International Journal of Organic Chemistry
Vol. 2 No. 3 (2012) , Article ID: 22753 , 9 pages DOI:10.4236/ijoc.2012.23031
Suggested New Terms for Describing Chiral States and the State-Dependent Behavior of Chiral Systems
Department of Chemistry, University of Turku, Turku, Finland
Email: klikakd@yahoo.co.uk
Received May 9, 2012; revised June 15, 2012; accepted June 29, 20123
Keywords: Stereochemistry; Terminology; Chirality; State-Dependent Behavior; Conglomerate; Racemate
ABSTRACT
Deficiencies in the terminology used to describe chiral systems exist for behaviors under various processes and thus a more general, robust terminology is considered. For example, the descriptions for characterizing melting point, solubility, and recrystallization behaviors were adopted well before it was realized that perturbation of the enantiomeric composition (ec) due to self-disproportionation could be effected by processes other than recrystallization such as sublimation, chromatography over achiral substrates, and even distillation. Thus, an endeavor has been made to address the question of universally describing behaviors under processes that effect, or are dependent on, the ec. The main terms that have been defined with respect to behavior are homomate (analogous to a conglomerate), heteromate, bimate (analogous to a racemic compound), and unimate (analogous to a solid solution) and they apply to melting point, solubility, recrystallization, sublimation, distillation, and chromatographic processes. Additionally, suggestions for improving the terminology for describing the states of chiral systems are also considered and the defined terms are: holemate (hol, ec = 100%), scalemate (scl, 50% < ec < 100%), and equimate (eqm, ec = 50%).
A clear consensus for describing chiral systems, both for processes, behaviors, and for the general state of systems, does not seem to be in effect despite the enormous amount of study devoted to chiral systems and their associated behaviors ever since the seminal experiments of Pasteur into the relationships between the crystalline state and chemical composition and their relation to optical rotation (OR) [1,2]—thus consequently the very embodiment of chirality and the subsequent inferences for homochirality, not to mention of course, the very report of the spontaneous resolution of a conglomerate itself [3]. Invariably, whatever particular description is adopted encounters objection from one quarter or another and hence the obvious question, though rhetorical in nature, is, what is the origin of the confusion and ambiguity that exists? In part, confusion and ambiguity arise because authors may be, for example, be referring to a single molecule or type of molecule or, on the other hand, an aggregation of molecules or a real sample analysis without taking due care to be precise in their meaning and simply assuming that the context of the discussion is sufficient to effect a distinction. Another source of confusion is the fact that as new frontiers were explored or developed, the terminology failed to keep pace and terms were simply borrowed out of convenience. There is no better example of this than the term homochiral1 [4-6]. Thus it is the intention of this report to help alleviate some of the deficiencies that have developed in the terminology in use with respect to chiral systems, especially since the systems that give rise to varying chiral behaviors have risen in number and complexity over the course of time.
One argument against new terminology, however, is that it in itself creates additional confusion and can be misleading, but this of course is not necessarily true. For example, the concept of inverse epimers [7] and the depiction of the relative stereochemistry [8] have been found to be most convenient and quite useful for the alnumycin Als [7,9,10] derived from bacterial sources [11]. To further illustrate the proposed [8] extension to the Natta projection system2, the following monoterpene systems can be considered. For the diepoxides of limonene (1a) [12] depicted in Figure 1 with three effective stereogenic centers3, the total number of possible stereoisomers is eight. The epoxidation of (+)-(4R)-limonene can thus yield four stereoisomers, but the depiction of all four of these stereoisomers (1b) in the one figure using conventional wavy-bond notation for indeterminate stereochemistry implies that in addition to 1S,2R and 1R,2S configurations, 1R,2R and 1S,2S configurations are also possible. Chemical sense prevails for the experienced reader, but for uninitiates and computer software, this may be less evident. Similarly for the cis and trans pair of stereoisomers depicted in 1c, but which can be portrayed unambiguously and errant-free by the depiction in 1d. Thus, for the depiction of all four stereoisomeric diepoxides of (+)-(4R)-limonene, structure 1e nicely fulfills the demands for clarity and consistency with chemical restraints.
Similarly, for 2a,9-dihydroxy-1,8-cineole [13], to depict both enantiomers of this compound unambiguously, structure 2a depicted in Figure 2 fulfills the demands in contrast to 2b which, due to the undefined stereo bonds at C1-C2 and C1-C6, implies the presence of one or other or both of the C-8 inverse epimers [7] {viz. (1S,2S,4R,8R) -2a,9-dihydroxy-1,8-cineole and (1R,2R,4S,8R)-2a,10- dihydroxy-1,8-cineole}, whilst 2c with undefined stereo bonds at C8-C9 and C8-C10, implies the presence of one or other or both of the C-8 epimers {viz. (1S,2S,4R,8R) -2a,9-dihydroxy-1,8-cineole and (1S,2S,4R,8S)-2a,10- dihydroxy-1,8-cineole}, and finally, 2d merely implies the presence of one or other or both of (1S,2S,4R,8R)-2a, 9-dihydroxy-1,8-cineole and (1S,2R,4R,8R)-2b,9-dihydroxy-1,8-cineole. Clearly there are advantages to using the prescribed notion outlined previously [8] since using the conventional wavy-bond notation for indeterminate stereochemistry cannot permit the depiction of enantiomers without at least also indicating other diastereomers in this instance. Of note, these bond depictions of the recently proposed extension to the Natta projection system, in addition to being clear and unambiguous, can also readily lend themselves to stereo designations in computer software.
