International Journal of Organic Chemistry
Vol.05 No.04(2015), Article ID:61926,7 pages
10.4236/ijoc.2015.54026
New Schiff Bases from 6,6’-Diformyl-2,2’-Bipyridyl with Amines Containing O, S, N and F: Synthesis and Characterization
Md. Razzak1, Mohammad R. Karim1*, Md. Rejaul Hoq1, Aminul H. Mirza2
1Department of Chemistry, Tennessee State University, Nashville, USA
2Department of Chemistry, Faculty of Science, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei Darussalam

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 29 October 2015; accepted 13 December 2015; published 16 December 2015

ABSTRACT
Eight novel Schiff Bases, from 6,6′-diformyl-2,2′-bipyridyl (1a, 43%)with O, N, S and F containing amines: Thiosemicarbazide (2a, 70%), 4-Ethyl-3-thiosemicabazide (2b, 75%), 4,4-Dimethyl-3-thio- semicarbazide (2c, 75%), S-benzyldithiocarbazide, SBDTC (2d, 80%), (Trifluromethyl) phenylhydrazine (2e, 80%), 4-Phenyl-3-thiosemcarbazide (2f, 80%), Thiocarbazide (2g, 70%), 2-Amino- thiophenol (2h, 65%), have been synthesized. The conventional method of synthesis of the Schiff bases involves refluxing the reaction mixture containing the diformyls and amines for 1 hour. The solid products that had formed were filtered off using suction filtration. In few reactions, 2 - 3 drops of conc. sulfuric acid were used to obtain high yield. The structures of all eight novel synthesized compounds have fully been characterized by spectroscopic (IR, NMR, MS) methods.
Keywords:
2,2’-Bipyridyl, Schiff Base, O, N, S and F Containing Amines, 6,6’-Diformyl

1. Introduction
Previously we have reported synthesis of several Schiff Bases derived from 1, 10-phenanthroline-2,9-dicarbal- dehyde [1] . Phenanthroline backbone rather produced structurally rigid, less flexible Schiff Bases. We therefore, decided to add more flexibility to the molecule by synthesizing similar Schiff Bases derived form 6,6’-difor- myl-2,2’-bipyridyl, thereby eliminating structural rigidity. Moreover, design and synthesis of organic chelating agents containing nitrogen and sulfur as donor atoms is our major focus. In this case, bi-dentate N,N chelating agent such as 2,2’-bipyridyl is expected to play a vital role in building many mixed-ligand complexes for their desired predictable co-ordination behavior and their electrochemical and photo-physical properties [2] - [4] . The 2,2’-bipyridyl and ligands derived from it also extensively used in different areas, such as molecular scaffolding, supramolecular assemblies, catalysis, biochemistry, electrochemistry, ring-opening metathesis polymerization and biochemistry [5] - [9] , biologically photoredox reactions [10] , synthetic, medicinal chemistry, biotechnology [11] and solar cell [12] [13] . Being more flexible, these Schiff bases are expected to form stable complexes with a wide variety of metal ions, which are expected to show interesting properties as observed in the literature for similar metal complexes [14] - [16] . In view of the importance of Schiff bases derived from 6,6’-formyl-2,2’-bipyridyl and O, S, N and F-containing amines in different fields, we report here synthesis and characterization of eight new Schiff bases from 6,6’-diformyl-2,2’-bipyridyl. We also plan to continue studies like anti-cancer, antibacterial, and antitumor activities with novel Schiff bases derived from 6,6’-formyl-2,2’-bipyridyl with O, S, N and F-containing amines.
