International Journal of Organic Chemistry
Vol.07 No.02(2017), Article ID:76827,8 pages
10.4236/ijoc.2017.72012
Synthesis and Spectroscopic Characterization of Eight Chloro Cyclopentadienyl Titanium Bis (O, O-Dialkyl and Alkylene Dithiophosphate) Compounds
Adnan A. S. El Khaldy1*, Alaa M. Abu Shanab2, Yannic Boni1
1Department of Physics, Chemistry, and Mathematics, College of Engineering, Technology and Physical Sciences, Alabama A & M University, Normal, AL, USA
2Chemistry Department, Al-Aqsa University, Gaza, Palestine

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 25, 2017; Accepted: June 9, 2017; Published: June 12, 2017
ABSTRACT
A series of new complexes, CpTiCl[S2P(OR)2]2 (where R) Et, n-Pr, i-Pr, Bui, Ph) and CpTiCl [S2POGO]2 (where G) -CH2CMe2CH2-, -CMe2CMe2-) have been prepared by the drop wise addition of the appropriate O, O’-dialkyl or -alkylene dithiophosphoric acid to cyclopentadienyl titanium trichloride in 1:2 molar ratio and refluxed in benzene solution. The new compounds were characterized by molecular weight measurements elemental analyses and spectroscopic studies (1H, 13C, and 31P NMR, and infrared). We suggest a distorted tetrahedron structure of these new complexes and the dithioligand behaves as bidentate ligand.
Keywords:
Chloro Cyclopentadienyl Titanium (IV) Bis Dialkyl, Alkylene Dithiophosphate

1. Introduction
Dithiophosphate complexes of both transition and non-transition elements have received considerable interest due to their wide diversity in chemical [1] - [6] and biological systems [7] [8] O, O-Dialkyl and alkylene dithiophosphate ligands can coordinate to metal atoms in a monodentate or anisobidentate fashion [9] [10] . More recent applications of thio compounds are in the production of nanoparticles of metal sulfides [11] [12] . Metal thio compounds are extensively used as pesticides [13] , corrosion inhibitors [14] , agricultural reagents [15] , and quite recently in therapy for HIV infections [16] . Moreover, some of these thio complexes are also known to show antitumor properties [17] [18] and their antioxidant properties could be of importance for treating Alzheimer’s disease [19] . A survey of literature on dithiophosphate derivatives of titanium and organotitanium reveals that only simple derivatives (e.g., those containing organic and halo substituents on titanium in addition to the dithiophosphate group) have been described [20] [21] . In this paper and continuation of our work we hereby report some novel cyclopentadienyl titanium chloro dithiophosphate complexes.
2. Experimental
All the reactions were carried out in air and moisture-free conditions. Solvents (benzene and chloroform) and alcohols (ethanol, n-propanol, isopropanol and iso-butanol) were dried before use, by standard methods. CpTiCl3 (Sigma Aldrich) was used as such. dialkyl and alkylenedithiophosphoric acids or their sodium/ammonium salts were prepared by the methods reported in the literature. Sulfur was estimated gravimetrically as barium sulfate. Chlorine estimated by Volhard’s method.
3. Measurements
Molecular weights were determined cryoscopically in benzene. IR spectra, using CsI cells, were recorded as neat liquids or in the form of Nujol mulls (in case of solid compounds) on a Perkin-Elmer 577 spectrometer in the range 4000 - 200 cm−1. 1H and 13C spectra were recorded on a Jeol-FT NMR spectrometer-LA300 spectrophotometer using TMS as an externa standard. 31P NMR (proton decoupled) spectra were recorded in CDCl3 using 85% H3P04 as an internal standard on the same instrument.
4. Results and Discussion
Chloro cyclopentadienyl titanium bis (dialkyl and alkylenedithiophosphate) have been prepared by the reaction of cyclopentadienyl titanium trichloride with acid or sodium salt of O, O-dialkyl and alkylenedithiophosphoric acids in 1:2 molar ratios in refluxing benzene as in Equations (1) and (2).
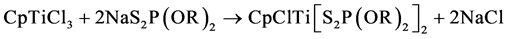 (1)
(1)
where R = Et, Pr-n, Pr-i, Bu-i and Ph
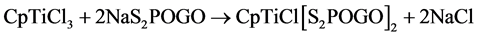 (2)
(2)
G = -CH2CMe2CH2-, -CH2CEt2CH2- and -CMe2CMe2.