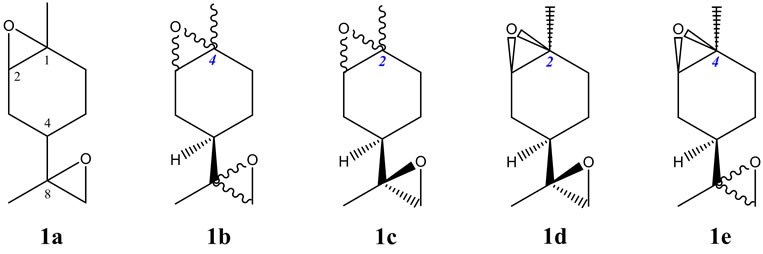
Figure 1. Various depictions of the stereoisomeric diepoxides of limonene.
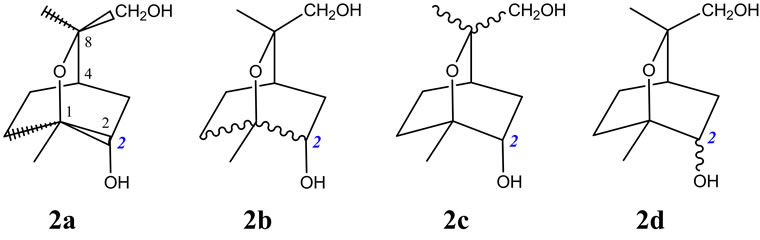
Figure 2. Various depictions of the stereoisomeric 2a,9/ 10-dihydroxy-1,8-cineoles.
Overall, the terminology in use for processes, behaviors, and for the general state of systems is multiple, restricted, on occasion inadequate, and sometimes ambiguous. Indeed, Gal [4,5], Eliel [6], Klika [8], Gawley [14], Kagan [15], Brewster [16], Heathcock [17], and the Cornforths [18], as well as many others, have recognized the need and urged for change. Two examples of ambiguous terms, homochiral and racemate, illustrate the point. Firstly, the original meaning of homochiral was defined by Lord Kelvin to refer to the stereochemical relationship between molecules (or between substituents, moieties, etc., within a molecule) that have the same sense of chirality, yet its meaning was, due to lack of a suitable alternative, expanded to incorporate the enantiomeric composition4 (ec) of a sample [4,5], i.e. it morphed from molecular designate into a macroscopic description leading to ambiguity and oft times confusion. Secondly, the term “racemate” can elicit confusion [19] since its use may be intended to refer to a sample mixture comprising of a 1:1 composition of the enantiomers, or the user may be referring to a sample which will crystallize out as a mixture of the enantiomers with a set ratio, e.g. 1:1, of the enantiomers in the unit cell. The former application of the term is an analytical statement, the latter usage a statement on the behavior of a system5. It is worth noting that crystallization of the “racemate” can readily occur for compositions other than, and indeed well away from, 50% ec.
To consider various processes, take for example the melting point behavior of a mixture of an enantiomer pair as the ec ranges from 0% - 100% for one enantiomer. The melting point diagrams may conform to a conglomerate (alternatively, “racemic mixture” or “racemic conglomerate”) as depicted in Figure 3, or a racemic compound (alternatively, “true racemate” or just “racemate”) as depicted in Figure 4. A third, very distinct, behavior is also possible, that of a solid solution (alternatively, “racemic solid solution”, “mixed crystal”, or “pseudoracemate”) with constant melting point. Whilst a plethora of terms is obviously undesirable, they do seem to, nonetheless, adequately describe idealized behavior.
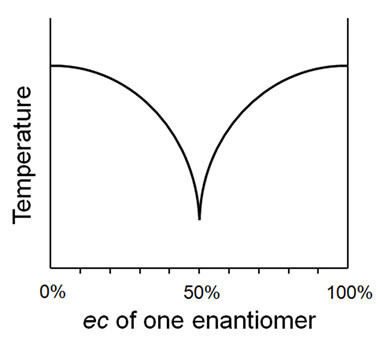
Figure 3. Ideal conglomerate melting point behavior.
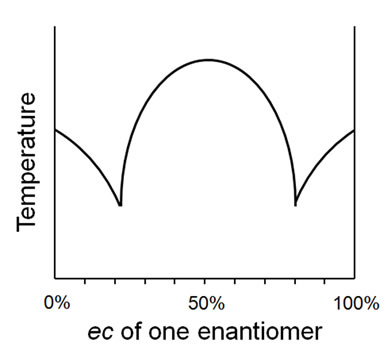
Figure 4. Ideal racemic compound melting point behavior.
However, for unconventional behavior such as depicted in Figure 5, should a compound behaving like this be described as a conglomerate or a racemic compound? Indeed, from either side of the 50% ec point, the linearity is suggestive of a solid solution. Thus, amendment to the terms currently in use could be worth considering for this point alone.