2. General Method and Procedures
2.1. Experimental
HPLC grade solvents were used in all the reactions. All reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and ACROS (drive Pittsburgh, PA, USA) and were used without further purification. Routine thin-layer chromatography (TLC) was performed on aluminum-backed Whatman, Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The conventional method of synthesis of the Schiff bases involves refluxing the reaction mixture containing the diformyls and amines for 1 hour. The solid product that had formed was filtered off using suction filtration. In some of the reactions, 2 - 3 drops of conc. sulfuric acid were used to obtain high yield. To obtain NMR spectra, all compounds were dissolved in DMSO-d6 and recorded on a Bruker Ascend 400 M Hz NMR spectrometer using TMS as an internal standard. To obtain Mass spectra, samples were dissolved in CH3CN:H2O:AcOH (50%:50%:0.1%) and injected by a direct infusion method. Data were recorded on a LTQ XL linear Ion Trap Mass spectrometer with electrospray ionization (ESI) mode. MS spectrophotometer was purchased from Thermo Scientific. All infra-red (IR) data were recorded (υmax in cm−1) on Smart iRT purchased from Thermo Scientific.
2.2. Synthesis of 6,6′-Diformyl-2,2′-Bipyridyl (1a) from 6,6′-Dimethyl-2,2′-Bipyridyl
6,6′-Diformyl-2,2′-bipyridyl (245 mg, 43%) 1a was synthesized from 6,6′-dimethyl-2,2′-bipyridyl (500 mg, 0.003 mol) through direct oxidation by SeO2 (3 g, 0.03 mol) in presence of glacial acetic acid (40 ml) following a previously reported procedure [17] . 1H NMR and 13C NMR data were consistent with the literature value (Scheme 1).
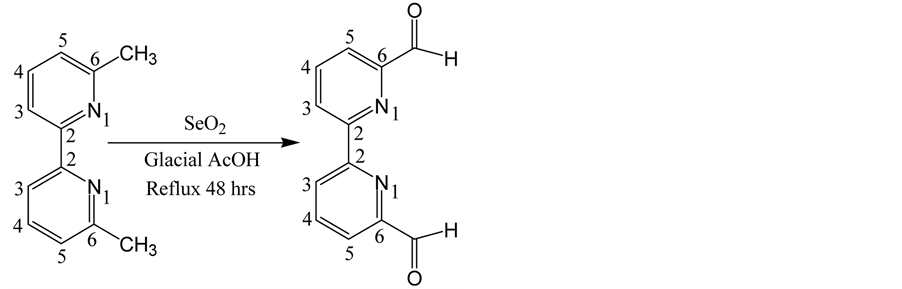
6, 6′-dimethyl-2, 2′-bipyridyl 1a, 43%
Scheme 1. Synthesis of 6,6′-diformyl-2,2′-bipyridyl (1a) from 6,6′- dimethyl-2,2′-bipyridyl.
2.3. General Procedure for Synthesis of Schiff Bases (2a-2h) from 6,6′-Diformyl-2,2′-Bipyridyl (1a)
3 equivalents of O, N, S and F containing amines were added to a solution of 1 equivalent of 6,6'-diformyl-2,2'- bipyridyl (1a) in 30 ml of MeOH. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum. For synthesizing compound 2d, S-benzyldithiosemicarbazide was prepared following a previously reported procedure [18] and 1H NMR and 13C NMR data were consistent with the literature value. 2 - 3 drops of conc. H2SO4 were added in the reaction mixture to obtain high yield of compound 2e (Scheme 2). The spectral data to confirm the structures of all eight desired Schiff bases have been shown in Table 1.
Table 1. Spectral data of the synthesized compounds.
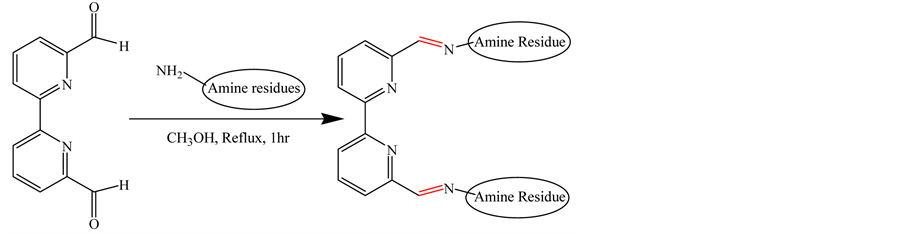
1a 2a-2h
Scheme 2. Synthesis of Schiff Bases from 1a with O, N, S and F containing amines. 2a, amine: Thiosemicarbazide (Y 70%); 2b, amine: 4-Ethyl-3-thiosemicarbazide (Y 75%); 2c, amine: 4,4-Di- methyl-3-thiosemicarbazide (Y 75%); 2d, amine: 4 with S-Benzyldithiosemicarbazide (Y 80%); 2e, amine: (Trifluromethyl) phenylhydrazine, (Y 80%); 2f, amine: 4-Phenyl-3-thiosemicarbazide (Y 80%); 2g, amine: Thiocarbazide (Y 70%); 2h, amine: 2-Aminothiophenol (Y 65%).
