 Psychology, 2015, 6, 965-988 Published Online June 2015 in SciRes. http://www.scirp.org/journal/psych http://dx.doi.org/10.4236/psych.2015.68095 How to cite this paper: Sugawara, Y., Shigetho, A., Yoneda, M., Tuchiya, T., Yamada, H., Matumura, T., & Hirano, M. (2015). Relationship between Mood Change, Odor and Its Psychophysiological Responses in Humans in Terms of the Sensory Eval- uation Spectrum. Psychology, 6, 965-988. http://dx.doi.org/10.4236/psych.2015.68095 Relationship between Mood Change, Odor and Its Psychophysiological Responses in Humans in Terms of the Sensory Evaluation Spectrum Yoshiaki Sugawara*, Asami Shigetho, Mai Yoneda, Tomoko Tuchiya, Hiroko Yamada, Tomomi Matumura, Miki Hirano Department of Health Science, Prefectural University of Hiroshima, Hi rosh i ma, Japan Email: *sugawara@pu-hiroshima.ac.jp Received 13 May 2015; accepted 23 June 2015; published 26 June 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativ ecommon s.org/l icenses/by/4. 0/ Abstract How does the brain ultimately identify more than 10,000 odorants? How precisely can humans and their olfactory system detect and discriminate a great variety of odors and subtle differences in the molecular structures of a given aroma? In a series of studies, we have attempted to examine the relationship between mood change, odor and its psychophysiological responses, by focusing on the possible verbal (semantic) and non-verbal (skin temperature) changes in humans induced by smelling the fragrances of essential oils as well as linalool and its enantiomers. In our experi- mental design, the perceived sensory attributes in the participants can be represented by a sen- sory spectrograph: a bar graph whereby the mean of the impressions is plotted against the setting semantic impression descriptors. This article is an overview of our verbal (semantic) research results over the past decade as well as the non-verbal outcomes, which suggest that our tests of task-related sensory perception and skin temperature changes are useful for shedding more light on the finer nuances of odor discrimination and psychophysiological responses to odorants in humans. So, such information may provide clues to our long-standing questions mentioned above. Keywords Essential Oils, Linalool and Its Enantiomers, Mood Change, Psychological Responses, Sensory Test, Sensory Evaluation Spectrum *  Y. Sugawara et al. 1. Introduction Sensory evaluation is a method of measuring consciousness developed primarily in experimental psychology and mathematical psychology (Coombs, 1964; Guilford, 1 954; Kling & Riggs, 1972; Stevens, 1951; Torgerson, 1958). Sensory experiences can be reported using verbal (semantic) methods. To elucidate relationship between mood change, odor and its psychophysiological responses in humans while inhaling the fragrances of essential oils and one monoterpenoid (linaloo l) as well as its enantiometr ic iso mers [(R)-(–)-, (S)-(+)- and ( RS)-(±)-fo rms ] , the authors have endeavored to develop a sensory (verbal) measure of the perceived odor quality for a given aroma and analyze it statistically (Satoh & Sugawara, 2003; Sugawara, 2001, 2008; Sugawara et al., 1998a, 1998b, 1999, 2000, 2003, 2006, 2008, 2009a, 2009b, 2013; Yamagata & Sugawara, 2014). In our experimental design, we asked the participants to complete a sensory questionnaire in which the per- ceptions of fragrances were evaluated using 13 contrasting pairs of adjectives. We assigned behavioral tasks to the participants. These included the Uchida-Kraepelin test as a mental arithmetic task and listening to environ- mental (natural) sounds as an auditory task. The sensory test was conducted twice, once before and once after the task. We then represented the sensory attributes of the perceived odors of essential oils as well as linalool and its enantiomers be fore and after the task via a sensory evaluation spectrum: a bar graph whereby the mean of the impressions was plotted against the semantic impression de scrip tors. These tests have been carried out for the past decade and the sensory features of 21 essential oils as well as linalool and its enant i omers were settled so far (mention late r). Amo ng the se, the yla ngyla ng senso r y eval uati on spectrum (in which the mean o f the impr ession s while i nhali ng the fra grance of ylangylang was plotted agai nst the descriptors) was shown to be task dependent. When using the auditory task involving listening to environ- mental (natural) sounds, the ylang yl ang spectrum was found to be the reverse of the spectrum recorded when undertaking mental arithmetic task. Similar task dependent effects