Furthermore, the aforementioned categorizations are, by well-entrenched custom, limited to melting point and solubility behaviors. But other processes, again also depending on the ec of the sample, can too exhibit divergent behavior or lead to perturbation of the ec due to the interaction of enantiomers with themselves (the term self-disproportionation of enantiomers (SDE) has been coined for such a process [20]) and these include sublimation, distillation, as well as chromatography over achiral substrates. Finally, spectroscopic measurements in solution can also be perturbed by the preference for either homochiral or heterochiral interactions.
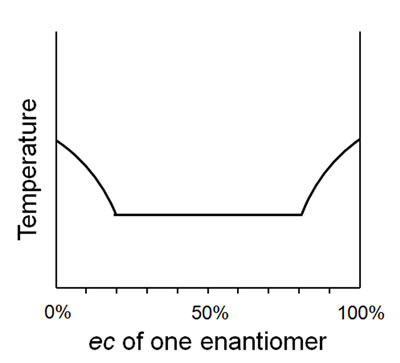
Figure 5. Less conventional melting point behavior displaying a mix of conglomerate and racemic compound/solid solution behaviors.
Sublimation has been shown to readily alter the ec of a sample in either the sublimate or the residue [21-30], but what constitutes a conglomerate or a racemic compound under such a process? For not only can the ec of the residue alter, but a tendency towards optical purification or the racemate can actually be dependent on the starting ec with, for example, high ec samples tending towards the optically pure state, i.e. the pure enantiomer, and conversely low ec samples tending towards the racemate. Clearly, eutectic points (ep), as for melting point/solubility behavior, exist for such systems. Of note, for some compounds exhibiting SDE via sublimation, there are cases which tend to either the pure enantiomer or the racemate irrespective of the starting ec. For the purposes of the definitions that follow, clearly the solid residue can be taken as the more stable state and the vapor/sublimate as the less stable. Thus, the former behavior is clearly akin to usual conglomerate behavior whilst the latter behavior is not comparable to any described behavior for recrystallization but can be considered to represent a type of reverse conglomerate behavior. Other mixed behaviors not clearly associated with a simple descriptor are also evident.
SDE via distillation [31,32], though exceedingly rare, can similarly exhibit one trend or another with respect to the change in the ec of the pot residue or the distillate. In fact, multiple trends across the ec can occur, i.e. ep are identifiable. Thus, what terms are preferable to describe the boiling point trends or the trends of the ec changes of either the pot residue or the distillate? For the purposes of the definitions that follow, clearly the pot residue can be taken as the more stable state and the vapor/distillate as the less stable. For one system [31], behavior in many respects resembles that of a racemic compound (an ep of lower stability with respect to both the pure enantiomer and the racemate) whilst inverted behavior also seems evident for another system [32] (an ep of higher stability with respect to both the pure enantiomer and the racemate).
SDE via chromatography, specifically referred to as enantiomer self-disproportionation on achiral chromatography (ESDAC) [33-37], bears some similarity to the aforementioned phase transitions, though some distinctions are apparent. Thus far, no examples exist of systems which are dependent on the starting ec with respect to the order of elution which always remains the same, i.e. either the enriched fraction elutes uniquely before or after the more racemic fraction (though one particularly spectacular example [38-40] does exist in which enriched fractions elute both before and after the more racemic fraction). For the purposes of the definitions that follow, the retarded portion of the eluting analyte is declared as the more stable state and the faster eluting portion as the less stable.
System bias can also be seen in many spectroscopic methods. For example, the phenomenon of self-induced diastereomeric anisochromism (SIDA) [33,41] in NMR where signal migrations over the course of an enantiomeric titration of the distinct signals for each enantiomer are able to indicate the preference of homoor heterochiral aggregates [42]. Even in cases where NMR signals do not split but nevertheless still migrate—referred to as atypical SIDA (aSIDA) [40]—system bias can still be inferred [33,40]. Also, a variety of other spectroscopic methods, for example OR, have been demonstrated to exhibit nonlinear behavior over the course of an enantiomeric titration (i.e., the responses are nonlinearly dependent on the ec of the sample) and thus these cases also need to be addressed in terms of suitable descriptors.
Finally, there is also considerable interest in examining the association of enantiomers by molecular modeling and rendering comparison between homochiral aggregations and heterochiral aggregations. Such studies have important relevance to the chromatographic case, whereby aggregates may be more stable if they are homochiral or heterochiral, though in ESDAC the elution order is dependent firstly on the thermodynamic preferences (homochiral vs. heterochiral), and then secondly on the chromatographic preferences (single molecule vs. aggregate) [33,39,40].