The color of the reaction medium changed yellow-brown color with the pro- gress of reaction. Chloro cyclopentadienyl titanium bis (dialkyl and alkylenedithiophosphate) derivatives are brown color solids in these complexes. The new complexes are soluble in common organic solvents like benzene, chloroform and dichloromethane. The molecular weight of all these compounds determined by cryoscopic method in benzene indicated the monomeric nature of these pro- ducts (Table 1).
4.1. IR Spectra
IR spectra of chloro cyclopentadienyl titanium bis (dialkyl and alkylenedithiophosphate), have been recorded in the region 4000 - 400 cm−1 [22] [23] . The band shown by the parent acids in the region 2544 - 2400 cm−1, due to SH stretching vibration, are absent for chloro cyclopentadienyl titanium bis (dialkyl and alkylenedithiophosphate) derivatives, indicating the formation of Ti-S bond with the appearance a new band in the regions 428 - 400 cm−1 [22] [24] . The bands present in the region 1104.0 - 1014.5 cm−1 and 937.3 - 800 cm−1 have been assigned to ν (P)-O-C and νP-O-(C) stretching vibrations respectively. Strong to medium bands in the region 995 - 921.9 cm−1 are due to dioxaphosphorinane and dioxaphospholane ring vibrations [25] [26] [27] . The bands shown in the region 704.0 - 638.0 cm−1 can be assigned to ν P=S vibrations [28] . The bands in medium weak intensities in the region 602 - 513.0 cm−1 may be attributed to vibration of νP-S asymmetric and symmetric vibrations [29] . Details regarding the individual bands have been included in Table 2.
4.2. 1H NMR Spectra
The 1H NMR spectrum of complexes 1 - 7 exhibit the characteristic resonance due to alkoxy and glycoxy (dithio moiety) protons (Table 3). The singlet peak at (3.1 - 3.5 ppm) in the parent ligand assigned to SH proton, is absent from the spectra of chloro cyclopentadienyl titanium bis (dialkyl and alkylenedithiophosphate) derivatives indicating deprotonation of SH group and forming of Ti-S bond [30] .
4.3. 13C NMR Spectra
The 13C NMR spectra of a selection of chloro cyclopentadienyl titanium bis (di-
Table 1. Physical Properties and Analytical Data of Chloro Cyclopentadienyl Titanium Bis (O,O-Dialkyl and Alkylene Dithiophosphate) Compounds.
Table 2. IR SPECTRAL Data (cm−1) of Chloro Cyclopentadienyl Titanium Bis(O,O-Dialkyl and Alkylene Dithiophosphate Compounds.
s = strong, m = medium, w = weak and b = broad absorption bands.
Table 3. 1H and 31P NMR Spectral Data of Chloro Cyclopentadienyl Titanium Bis (O, O-Dialkyl and Alkylene Dithiophosphate) Compounds.
alkyl and alkylenedithiophosphate) derivatives were recorded in deuterated chlo- roform at ambient temperature. These compounds did not show any shift compared to corresponding carbons in the dithiophosphoric acid and salt (Table 4).
4.4. 31P NMR Spectra
Decoupled 31P NMR spectra for these products give a singlet. The observation of only one 31P singlet for all compounds reflects the equivalent nature of phosphorous nuclei and the purity of the compound. The values of chemical shifts according to Glidewell’s [31] observation indicates bidentate chelating behavior of the ligand (Table 3).
5. Structural Elucidation
Molecular weight determination of chloro cyclopentadienyl titanium bis (dialkyl and alkylenedithiophosphate) derivatives showed monomeric nature of these compounds in benzene. Thus, on the basis of our observations for IR, NMR (1H,13C and 31P) and molecular weight determinations, the following structures are proposed for these new complexes, as shown in Figure 1 and Figure 2.
6. Conclusion
We have successfully prepared and characterized the chloro cyclopentadienyl ti-
Table 4. 13C NMR Spectral Data of Some Chloro Cyclopentadienyl Titanium Bis (O, O- Dialkyl and Alkylene Dithiophosphate) Compounds.
s = singlet, d = doublet and t = triplet.