3. Results and Discussions
The synthesis of novel Schiff Bases were outlined in Scheme 3. All compounds were obtained from 6, 6'-diformyl-2, 2'-bipyridyl and confirmed by the help of infrared, 1H-NMR, 13C-NMR and Massspectrometry. In IR spectrum of 2a, bands appeared at 3433 cm−1, 3240 cm−1 and 3154 cm−1 were identified as stretching frequencies for the presence of NH and NH2 group. The IR spectrum of 2a revealed C=N stretching band of imine group at 1522 cm−1. Band at 1595 cm−1 was for aromatic C=C bond. Band at 1114 cm−1 was due to the presence of C=S bond in amine residue. 1H-NMR spectrum of compound 2a showed peak at 11.77 ppm (highly deshielded) as singlet for the presence of secondary amine >N-H which confirms the formation of imine C=N bond. 6 pyridyl protons appeared between 8.45 - 8.30 ppm as multiplet. Total number of 13C-NMR peaks were 7 in which peak at 178 ppm was for the presence of C=S group. Other peaks appeared between 154 and 121. In LC-MS, the molecular ion as base peak appeared at m/z 381 (M+Na).
IR spectrum of compound 2b showed two bands 3314 cm−1 and 3155 cm−1 due to NH group. Bands at 2960 cm−1 and 2810 cm−1 stretching frequencies were for >CH group. The imine C=N bond showed IR band at 1515 cm−1 whereas 1573 cm−1 for aromatic C=C bond. Thiol (C=S) band appeared at 1076 cm−1. In 1H-NMR for the compound 2b, two singlet peaks appeared at 11.80 ppm and 8.20 ppm due to 4 N-H groups. 2 pyridine protons showed triplet at 8.79 ppm with coupling constant value of 4 Hz. 4 pyridine ring and 2 imine protons showed peaks as multiplet between 8.40 - 8.3 ppm. Other 2 pyridine ring protons showed triplet at 8.03 ppm with coupling constant, J1 = J2 = 8 Hz. 4 methylene protons appeared as multiplet at 3.65 ppm whereas 6 methyl protons at 1.2 ppm with coupling constant of 7 Hz. 9 peaks were observed for compound 2b in 13C-NMR spectrum with 177 ppm for thiol carbon and remaining peaks appeared for pyridine and aliphatic carbons. Molecular ion peak appeared at m/z 437 (M + Na) and M+H ion peaks at m/z 415 for compound 2b.
Compound 2c, IR spectrum showed bands at 3415 cm−1, 3345 cm−1 and 3220 cm−1 for the group N-H’s stretching frequencies. >CH group’s stretching bands appeared at 2910 cm−1 and 2870 cm−1. Imine band appeared at 1470 cm−1. The bands at 1557 cm−1 and 911 cm−1 were for C=C bond (aromatic) and thiol bond C=S, respectively. In compound 2c, the indication of imine bond formation in 1H-NMR spectrum was found at 11.40 ppm due to two N-H protons which is in highly deshielded region. 6 pyridine ring protons and 2 imine proton peaks appeared between 8.40 - 8.3 ppm and 8.10 - 7.90 ppm as multiplet. 12 methyl protons peak appeared at 3.35 ppm as singlet. Number of peaks in 13C-NMR spectrum for compound 2c were 8, including thiol carbon at 180 ppm. The molecular ion peak for compound 2c appeared at m/z 437 (M + Na).