Thus, how to describe all manner of systems with a uniform and generalized system of description? What is desired is to have a terminology that takes into account an expanded set of processes and the suggestion herein is that new terms be adopted reflecting the stability preference for the system under the particular conditions at hand for the particular process in effect and their response to the change in ec. Firstly though, with particular respect to the ec of the systems, occasionally there is confusion, and often unwieldy nomenclature with divergent opinions on how to express the ec, particularly in consideration to behavior. Thus it is suggested that the categorization of samples (or states) with respect to their ec can be re-termed as follows: holemate6 for an optically pure sample (i.e. a sample for which ec = 100%), equimate for a “racemate” or racemic sample (i.e. a sample for which ec = 50%), and scalemate6 for anything in between these two limits, i.e. 50% < ec < 100%. Suitable abbreviations of these terms for use as prefixes in names would be hol, eqm, and scl, respectively, with the corresponding adjectival forms being, holemic, equimatic, and scalemic, respectively, and the property names being holemicity, equimicity, and scalemicity, respectively. Such terms are perhaps preferable to terms such as “pure enantiomer”, “racemate”, “racemic (mixture)”, “partial racemate”, “partially racemic mixture”, and “non-racemic mixture” which do not seem to find universal favor. The term “pure enantiomer” has, of course, undesirable connotations for cases which are not chemically pure and vice versa whilst “racemate” and “racemic” are confusing with respect to melting point behavior and “partial racemate” and “partially racemic mixture” etc. are often just frowned upon for ec lying in the range 50% - 100%. For systems which are not enantiomeric, the prefix quasi can readily be utilized, or simply the terms diastereomers, stereoisomers, etc. utilized as appropriate. A compilation of preferred terms and various old or alternate terms, including disfavored terms and terms to be avoided or restricted in their use, is presented in Table 1.
Regarding behavior, the suggested terms are: heteromate for when the equimatic state is more stable and the addition of the enantiomer of lower ec to an scalemic sample enhances the stability of the system; homomate for when the holemate is preferred and addition of the enantiomer of higher ec accordingly leads to greater system stability; unimate for when the system is indifferent to the ec; bimate for when the system displays both heteromatic and homomatic behaviors depending on the ec with the implicit assumption that the state below the ep is heteromatic and the state above it is homomatic; and trimate for when the system displays all three behaviors with the implicit assumption that the state below the first ep is heteromatic, the state above the second ep is homomatic, and the state in between is unimatic. Subindices can be used to indicate ep, e.g. n in the case of a bimate and n and m in the case of a trimate (with n < m). Reverse order behavior in the case of a bimate can be indicated by a negative value for n whilst reverse order in the case of a trimate can be indicated by an interchange of n and m. For unconventional behaviors or limited examinations, regions can be indicated by subindices such as n–m, n®, or ®n where n and m represent ep and the arrows imply from n to 100% and from 50% to n, respectively. For systems which are not enantiomeric but which display similar composition dependent behaviors, the prefix quasi can similarly be readily utilized, or not with the implication that one is referring to diastereomers, stereoisomers, etc. The terms homomate, heteromate, and unimate can also apply equally well to the relative stabilities of particular macroscopic states without a process being applied.
Thus, the conglomerate behavior depicted in Figure 3 is unequivocally described by the term homomate whilst the racemic compound behavior of Figure 4 is fully enunciated by the term bimate80. The system of Figure 5 may be described quite comprehensively as a unimate®80 (thus equating to a solid solution within this prescribed range) and as a homomate80® whilst the behavior depicted in Figure 6 can succinctly be described as a
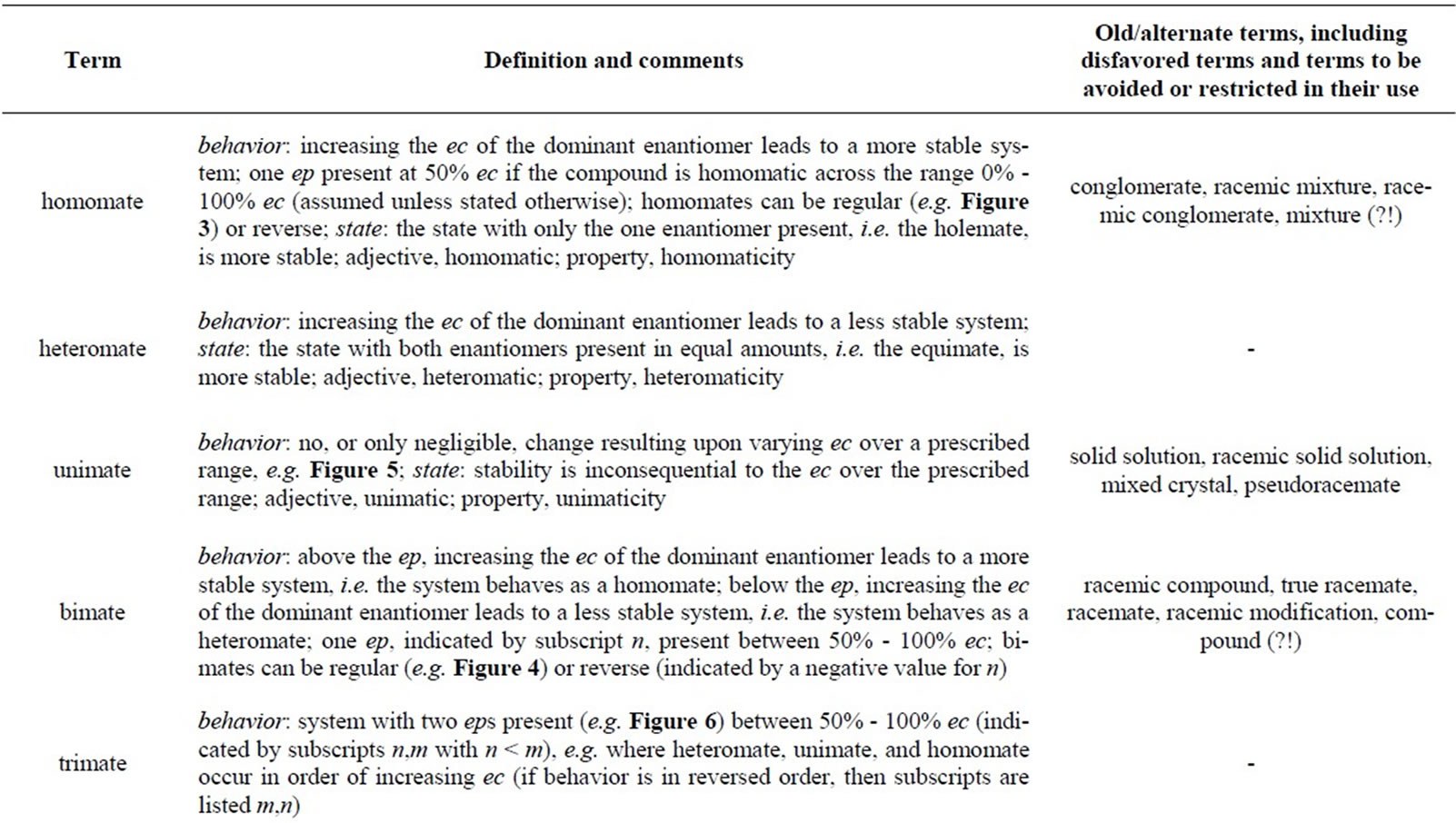

Table 1. Definitions and summary of preferred terms for chiral-based behaviors and states.