Figure 1. Suggested structure for chloro cyclopentadienyl titanium bis (dialkyldithiophosphate) complexes.
Figure 2. Suggested structure for chloro cyclopentadienyl titanium bis (alkylene dithiophosphate) complexes.
tanium bis (alkylene dithiophosphate) complexes. The IR, 1H, 13C and 31P NMR spectra of all of these titanium complexes and the molecular structures of open chain CpTiCl[S2P(OR)2]2 and cyclic compounds Cp TiCl [S2POGO]2 were determined. There are chemical shift differences between the ligand acids and the organotitanium complexes. These changes can be attributed to bidentate phosphorodithioate.
Acknowledgements
The authors are thankful for financial support from REU summer program. The authors also wish to express their profound appreciation for the support received from Dr. Aggarwal, head of the chemistry department. Dean Chance M. Glenn and Shonda Scott.
Cite this paper
El Khaldy, A.A.S., Shanab, A.M.A. and Boni, Y. (2017) Synthesis and Spectroscopic Characterization of Eight Chloro Cyclopentadienyl Titanium Bis (O, O-Dialkyl and Alkylene Dithiophos- phate) Compounds. International Journal of Organic Chemistry, 7, 145-152. https://doi.org/10.4236/ijoc.2017.72012
References
- 1. Ivanov, A.V., Korneeva, E.V., Lutsenko, I.A., Gerasimenko, A.V., Antzutkin, O.N., Larsson, A.-C. and Sergienko, V.I. (2013) A Fixation Mode of Gold from Solutions Using Heterogeneous Reaction of Cadmium Dicyclohexyl Dithiophosphate with H[AuCl4]. Structural and (13C, 31P) CP/MAS NMR Studies and Thermal Behaviour of Crystalline Polymeric Gold(I) Dicy-clohexyl Dithiophosphate and bis(dicyclohexylthiophosphoryl) Disulphide. Journal of Molecular Structure, 1034, 152-161.
https://doi.org/10.1016/j.molstruc.2012.08.052 - 2. Rodina, T.A., Korneeva, E.V., Antzutkin, O.N. and Ivanov, A.V. (2015) Supramolecular Self-Organisation and Conformational Isomerism of a Binuclear O,O0-Dipropyl Dithi-ophosphate Gold(I) Complex, [Au2{S2P(OC3H7)2}2]: Synthesis, 13C and 31P CP/MASNMR Spectroscopy, Single-Crystal X-Ray Diffraction Study and Ther- mal Behavior. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 149, 881-888.
https://doi.org/10.1016/j.saa.2015.04.068 - 3. Ma, N., Li, Y., Xu, H., Wang, Z. and Zhang, X. (2010) Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to pH and cis-Diols. Journal of the American Chemical Society, 132, 442-443.
https://doi.org/10.1021/ja908124g - 4. El Khaldy, A.A.S., Abushanab, A.M. and Abu Alkhair, E. (2011) Synthesis and Antimicrobial Studies of Bis (O, O’-Dialkyl and Alkylene Dithiophosphoric Acids) Adducts of Diphenyl Diselenide. Applied Organometallic Chemistry, 25, 487-595.
- 5. Maheshwari, S., Drake, J.E., Kori, K., Light, M.E. and Ratnani, R. (2009) Synthesis and Spectroscopic Characterization of Tris(O, O’-Ditolyldithiophosphato) Arsenic/Anti- mony/Bismuth(III) Compounds: Crystal Structures of [As{S2P(OC6H4Me-m)(2)}(3)] Center Dot 0.5C(6)H(14), [Sb{S2P(OC6H4Me-m) (2)}(3)] and [Bi{S2P(OC6H4Me-m) (2)}(3)]. Polyhedron, 28, 689-694. https://doi.org/10.1016/j.poly.2008.12.017
- 6. Bingham, A.L., Drake, J.E., Hursthouse, M.B., Light, M.E., Nirwan, M. and Ratnani, R. (2007) Synthesis, Characterization and Spectral Studies of Nitrogen Base Adducts of bis (O, O’-Ditolyldithiophosphato) Nickel(II). Crystal Structur of Ni[S2P(OC6H4Me-p)2]2?C10H8N2 and Ni[S2P(OC6H4Me-o)2]2?C14H12N2?C6H6. Polyhedron, 26, 2672-2678.