Compound 2d in IR spectrum showed only 1 band at 3115 cm−1 for the group NH. The stretching frequencies at 2988 cm−1 and 2849 cm−1showed the presence of =CH groups. The imine and thiol bands appeared at 1510 cm−1 and 1041 cm−1 whereas aromatic C=C bond frequency appeared at 1580 cm−1. Peak at 13.70 ppm in 1H-NMR spectrum appeared for two N-H groups as singlet which is highly deshielded region and implies the imine bond formation. Two doublets at 8.40 ppm and 7.98 ppm with J = 8 Hz appeared for 4 pyridine ring protons. The imine proton peak appeared at 8.35 ppm as singlet for compound 2d. Another peak at 8.03 ppm as triplet showed for 2 pyridine ring protons. 8 benzene protons appeared at 7.30 ppm and 7.35 ppm as triplet with coupling constant J1 = J2 = 8 Hz where as other two at 7.45 ppm as doublet with J = 8 Hz. There were twelve
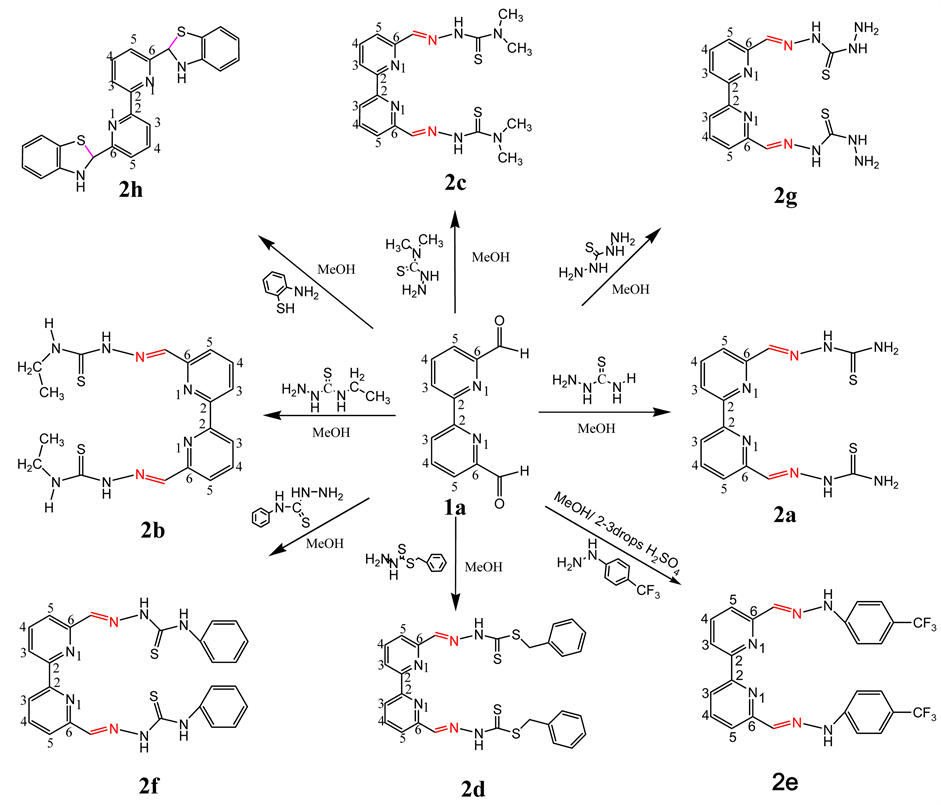
Scheme 3. Synthetic pathways of Schiff Bases from 1a with different O, N, S and F containing amines.
13C-NMR peaks appeared in 2d compound spectrum with thiol peak at 198 ppm. Other peaks were for aliphatic, pyridine and benzene rings. The molecular ion peak for compound 2d was shown at m/z 594.83 in LC-MS.