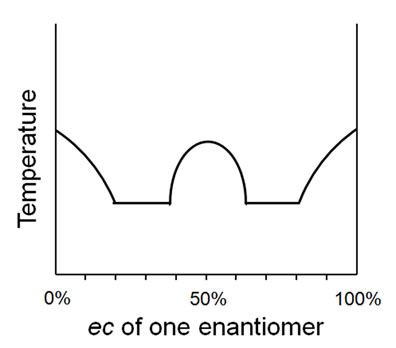
Figure 6. Melting point behavior of a trimate60,80.
trimate60,80. If need be, other conditions can also be indicated, e.g. sodium ammonium tartrate behaves as a homomate when crystallized below 27˚C and a bimate when crystallized above 27˚C and thus it can be conveniently described as both a homomate<27 and a bimate>27. Similarly for rubidium tartrate, which is both a homomate>40 and a bimate<40.
The extension of the terminology to other phase transitions and processes is henceforth quite evident. For melting point behavior, it seems natural to associate greater stability, i.e. higher melting point, with the allotted term and this convention can be followed analogously for the other systems whereby reference is made to the more stable state or condensed state as the defining property. Hence for sublimation and distillation, the change in ec of the pot residue can convey the sense of the system. Thus, if the pot residue moves towards the holemic state as a consequence of the process, the system can be described as homomatic at that point or over the prescribed range of the ec. In the case of ESDAC, the more retarded fraction is declared the more “stable” state though the retarded fraction is not necessarily the more energetically favored with respect to aggregation of the analytes since chromatographic properties are also a determinant factor [39,40]. Hence, if later fractions move towards a holemic state, then the system is likewise described as homomatic and conversely, if the later fractions move towards the equimatic state, then it is described as a heteromate. For spectroscopic measurements and modeling studies, if there is no dependency on the ec of the system, then simply the system is unimatic. With a bias towards either formation or greater stability of the equimate, then the system is heteromatic whilst a tendency towards the holemate represents a homomate.
Finally, it is worth encouraging the use of notation indicating the wavelength used for the measurement of OR in the name, e.g. “(+)589-(aR)-3,3’-diphenyl-[2,2’- binaphthalene]-1,1’-diol” [43]. The notation can be even more comprehensive, showing not only the wavelength of the measurement used, but also the measured value in addition to the sign. As well, the temperature and concentration as per the convention applied for the reporting of specific rotation can also be incorporated, e.g. “(+11.8)2d5, 0.27-(8S)-9-hydroxycineole [30]”. Use of such notation will no doubt alleviate some of the confusion and mistakes that arise due to the dispersive and other state-dependent properties of the compound at hand with regards to OR.
In summary, there is room for improvement and expanded application for the terms currently in use to describe chiral systems regarding both processes and states. Though the current terms for processes are well entrenched and amendment may be overdue, the terms associated with melting point behavior were of course long established by the time it came to be realized that other processes such as sublimation, chromatography over achiral substrates, or even distillation can also effect SDE behavior. Thus, with broad applicability and less contentiousness, the new terms suggested for processes may well represent a useful advantage if adopted with regards to diminishing ambiguity and expanding application (particularly with respect to the growing number of examples of processes which exhibit SDE) as well as being substantially more informative. Additionally, the proposed terms for describing the states of chiral systems are also likely to reduce the amount of ambiguity, or at least dissention with regards to the “correct terminology” that should be used. It is also worth emphasizing the need [4,5] to have a clear distinction between macroscopic (experimental analysis) and molecular (conceptual) domains as well as the state-dependent behavior of systems and the interactions or relationships between molecular entities. Thus, the molecular level is described by R and S nomenclature etc., the macroscopic state and aggregations by the ec (and terms such as holemate, scalemate, and equimate), the conceptual level and relationships by terms such as homochiral and heterochiral, and the behavior of systems and interactions by terms such homomate, heteromate, bimate, unimate, etc. A summary of the terms defined herein as well as other associated terminology is presented in Table 1 and it is hoped that some of these suggestions will be adopted by the chemical community.