were found for bergamot, peppermint and sandal wood esse ntial oils a s well as linalool. T hese findings imply that the perceived odour quality depends on which behavioral task is assigned to the participants, if the sa me fragrance given to the participants. Moreover, same task-dependence of the sensory evaluation spectrum was found for (RS)-(±)-linalool. Besides, the ( R)-(–)- and ( S)-(+)-forms of linalool resulted in different sensory spectra when subjects were undertaking mental arith- metic, but gave identical spectra when the auditory task was used. Based on these findings, it can be speculated that enantiomers of linalool may evoke different odour perceptions not only based on chirality but also based on beha vioura l tasks. Humans can detect and discriminate a vast number of odors. The number perceived to be distinguishably dif- ferent is estimated to range from a few thousand to more than ten thousand (Roderick, 1966; Firestein, 1991). Humans are capable of distinguishing even slight alterations in the structure of an odorous molecule, such as the difference between a pair of enantiomers, which possess the same molecular structure except for the chiral posi- tion. Such small molecular differences can lead to profound changes in the perceived odor quality. For i nsta nce, with the use of highly purified compounds by gas-liquid chromatography (GLC), it has been shown that (+)- carvone is characterized as a caraway-like scent, while (−)-carvone as a spearmint-like herbal odor (Friedman & Miller, 1971; Leitereg et al., 1971). Enantiomers of carvotanacetone and trans-dehydrocarvone, both synthe- sized from (+)-carvone, are caraway-like, whereas those prepared from (−)-carvone are spearmint-like (Russell & Hills, 1971). Similarly, (+)- and (−)-linalool are petitgrain-like and lavender-like, respectively (Ohloff & Klein, 1962). How precisely can humans and their olfactory system detect and discriminate such a great variety of odors and such subtle differences in the molecular structures? A number of proposals and theories have been presented to explain this issue, with a var iet y of views in vol ving vibr ational ener g y levels, in ter molec ular inte ractio ns, and molecular size and shape (Ro derick, 1966). However, no physiological theories have followed from these ideas. In 1991, a breakthrough was made by Buck and Axel, who identified the genes coding for olfactory receptors (Buck & Axel, 1991). Since this initial discover y in rat, it has be en establis hed that the detectio n of odora nts is mediated by a large number of G protein-coupled odorant receptors (Ors) encoded by a multigene family (Buck, 1996; Mombaerts, 1999). T he number of Ors is estimated at ~1000 in mouse and rat, ~500 - 7 50 in human and ~100 in fish (Zhang & Firestein, 2002). In mammals, Ors are found in the hair-like cilia of olfactory neurons, which are located in the olfactory epithelium at the back of the nose. Thus, initial o dor detection is mediated by a vast number of different odorant receptors, located at the entrance of the olfactory system in direct contact with the air, and signals of smell are transmitted to the brain. Under the assumption that the mammalian DNA contains around 100,000 genes, 1% or more of the genome is considered to be devoted to the detection of odors,  Y. Sugawara et al. making the Ors family the largest gene family thus far identified in mammals (Axel, 1995). The limbic system, which is related to mood, memory, and sexual activity, is closely associated with the ol- factory system and is likely to play a principal role not only in discrimination of a great variety of odors and subtle differences in the molecular structures but also in the diverse odor reaction depending on internal and extraneous conditions of the persons while inhaling fragrant ingredients. The scent of different odors is known to alter mood, alertness, and sexual arousal (Billot & Wells, 1975; Morr is, 1984; Valnet, 1982). Perfumes, room fragrances, and incense have been used for self-adornment and modification of living spaces since ancient times. The practice known as Aromatherapy, which began in France in the early 20th century, has been increasing in popularity up to the present day. In medicine, interest in the use of essential oils has grown considerably, such that essential oils are currently used worldwide for the management of chronic pain, depression, anxiety, and cognitive, sleep-, and stress-related disorders (Buchbaue r & Jirovetz, 1994; Buckle, 1999, 2004; Edge, 2003; Holmes et al., 2002; Kyle, 2006; Perry & Perry, 2006; Price & Price, 2007; Smallwood et al., 2001). Essential