There were 2 IR bands at 3334 cm−1 and 3089 cm−1 for the presence of NH for compound 2e. 1 stretching frequency observed at 2940 cm−1 due to =CH. For imine bond and aromatic C=C bond, bands appeared at 1505 cm−1 and 1615 cm−1 respectively. A singlet in 1H-NMR spectrum in highly deshielded region at 11.20 ppm of two N-H groups helps to determine the formation of imine bond. 2 doublets appeared at 7.60 ppm and 7.30 ppm of 8 benzene ring protons with J value 6 Hz for compound 2e. Between 8.11 - 8.05 ppm, peak of 4 of pyridine ring protons appeared as multiplet whereas 2 pyridine ring protons at 8.35 ppm appeared as doublet with J = 6 Hz and remaining 2 pyridine ring protons appeared at 8.02 ppm as triplet with J1 = J2 = 6 Hz. 13C-NMR spectrum for compound 2e showed 11 peaks all with pyridine, benzene and aliphatic regions. In LC-MS spectrum, the molecular ion peak for the compound 2e appeared at m/z: 529.01 (M + H).
There were 3 IR stretching frequencies 3290 cm−1, 3149 cm−1 and 3000 cm−1 appeared for NH group for compound 2f. 1 stretching frequency at 2940 cm−1 appeared for =CH group whereas imine, aromatic and thiol bond frequencies were observed at 1550 cm−1 , 1595 cm−1 and 1163 cm−1 respectively. For compound 2f, a singlet in highly deshielded region peat appeared at 12.20 ppm due to 2N-Hgroups which helps to identify the formation of imine bond. Peak at 10.30 ppm appeared for 2N-H groups as singlet. 4 doublets appeared at 8.55 ppm, 8.40 ppm, 8.3 ppm and 7.55 ppm with coupling constant value 8 Hz in which first 2 of them were pyridine ring protons, imine protons and benzene ring protons respectively. 3 triplets appeared at 8.11 ppm, 7.40 and 7.25 ppm with coupling constant value J1 = J2 = 8 Hz for 2 pyridine ring protons, 4 benzene ring protons and 2 benzene ring protons respectively. In total 11 peaks appeared in 13C-NMR spectrum for the compound 2f with all in the pyridine carbon and benzene carbon regions. LC-MS spectrum showed molecular ion as base peak at m/z: 551 (M + K).
2 stretching frequencies observed in IR spectrum for the compound 2g at 3264 and 3120 cm−1 due to the presence of NH group. 2 stretching frequencies at 2946 cm−1 and 1556 appeared for =C-H and aromatic C=C groups. The imine and thiol bands appeared at 1505 cm−1 and 1042 cm−1 respectively. Compound 2g in 1H-NMR spectrum showed peak at 11.80 ppm as singlet due to 2N-H groups which is in highly deshielded region and thus indicated the imine bond formation. Other 2N-H groups’ singlet peaks appeared at 10.10 ppm which was also relatively deshielded region. 3 doublet peaks observed at 8.48 ppm, 8.33 ppm and 8.14 ppm for 6 pyridine ring protons and imine protons with the value of coupling constant 8 Hz whereas 1 triplet peak appeared at 8.00 ppm for 2 pyridine protons with J value 8 Hz equally. There was a broad singlet observed at 5.10 ppm due to presence of 2 NH2 groups in compound 2g. In 13C-NMR spectrum for compound 2g, 7 peaks were observed with thiol carbon at 176 ppm. Mass analyses showed base peak at 389 (M + H).