REFERENCES
- L. Pasteur, “Essay on the Relationship between the Crystalline State and Chemical Composition and Their Effect on Optical Rotation,” Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences, Vol. 26, No. 21, 1848, pp. 535-538.
- L. Pasteur, “On the Relationships between the Crystalline Form, Chemical Composition and the Direction of Optical Rotation,” Annales de Chimie et de Physique, Vol. 24, No. 6, 1848, pp. 442-459.
- L. Pasteur, “Transformation of Tartaric Acid into Racemic Acid. The Discovery of Inactive Tartaric Acid. A New Method for the Separation of Racemic Acid into Right and Left Tartaric Acids,” Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences, Vol. 37, 1853, pp. 162-166.
- J. Gal, “On the Meaning and Use of Homochiral,” Journal of Chromatography A, Vol. 829, No. 1-2, 1998, pp. 417-418. doi:10.1016/S0021-9673(98)00845-0
- J. Gal, “Problems of Stereochemical Nomenclature and Terminology. 1. The Homochiral Controversy. Its Nature and Origins, and a Proposed Solution,” Enantiomer, Vol. 3, No. 3, 1998, pp. 263-273.
- E. L. Eliel, “Infelicitous Stereochemical Nomenclatures,” Chirality, Vol. 9. No. 5-6, 1997, pp. 428-430. doi:0.1002/(SICI)1520-636X(1997)9:5/6<428::AID-CHIR5>3.0.CO;2-1 http://www.uottawa.ca/publications/interscientia/inter.4/eliel/eliel.html
- P. Tähtinen, T. Oja, N. Dreiack, P. Mäntsälä, J. Niemi, M. Metsä-Ketelä and K. D. Klika, “Epimers vs. Inverse Epimers: The C-1 Configuration in Alnumycin Al,” RSC Advances, Vol. 2, 2012, pp. 5098-5100. doi:10.1039/C2RA20537H
- K. D. Klika, “Proposed Extension to the Natta Projection Notation System for Enabling an Indication of Relative Stereochemistry and the Stereochemical State,” International Journal of Organic Chemistry, Vol. 1, No. 4, 2011, pp. 215-217. doi:10.4236/ijoc.2011.14031
- T. Oja, P. Tähtinen, N. Dreiack, P. Mäntsälä, J. Niemi, M. Metsä-Ketelä and K. D. Klika, “Alnumycins A2 and A3, New Inverse-Epimeric Pairs Stereoisomeric to Alnumycin A1,” Tetrahedron: Asymmetry, Vol. 23, No. 9, 2012, pp. 670-682. doi:10.1016/j.tetasy.2012.05.001
- K. D. Klika, P. Tähtinen, P. Mäntsälä, J. Niemi and M. Metsä-Ketelä, “The Potential of VCD to Resolve the Epimer vs. Inverse Epimer Quandary,” Computational and Theoretical Chemistry, Vol. 992, 2012, pp. 156-163. doi:10.1016/j.comptc.2012.05.031
- T. Oja, K. D. Klika, L. Appassamy, J. Sinkkonen, P. Mäntsälä, J. Niemi and M. Metsä-Ketelä, “Biosynthetic Pathway toward Carbohydrate-Like Moieties of Alnumycins Contains Unusual Steps for C-C Bond Formation and Cleavage,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 109, No. 16, 2012, pp. 6024-6029. doi:10.1073/pnas.1201530109
- R. M. Carman and K. D. Klika, “The Four Diepoxides of (R)-(+)-Limonene,” Australian Journal of Chemistry, Vol. 44, No. 12, 1991, pp. 1803-1808. doi:10.1071/CH9911803
- R. M. Carman and K. D. Klika, “2,9-Dihydroxyand 2,10-Dihydroxy-1,8-Cineole. Two New Possum Urinary Metabolites,” Australian Journal of Chemistry, Vol. 47, No. 8, 1994, pp. 1509-1521. doi:10.1071/CH9941509
- R. E. Gawley, “Do the Terms ‘% ee’ and ‘% de’ Make Sense as Expressions of Stereoisomer Composition or Stereoselectivity?” The Journal of Organic Chemistry, Vol. 71, No. 6, 2006, pp. 2411-2416. doi:10.1021/jo052554w
- H. B. Kagan, “Is There a Preferred Expression for the Composition of a Mixture of Enantiomers?” Recueil des Travaux Chimiques des Pays-Bas, Vol. 114, No. 4-5, 1995, pp. 203-205. doi:10.1002/recl.19951140412
- J. H. Brewster, “Racemic, Scalemic, Holemic,” Chemical & Engineering News, Vol. 70, No. 20, 1992, pp. 2-3. doi:10.1021/cen-v070n020.p002
- C. H. Heathcock, “Alternative to Homochiral,” Chemical & Engineering News, Vol. 69, No. 5, 1991, pp. 2-3. doi:10.1021/cen-v069n005.p002
- R. H. Cornforth and J. W. Cornforth, “How to be Right and Wrong,” Croatica Chemica Acta, Vol. 69, No. 2, 1996, pp. 427-433.