oils are now extensively utilized in the context of aromatherapy (specifically alternative medicine) and aroma wellness. The following questions arise: How does the brain prompt the range of emotional or behavioral res- ponses that aromas often provoke? To what extent is a particular behavior or mood governed by the perception of odors? We have attempted to examine the relationship between mood change, odor and its psychophysiological res- ponses, by foc using o n the possible verbal (semantic) and non-verbal (skin temperature) changes i n huma ns in- duced by smelling the fragrances of essential oils as well as linalool and its enantiomers . In a series of our re- search activities, sensory evaluation has been a key component of our studies. In this article, we provide an overview of our semantic (verbal) research achievements over the past decade as well as the non-verbal (skin temperature) outcomes, which suggest that our tests of task-related sensory perception and skin temperature chan ges are us eful fo r shed ding mor e light o n the fine r nuanc es of o dor di scriminat ion a nd psycho physi olo gical responses to odorants in humans. We believe that this approach has the potential to be highly informative, so that such information may provide clues to the following long-standing issues: How does the brain ultimately identify more than 10,000 odorants? How precisely can humans and their olfactory system detect and discrimi- nate a great variety of odors and subtle differences in the molecular structures of a given aroma? How does the perception of odors usually associate with pleasant or unpleasant emotions? How does the brain prompt the range of emotional or behavioral responses that aromas often provoke? To what extent is a particular behavior or mood governed by the perception of odors? 2. Sensory Profiling for Verbal Responses in Perceived Odor Quality in Humans The sensory evaluation spectrum method is first discussed in reference to one of our senso ry stud ie s (Sugawara, 2008; Sugawara et al., 2009a, 2013; Yamagata & Sugawara, 2014), whereby the target sensory property was to determine the perceived odor quality in participants after inhalation of a given aroma on the basis of the task-dependent sensor y questionnaire assessments. Essential oils were purchased from Fleur (London, UK). Linalool was a product of Kanto Kagaku Co., Ltd. (Tokyo, Japan). To achieve the optimal concentration of each odorant for inhalation experiments, we presented a series of diluted solutions, which comprised the odorants mixed with deodorized diethyl phthalate (1/1, 1/5, 1/10, 1/50, 1/100, 1/1000, and 1/10,000), to several judges (usually five) via an inhalator consisting of a glass inhal a- tor device and a 300-mL flask with a ground-glass stopper. The inhalator flask was loaded by applying 200 μL of diluted od orant to a small strip of filter paper placed at the bottom of the flask. The flas k was sealed with the ground-glass stopper. The judges were asked to give each test solution a score from 0 to 5 based o n the follow- ing scale: 0, odorless; 1, odor barely detectable and the nature of the odor cannot be ascertained; 2, very weak odor but the nature of the odor can be discriminated; 3, weak odor but the odor can be readily detected; 4, strong odor; and 5, odor so strong that it cannot be tolerated. Concentrations receiving a score of 3 or above were used for e xper ime nts. For example, we used dilutions of 1/5 for lemon, 1/10 for bergamot , ca r d amom, c i n n a mo n , cy- press, geranium, ho leaf/wood, juniper, lime, orange, palmarosa, sandalwood, spearmint and ylangylang, and one monoterpenoid (linalool), and 1/100 for basil, chamomile, clove, lavender, marjoram, pepper min t and ro- sema ry. The op timal concentra tion of optically active linalools will be detailed la te r . Incidental ly, Lorig and Schwartz (1988) stated that odors a ct as neurop hysiological stimul i by causing differ- ent perceptions, which, in turn, lead to diverse odor reactions, depending on the internal and extra neous condi-  Y. Sugawara et al. tions of the participants. To investigate this notion, we assigned different behavioral tasks (“extraneous condi- tions”) to the participants. We used the Uchida-Kraepelin test as a mental arithmetic task and listening to envi- ronmental (natural) sounds as an auditory task. In the Uchida-Kraepelin test, the participant is given a sheet of paper with adjacent rows of numbers (100 numbers per row). They are asked to perform simple additions using the numbers within a row. We gave our pa rticipants 40 s to work on each row before changin g to