https://doi.org/10.1016/j.poly.2007.01.003� - 7. Plante, O.J., Palmacci, E.R., Andrade, R.B. and Seeberger, P.H. (2001) Oligosaccharide Synthesis with Glycosyl Phosphate and Dithiophosphate Triesters as Glycosylating Agents. Journal of the American Chemical Society, 123, 9545-9554.
https://doi.org/10.1021/ja016227r - 8. Henis, N.B.H., Author Links Open the Author Work Space, Busch, K.L. (1981) Methane Enhanced Negative Ionization Mass Spectra of Some Bio-logically Important Chelate Compounds. Inorganica Chimica Acta, 53, L31-L33.
https://doi.org/10.1016/S0020-1693(00)84733-7 - 9. Chauhan, H.P.S., Singh, U.P., Shaik, N.M., Mathur, S. and Huch, V. (2006) Synthetic, Spectroscopic, X-Ray Structural and Antimicrobial Studies of 1,3-Dithia- 2-Stibacyclo-pentane Derivatives of Phosphorus Based Dithiolato Ligands. Polyhedron, 25, 2841-2847.
https://doi.org/10.1016/j.poly.2006.04.027 - 10. Drake, J.E., Gurnani, G., Hursthouse, M.B., Light, M.E., Nirwan, M. and Ratnani, R. (2007) Synthesis and Spectroscopic Characterization of Dimethyl/di(n-butyl) tin(IV) bis (O, O’-Ditolyl Di-thiophosphate) Complexes. Crystal Structures of Me2Sn [S2P(OC6H4Me-o)2]2 and n-Bu2Sn[S2P(OC6H4Me-o)2]2. Applied Organometallic Chemistry, 2, 539-544.
https://doi.org/10.1002/aoc.1265 - 11. Xu, K. and Ding, W. (2008) Controlled Synthesis of Spherical CuS Hierarchical Structures. Materials Letters, 62, 4437-4439.
- 12. Han, Q., Chen, J., Yang, X., Lu, L. and Wang, X. (2007) Preparation of Uniform Bi2S3 Nanorods Using Xanthate Complexes of Bismuth (III). The Journal of Physical Chemistry C, 111, 14072-14077.
https://doi.org/10.1021/jp0742766 - 13. Doane, W.M., Sha-sha, B.S. and Russel, C.R. (1977) Encapsulation of Pesticides Withinstarch Matrix. Controlled Release Pesticides, 53, 74-83.
https://doi.org/10.1021/bk-1977-0053.ch007 - 14. Scendo, M. (2005) Potassium Ethyl Xanthate as Corrosion In-hibitor for Copper in Acidicchloride Solutions. Corrosion Science, 47, 1738-1749.
- 15. Orts, W.J., Sojka, R.E. and Glenn, G.M. (2002) Polymer Additives in Irrigation Water Toreduce Erosion and Better Manage Water Infiltration. Agro Food In-dustry, 13, 37-41.
- 16. Gorgulu, O.A., Arslan, M. and Cil, E. (2006) Synthesis and Characterization of Potassium1,3-Bis(N-methylpiperazino) Propan-2-O-xanthate and the Complexes of Co(II), Ni(II) and Cu(I) Ions. Journal of Coordination Chemistry, 59, 637-642.
https://doi.org/10.1080/00958970500393356 - 17. Larsson, A.-C. and Oberg, S. (2011) Study on Potassium Iso-Propylxanthate and Its Decomposition Products: Experimental 13C CP/MAS NMR Combined with DFT Calculations. The Journal of Physical Chemistry A, 115, 1396-1407.
https://doi.org/10.1021/jp110233d - 18. Amtmann, E. (1996) The Antiviral, Antitumoural Xanthate D609 Is a Competitive Inhibitor of Phosphatidylcholine-Specific Phospholipase C. Drugs under Experimental and Clinical Research, 22, 287-294.
- 19. Perluigi, M., Joshi, G., Sultana, R., et al. (2006) In Vivo Protection by the Xanthatetricyclodec-an-9-yl-Xanthogenate against Amyloid β-Peptide (1-42)-Induced Oxidativestress. Neuroscience, 138, 1161-1170.