For compound 2h, 2 bands appeared for N-H group at 3360 cm−1 and 3053 cm−1 due to stretching vibrations. 1 band appeared at 2920 for the presence of =CH group. Aromatic C=C bond frequency appeared at 1562 cm−1. Compound 2h showed twisted geometry that appeared in both 1H-NMR and 13C-NMR spectrums. In 1H-NMR spectrum of compound 2h, 3 singlet peak appeared: at 8.32 ppm for 1 thiazolyl ring C-H proton, at 6.5 ppm as singlet for 1 thiazolyl ring N-H proton and 7.25 ppm for another thiazolyl ring N-H proton. 4 doublet peaks observed at 8.52 ppm, 7.70 ppm, 7.05 ppm and 6.75 ppm for 1 pyridine ring proton and later 3 peaks for benzene ring protons. 2 triplet peaks appeared at 6.90 ppm and 6.67 ppm due to 2 benzene ring protons. 2 multiplet peaks were observed between 8.38 - 8.5 ppm for 2 pyridine ring protons and between 8.30 - 8.08 ppm for 3 pyridine protons and 1 thiazolyl C-H protons. 1 multiplet peak between 7.65 - 7.50 ppm due to 3 benzene ring protons. In 1H-NMR spectrum of compound 2h, all coupling constant value were observed at J = 4 Hz. In 13C-NMR spectrum, 22 peaks of carbon were observed due to twisted geometry of the product which made 2h carbons diastereotopic in nature. All carbons appeared in pyridine ring, benzene ring regions. Molecular ion peak appeared in LC-MS spectrum at m/z: 449 (M + Na).
4. Conclusion
Eight novel Schiff bases of 6,6’-diformyl-2,2’-Bipyridyl with O, S, N and F containing amines have been successfully synthesized. Conc. sulfuric addition has been found to significantly enhance the yields of the products. In some cases, sulfuric acid was not added to avoid salt formation with nitrogen and amine group. However, it was observed that the yield increased significantly when the reaction was carried out under mild acidic conditions. This is due to the fact that protonation of the carbonyl group (C=O) enhances the nucleophilic attack-NH2 group of the amine.
Acknowledgements
We thank the Department of Chemistry at Tennessee State University for providing the necessary support to carry out the research. We also thank the Department of Education, Title III funds for providing instrumental support.
Cite this paper
Md.Razzak,Mohammad R.Karim,Md. RejaulHoq,Aminul H.Mirza, (2015) New Schiff Bases from 6,6’-Diformyl-2,2’-Bipyridyl with Amines Containing O, S, N and F: Synthesis and Characterization. International Journal of Organic Chemistry,05,264-270. doi: 10.4236/ijoc.2015.54026
References
- 1. Jaman, Z., Karim, M.R., Siddiquee, T.A., Mirza, A.H. and Ali, M.A. (2013) Synthesis of 5-Substituted 2, 9-Dimethyl-1,10-Phenanthroline Dialdehydes and Their Schiff Bases with Sulfur-Containing Amines. International Journal of Organic Chemistry, 3, 214-219. http://dx.doi.org/10.4236/ijoc.2013.33029
- 2. Siebert, R., Winter, A., Dietzek, B., Schubert, U.S. and Popp, J. (2010) Dual Emission from Highly Conjugated 2,2':6':2"-Terpyridine Complexes—A Potential Route to White Emitters. Macromolecular Rapid Communications, 31, 883-888.
http://dx.doi.org/10.1002/marc.200900894 - 3. Siebert, R., Winter, A., Schubert, U.S., Dietzek, B. and Popp, J. (2010) Excited-State Planarization as Free Barrierless Motion in a π-Conjugated Terpyridine. The Journal of Physical Chemistry C, 114, 6841-6848.
http://dx.doi.org/10.1021/jp100313x - 4. Siebert, R., Winter, A., Schubert, U.S., Dietzek, B. and Popp, J. (2011) The Molecular Mechanism of Dual Emission in Terpyridine Transition Metal Complexes—Ultrafast Investigations of Photoinduced Dynamics. Physical Chemistry Chemical Physics, 13, 1606-1617.
http://dx.doi.org/10.1039/C0CP01134G - 5. Binnemans, K., Lenaerts, P., Driesen, K. and Gorller-Walrand, C. (2004) A Luminescent Tris(2-thenoyltrifluoroace- tonato)europium(III) Complex Covalently Linked to a 1,10-Phenanthroline-Functionalised Sol-Gel Glass. Journal of Materials Chemistry, 14, 191-195.
http://dx.doi.org/10.1039/b311128h - 6. Larsson, K. and Ohrstrom, L. (2004) X-Ray and NMR Study of the Fate of the Co(1,10-phenanthroline-5,6-diketone)33+ Ion in Aqueous Solution: Supramolecular Motifs in the Packing of 1,10-Phenanthroline-5,6-Diketone and 1,10-Phe- nanthroline-5,6-Diol Complexes. Inorganica Chimica Acta, 357, 657-664.
http://dx.doi.org/10.1016/j.ica.2003.07.001 - 7. Steed, J.W. and Atwood, J.L. (2000) Supramolecular Chemistry. Wiley, Chichester.
