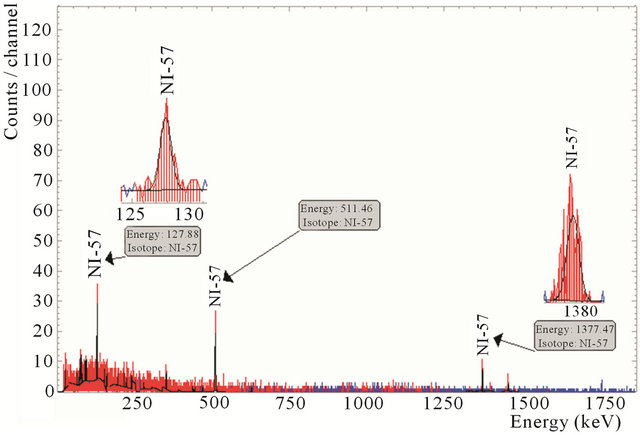
Figure 11. Gamma spectra of the separated Ni.
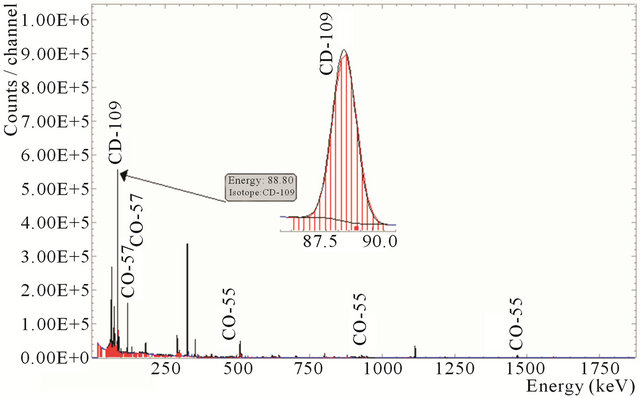
Figure 12. Gamma spectra of the resin containing 109Cd and radioactive cobalt.
7. Acknowledgements
The authors are grateful to the IAEA for supporting this research through CRP 15937. Thanks to prof. I. Othman (GD of AECS) for the encouragement and support. Thanks to Raad A., Ibrahim W., Freihat S., Orabi H., Sahar M., Abdel hadi M. Abbas I., Al Enaya F., and Zakkar N. for their technical support.
REFERENCES
- C. P. J. Blower, J. S. Lewis and J. Zweit, “Copper Radionuclides and Radiopharmaceuticals in Nuclear Medicine,” Nuclear Medicine and Biology, Vol. 23, No. 8, 1996, pp. 957-980. doi:10.1016/S0969-8051(96)00130-8
- T. J. Wadas, E. H. Wong, G. R. Weisman and C. J. Anderson, “Copper Chelation Chemistry and Its Role in Copper Radiopharmaceuticals,” Current Pharmaceutical Design, Vol. 13, No. 1, 2007, pp. 3-16. doi:10.2174/138161207779313768
- A. Obata, S. Kasamatsu, J. S. Lewis, T. Furukawa, S. Takamatsu, J. Toyohara, T. Asai, M. J. Welch, S. G. Adams, H. Saji, Y. Yonekura and Y. Fujibayashi, “Basic Characterization of 64Cu-ATSM as a Radiotherapy Agent,” Nuclear Medicine and Biology, Vol. 32, No. 1, 2005, pp. 21-28. doi:10.1016/j.nucmedbio.2004.08.012
- A. Obata, M. Yoshimoto, S. Kasamatsu, H. Naiki, S. Takamatsu, K. Kashikura, T. Furukawa, J. S. Lewis, M. J. Welch, H. Saji, Y. Yonekura and Y. Fujibayashi, “Intra-Tumoral Distribution of 64Cu-ATSM: A Comparison Study with FDG,” Nuclear Medicine and Biology, Vol. 30, No. 5, 2003, pp. 529-534. doi:10.1016/S0969-8051(03)00047-7
- P. McQuade, K. E. Martin, T. C. Castle, M. J. Went, P. J. Blower, M. J. Welch and J. S. Lewis, “Investigation into 64Cu-labeled Bis(selenosemicarbazone) and Bis(thiosemicarbazone) Complexes as Hypoxia Imaging Agents,” Nuclear Medicine and Biology, Vol. 32, No. 2, 2005, pp. 147-156. doi:10.1016/j.nucmedbio.2004.10.004
- A. Obata, S. Kasamatsu, D. W. McCarthy, M. J. Welch, H. Saji, Y. Yonekura and Y. Fujibayashi, “Production of Therapeutic Quantities of 64Cu Using a 12 MeV Cyclotron,” Nuclear Medicine and Biology, Vol. 30, No. 5, 2003, pp. 535-539. doi:10.1016/S0969-8051(03)00024-6
- M. A. Avila-Rodriguez, J. A. Nyeb and R. J. Nickles, “Simultaneous Production of High Specific Activity 64Cu and 61Co with 11.4 MeV Protons on Enriched 64Ni Nuclei,” Applied Radiation and Isotopes, Vol. 65, No. 10, 2007, pp. 1115-1120. doi:10.1016/j.apradiso.2007.05.012
- X. Hou, U. Jacobsen and J. C. Jorgensen, “Separation of No-Carrier-Added 64Cu from a Proton Irradiated 64Ni Enriched Nickel Target,” Applied Radiation and Isotopes, Vol. 57, No. 6, 2002, pp. 773-777. doi:10.1016/S0969-8043(02)00170-7
- D. W. McCarthy, R. E. Shefer, R. E. Klinkowstein, L. A. Bass, W. H. Margeneau, C. S. Culter, C. J. Anderson and M. J. Welch, “Efficient Production of High Specific Activity 64Cu Using a Biomedical Cyclotron,” Nuclear Medicine and Biology, Vol. 24, No. 1, 1997, pp. 35-43. doi:10.1016/S0969-8051(96)00157-6
- A. H. Al Rayyes and Y. Ailouti, “Routine Simultaneous Production of No-Carrier Added High Purity 64Cu and 67Ga,” Nukleonika, Vol. 56, No. 4, 2011, pp. 259-262.
- S. V. Smith, D. J. Waters and N. D. Bartolo, “Separation of 64Cu from 67Ga Waste Products Using Anion Exchange and Low Acid Aqueous/Organic Mixtures,” Radiochimica Acta, Vol. 75, No. 2, 1996, pp. 65-68.
NOTES
*Corresponding author.