- 20. El khaldy, A.A., Hussien, A.R., Abushanab, A.M. and Wasse, M.A. (2011) Synthesis and Characterization of Chloro-Bis (Cyclopentadienyl) Titanium(IV) and Zirconium(IV) O,O’-Dialkyl and Alkylene Dithiophosphates. Phosphorus, Sulfur, and Silicon and the Related Elements, 186, 589-597.
- 21. El Khaldy, A.A.S., Okafor, F. and Abu Shanab, A.M. (2014) Synthesis, Spectraland Antimicrobial Studies of Bis (Cyclopentadienyl) Titanium (IV) Bis (O,O’-Dialkyl and Alkylenedithiophosphate) Complexes. International Journal of Organic Chemistry, 4, 339-346. https://doi.org/10.4236/ijoc.2014.45037
- 22. Kato, S., Hori, A., Shiotani, H., Mizuta, M., Hayashi, N. and Takakuwa, T. (1974) Infrared and Raman Spectra of (Thioacetoxythio) Triorgano Derivatives of Silicon, Germanium, Tin and Lead. Journal of Organometallic Chemistry, 82, 223-228.
- 23. Sowerby, D.B., Haiduc, I., Barbul-Rusu, A. and Salajan. M. (1983) Antimony (III) Diorganophosphoro- and Diorga Nophospinodithioates: Crystal Structure of Sb[S2P(OR)2]3 (R = Me and i-Pr). Inorganica Chimica Acta, 162, 87-96.
- 24. Pavia, D.L., Lampman, G.M. and Kris, G.S. (1996) Introduction to Spectroscopy. 2nd Edition, Saunders Golden Sunburst Series, Orlando.
- 25. Corbridge, D.E.C. (1969) Infra-Red Spectra of Phosphorus Compounds. Topics in Phosphorus Chemistry, 6, 235-366.
- 26. Ohkaku, N. and Nakamoto, N. (1973) Metal Isotope Effect on Metal-Liquid Vibrations. X Far-Infrared Spectra of Trans Adducts of Tin (IV) Tetrahalide with Unidentate Ligands. Inorganic Chemistry, 12, 2440-2446.
https://doi.org/10.1021/ic50128a043 - 27. Lockhart, T.P. and Manders, W.P. (1986) Structure Determination by NMR Spectroscopy. Dependence of |2J (119Sn, 1H)| on the Me-Sn-Me Angle in Methyltin(IV) Compounds. Inorganic Chemistry, 25, 892-895.
https://doi.org/10.1021/ic00227a002 - 28. Drew, M.G.B., Baricalli, P.J., Mitchell, P.C.H. and Read, A.R. (1983) Crevice Coordination: Binding of a Ligand Molecule in a Molecular Crevice. Crystal and Mo-lecularStructures of μ-Oxo-μ-pyridine-μ-sulphido-bis[(OO’-diisopropyl phosphorodithioato) oxo-molybdenum(V)] and μ-Oxo-μ-pyridazine-μ-sulphido-bis[(OO’- diisopropyl phosphorodithioato) oxomolybdenum(V)]. Journal of the Chemical Society, 649-655. https://doi.org/10.1039/DT9830000649
- 29. Gupta, R.K., Rai, A.K., Mehrotra, R.C. and Jain, V.K. (1984) Cyclic O, O Alkylenedithiophosphates of Phenyl-Arsenic and -Antimony. Inorganica Chimica Acta, 88, 201-207.
- 30. Gupta, R.K., Rai, A.K., Mehrotra, R.C., Jain, V.K., Hoskins, P.F. and Tiekink, E.R.T. (1985) Phenylarsenic (III) and Phenylantimony (III) Bis (dialkyl dithiophosphates): Synthesisand Multinuclear (Proton, Carbon-13, Phosphorus-31) NMR and Mass Spectral Studies. Crystal and Molecular Structures of C6H5M [S2P(OCHMe2)2]2 [M = Sb(III) and As (III)]. Inorganic Chemistry, 24, 3280-3284.
https://doi.org/10.1021/ic00214a037 - 31. Glidewell, C. (1977) Ambident Nucleophiles: VI. Solution Metal-Ligand Binding Modes in Phosphorodithioate Complexes. A Phosphorus-31 N.M.R. Study. Inorganica Chimica Acta, 25, 159-163.