- F. Faigl, E. Fogassy, M. Nógrádi, E. Pálovics and J Schindler, “Separation of Non-Racemic Mixtures of Enantiomers: An Essential Part of Optical Resolution,” Organic & Biomolecular Chemistry, Vol. 8, No. 5, 2010, pp. 947-959. doi:10.1039/b917564d
- V. A. Soloshonok and D. O. Berbasov, “Self-Disproportionation of Enantiomers on Achiral Phase Chromatography. One More Example of Fluorine’s Magic Powers,” Chimica Oggi/Chemistry Today, Vol. 24, No. 3, 2006, pp. 44-47.
- G. Pracejus, “Optical Activation of N-Phthalyl-a-amino Acid Derivatives by Tert.-Base-Catalysis,” Justus Liebigs Annalen der Chemie, Vol. 622, No. 1, 1959, pp. 10-22. doi:10.1002/jlac.19596220104
- H. Kwart and D. P. Hoster, “Separation of an Enantiomorph and Its Racemate by Sublimation,” The Journal of Organic Chemistry, Vol. 32, No. 6, 1967, pp. 1867-1870. doi:10.1021/jo01281a037
- D. L. Garin, D. J. C. Greco and L. Kelley, “Enhancement of Optical Activity by Fractional Sublimation. An Alternative to Fractional Crystallization and a Warning,” The Journal of Organic Chemistry, Vol. 42, No. 7, 1977, pp. 1249-1251. doi:10.1021/jo00427a033
- V. A. Soloshonok, H. Ueki, M. Yasumoto, S. Mekala, J. S. Hirschi and D. A. Singleton, “Phenomenon of Optical Self-Purification of Chiral Non-Racemic Compounds,” Journal of the American Chemical Society, Vol. 129, No. 40, 2007, pp. 12112-12113. doi:10.1021/ja065603a
- M. Yasumoto, H. Ueki and V. A. Soloshonok, “SelfDisproportionation of Enantiomers of a-Trifluoro-methyl Lactic Acid Amides via Sublimation,” Journal of Fluorine Chemistry, Vol. 131, No. 4, 2010, pp. 540-544. doi:10.1016/j.jfluchem.2009.11.010
- M. Yasumoto, H. Ueki and V. A. Soloshonok, “SelfDisproportionation of Enantiomers of 3,3,3-Tri-fluorolactic Acid Amides via Sublimation,” Journal of Fluorine Chemistry, Vol. 131, No. 2, 2010, pp. 266-269. doi:10.1016/j.jfluchem.2009.10.002
- M. Yasumoto, H. Ueki, T. Ono, T. Katagiri and V. A. Soloshonok, “Self-Disproportionation of Enantiomers of Isopropyl 3,3,3-(Trifluoro)Lactate via Sublimation: Sublimation Rates vs. Enantiomeric Composition,” Journal of Fluorine Chemistry, Vol. 131, No. 4, 2010, pp. 535- 539. doi:10.1016/j.jfluchem.2009.11.026
- M. Albrecht, V. A. Soloshonok, L. Schrader, M. Yasumoto and M. A. Suhm, “Chirality-Dependent Sublimation of a-(Trifluoromethyl)-Lactic Acid: Relative Vapor Pressures of Racemic, Eutectic, and Enantiomerically Pure Forms, and Vibrational Spectroscopy of Isolated (S,S) and (S,R) Dimers,” Journal of Fluorine Chemistry, Vol. 131, No. 4, 2010, pp. 495-504. doi:10.1016/j.jfluchem.2009.11.015
- R. H. Perry, C. Wu, M. Nefliu and R. G. Cooks, “Serine Sublimes with Spontaneous Chiral Amplification,” Chemical Communications, No. 10, 2007, pp. 1071-1073. doi:10.1039/b616196k
- R. M. Carman and K. D. Klika, “Partially Racemic Compounds as Brushtail Possum Urinary Metabolites,” Australian Journal of Chemistry, Vol. 45, No. 4, 1992, pp. 651-657. doi:10.1071/CH9920651
- B. Koppenhoefer and U. Trettin, “Is It Possible to Affect the Enantiomeric Composition by a Simple Distillation Process?” Fresenius’ Zeitschrift für Analytische Chemie, Vol. 333, No. 7, 1989, p. 750. doi:10.1007/BF00476607
- T. Katagiri, C. Yoda, K. Furuhashi, K. Ueki and T. Kubota, “Separation of an Enantiomorph and Its Racemate by Distillation: Strong Chiral Recognizing Ability of Trifluorolactates,” Chemistry Letters, Vol. 25, No. 2, 1996, pp. 115-116. doi:10.1246/cl.1996.115
- V. Nieminen, D. Yu. Murzin and K. D. Klika, “NMR and Molecular Modeling of the Dimeric Self-Association of the Enantiomers of 1,1’-Bi-2-Napthol and 1-Phenyl-2,2, 2-Trifluoroethanol in the Solution State and Their Relevance to Enantiomer Self-Disproportionation on Achiral Phase Chromatography (ESDAC),” Organic & Biomolecular Chemistry, Vol. 7, No. 3, 2009, pp. 537-542. doi:10.1039/b814905d
- V. A. Soloshonok, “Remarkable Amplification of the Self-Disproportionation of Enantiomers on Achiral-Phase Chromatography Columns,” Angewandte Chemie, International Edition, Vol. 45, No. 5, 2006, pp. 766-769.