- 8. Chow, C.S. and Bogdan, F.M. (1997) A Structural Basis for RNA—Ligand Interactions. Chemical Reviews, 97, 1489-1514.
http://dx.doi.org/10.1021/cr960415w - 9. Sammes, P.G. and Yahioglu, G. (1994) 1,10-Phenanthroline: A Versatile Ligand. Chemical Society Reviews, 23, 327-334.
http://dx.doi.org/10.1039/cs9942300327 - 10. Balzani, V., Juris, A., Venturi, M., Campagna, S. and Serroni, S. (1996) Luminescent and Redox-Active PolynuclearTransition Metal Complexes. Chemical Reviews, 96, 759-834.
http://dx.doi.org/10.1021/cr941154y - 11. Daniel, S. and Gnana Raj, G.A. (2013) Photoinduced Electron-Transfer Reactions of Tris(4,4'-dinonyl-2,2'-bipyridyl) Ruthenium(II) Cation with Phenolate Ions in Aqueous Acetonitrile. Journal of Chemical and Pharmaceutical Research, 5, 220-227.
- 12. Smith, N.A. and Sadler, P.J. (2013) Photoactivatable Metal Com-plexes: From Theory to Applications in Biotechnology and Medicine. Philosophical Transactions of the Royal Society A, 373, Article ID: 20120519.
http://dx.doi.org/10.1098/rsta.2012.0519 - 13. Monat, J.E., Rodriguez, J.H. and McCusker, J.K. (2002) Ground- and Excited-State Electronic Structures of the Solar Cell Sensitizer Bis(4,4'-dicarboxylato-2,2'bipyridine)bis(isothiocyanato)ruthenium(II). Journal of Physical Chemistry A, 106, 7399-7406.
http://dx.doi.org/10.1021/jp020927g - 14. Gomathi, V., Selvameena, R., Subbalakshmi, R. and Valarmathy, G. (2013) Synthesis, Spectral Characterization and Antimicrobial Screening of Mn(ll) and Zn(ll) Complexes Derived from (E)-1-((p-tolylimino)methyl)naphthalene-2-ol. Oriental Journal of Chemistry, 29, 533-538.
http://dx.doi.org/10.13005/ojc/290220 - 15. Wang, J., Onions, S., Pilkington, M., Stoeckli-Evans, H., Halfpenny, J.C. and Wallis, J.D. (2007) Metal Catalyzed Rearrangement of a 2,2-Bipyridine Schiff-Base Ligand to a Quaterpyridine-Type Complex. Chemical Communications, 2007, 3628-3630.
http://dx.doi.org/10.1039/b705555b - 16. Hodacova, J. and Budesmsky, M. (2007) New Synthetic Path to 2,2'-Bipyridine-5,5'-Dicarbaldehyde and Its Use in the [3+3] Cyclocondensation with Trans-1,2-Diaminocyclohexane. Organic Letters, 9, 5641-5643.
http://dx.doi.org/10.1021/ol702612t - 17. Newkome, G.R. and Lee, H.-W. (1983) 18[(2,6) Pyridin6coronand-6: “Sex-ipyridine”. Journal of the American Chemical Society, 105, 5956-5957.
http://dx.doi.org/10.1021/ja00356a061 - 18. Mughrabi, F.F., Hashim, H., Ameen, M., Khaledi, H. and Ali, H.M. (2011) Cytoprotective Effect of Benzyl N'-(indol- 3-ylmethylidene)-Hydrazinecarbodithioate against Ethanol-Induced Gastric Mucosal Injury in Rats. African Journal of Pure and Applied Chemistry, 5, 34-42. |
http://www.academicjournals.org/AJPAC.
NOTES
*Corresponding author.