- R. Stephani and V. J. Cesare, “Enantiomeric Enrichment of Non-Racemic Antihistamines by Achiral High-Performance Liquid Chromatography,” Journal of Chromatography A, Vol. 813, No. 1, 1998, pp. 79-84. doi:10.1016/S0021-9673(98)00339-2
- R. M. Carman and K. D. Klika, “The Optical Fractionation of a Partially Racemic Natural Product by Chromatography over an Achiral Substrate,” Australian Journal of Chemistry, Vol. 44, No. 6, 1991, pp. 895-896. doi:10.1071/CH9910895
- V. A. Soloshonok and D. O. Berbasov, “Self-Disproportionation of Enantiomers of (R)-Ethyl 3-(3,5-Dinitrobenzamido)-4,4,4-Trifluorobutanoate on Achiral Silica Gel Stationary Phase,” Journal of Fluorine Chemistry, Vol. 127, No. 4-5, 2006, pp. 597-603. doi:10.1016/j.jfluchem.2005.11.004
- M. Suchý, P. Kutschy, K. Monde, H. Goto, N. Harada, M. Takasugi, M. Dzurilla and E. Balentová, “Synthesis, Absolute Configuration, and Enantiomeric Enrichment of a Cruciferous Oxindole Phytoalexin, (S)-(−)-Spirobrassinin, and Its Oxazoline Analog,” The Journal of Organic Chemistry, Vol. 66, No. 11, 2001, pp. 3940-3947. doi:10.1021/jo0155052
- K. D. Klika, M. Budovská and P. Kutschy, “NMR Spectral Enantioresolution of Spirobrassinin and 1-Methoxyspirobrassinin Enantiomers Using (S)-(−)-Ethyl Lactate and Modeling of Spirobrassinin Self-Association for Rationalization of Its Self-Induced Diastereomeric Anisochronism (SIDA) and Enantiomer Self-Disproportionation on Achiral-Phase Chromatography (ESDAC) Phenomena,” Journal of Fluorine Chemistry, Vol. 131, No. 4, 2010, pp. 467-476. doi:10.1016/j.jfluchem.2009.10.018
- K. D. Klika, M. Budovská and P. Kutschy, “Enantiodifferentiation of Phytoalexin Spirobrassinin Derivatives Using the Chiral Solvating Agent (R)-(+)-1,1’-Bi-2- Naphthol in Conjunction with Molecular Modeling,” Tetrahedron: Asymmetry, Vol. 21, No. 6, 2010, pp. 647-658. doi:10.1016/j.tetasy.2010.03.035
- A. B. Ouryupin, M. I. Kadyko, P. V. Petrovskii, E. I. Fedin, A. Okruszek, R. Kinas and W. J. Stec, “Enantiomeric 2-Anilino-2-oxo-1,3,2-oxazapho-sphorinanes: Synthesis and NMR-Investigation of Their Non-Racemic Mixtures,” Tetrahedron: Asymmetry, Vol. 6, No. 7, 1995, pp. 1813-1824. doi:10.1016/0957-4166(95)00227-G
- C. Luchinat and S. Roelens, “Enantiomeric Purity Determination of 1,2-Diols through NMR Spectroscopy without Chiral Auxiliaries,” Journal of the American Chemical Society, Vol. 108, No. 16, 1986, pp. 4873-4878. doi:10.1021/ja00276a027
- P. L. Polavarapu, A. G. Petrovic, S. E. Vick, W. D. Wulff, H. Ren, Z. Ding and R. J. Staples, “Absolute Configuration of 3,3’-Diphenyl-[2,2’-Binaphthalene]-1,1’-Diol Revisited,” The Journal of Organic Chemistry, Vol. 74, No. 15, 2009, pp. 5451-5457. doi:10.1021/jo901013z
NOTES
1Perhaps somewhat enigmatically, the author does not feel compelled to push for the abandonment of the term homochirality. Homochiral is a general adjective and its use should be restricted to the molecular domain as per its originally intended use rather then also being used to describe macroscopic domains given the problems outlined by a number of authors [4-6]. Homochirality, on the other hand, though ostensibly the noun form of homochiral, has become thoroughly entrenched as a specific concept, that of the predominance of D-sugars and L-amino acids in the biosphere. Since there does not seem to be any need for use of the noun in the general sense, its continued use is therefore unproblematic.
2Giulio Natta, 1903-1979.
3The geometric constraints of the fused-epoxide ring limits the C-1 and C-2 stereogenic centers to just 1S,2R and 1R,2S configurations.
4The recommendations of Gawley [14] and Kagan [15] are preferred with regards to the use of enantiomeric composition (ec as the percentage of one enantiomer) over enantiomeric excess (ee).
5Arguments that the meaning of the term “racemate” is always clear from the context fail to impress this author.
6Holemate and scalemate are derived from the already defined [16,17] (though generally not in widespread use) adjectives holemic and scalemic, respectively.