t he next row (5 min total). The a uditory task (5 min total) consisted of sitti ng on a chair and listening to natural so unds, such as bir ds singi ng a nd the mur mur ing o f a small stream. This was accomplished via headphones connected to a com- pact disc player. As a measure (sensory profiling) of the perceived odor quality after inhalation of a given aroma, we asked the participants to complete a sensory questionnaire assessment (Sugawa ra, 2008; Sugawara et al., 2009a, 2013; Yamagata & Sugawara, 2014). In the questionnaire, aroma perception was evaluated using 13 descriptors com- prising the following pairs of contrasti ng adjectives: fres h-stale, soo thing-activating, airy-heavy, plain-rich, nat- ural-unnatural, elegant-unrefined, soft-strong, pleasant-unpleasant, warm-cool, comfortable-uncomfortable, woodsy-not woodsy, floral-peppery, lively-dull. The 13 descriptors were scored on an 11-point scale (–5 to +5), with 0 as the middle score. There were no symbolic representations associated with the numbers, similar to the Likert scale (Li nkert, 1932). We conducted the sensory test twice, before and after the task. We evaluated the differences between the pre- and post-task score s (post-task scores minus pre-task scores) for each of the descriptors using Student’s t-tests. The mean dif- ference in the score of each descriptor was plotted against the 13 descriptors, producing a sensory evaluation spectrum. The statistical significance of each descriptor was marked and scored as follows: a score was given an * (asterisk) and a significance score of 1 if the difference was significant such that p < 0.05; a ± and a signific- ance score of 0.5 if the difference was significant such that p = 0. 0 5 - 0.1; and no symbol and a significance score of 0 if the difference was not significant such that p ≥ 0.1. The sum of these scores was the total signific- ance score = Σ13i = 1 (significance score of descriptor)i. We used this value as an index of whether the relevant sensory spectrum could be considered to be statistically significant across the spectrograph (Suga wara, 2008; Sugawara et al., 2009a, 2013; Yamagata & Sugawara, 2014). To confirm this, we used a sign test with n = 13, corresponding to the number of descriptors used in our sensory test. We found that the spectra could be expected to reach significance (p < 0.05) when the number of descrip tors regarded as significant at a pr obability value of p < 0.05 (according to the t-test) was greater than 10 out of the 13 descriptors. In contrast, when this value was less than 3, the null hypothesis could be rejected. In the verbal tests so far examined, we were able to establish appropriate parameters for assessing the sensor y attributes o f the follo wing 21 essential oil s, and one monoter penoid ( linalool) and its enantio metric iso mers as a functi on o f the two be havioral tasks ( mental arithmetic a nd auditory) (Ta ble 1): basil (Ocimum basilicum), ber- ga mot (Citrus bergamia), cardamom (Elettaria cardamomum), chamomile (Matrica ria chamomilla), cinnamon (Cinnamomum zeylanicum), clove (Syzygium aromaticum), cypress (Cupressus sempervirens), geranium (Pe- largonium graveolens), ho leaf/wood (Cinnamomum camphora), juniper (Juniperus communis), lavender (La- vandula angustifolia), lemon (Citrus limon), lime (Citrus latifolia), marjoram (Origanum majorana), orange (citrus sinensis), palmarosa (Cymbopogen martini), peppermint (Mentha piperita), rosemary (Rosmarinus offi- cinalis), sandalwood (Santalum album), spearmint (Mentha spicata) and ylangylang (Cananga odorata). To this e nd, 1144 participants formed the sensory test panel. Among thes e, 55 were male students fro m Hiro- shima University ranging in age from 18 to 24 years. The remaining participants were female students at Hiro- shima University, Prefectural University of Hiroshima (formerly Hiroshima Prefectural Women’s University, but renamed as of April 1, 2005) and Suzugamine Women’s Coll ege, ra ngin g in age fr om 1 8 to 2 2 years. Thus, the proportion of male participants was only about 5%. Each participant completed one sensory profile only. The detailed number of participants for each sensory profile can be found in Tabl e 1. 3. Sensory Profiles of Lemon and YlangYlang Essential Oils and Linalool as a Function of Behavioral Task (Extraneous Condition Assigned to the Participants) We employed a sensory evaluation spectrum as a measure (sensory profiling) of the perceived odor quality in participants after inhalation of a given aroma on the basis of the task-dependent sensory questionnaire assess- ments. It is a bar graph in which the mean of the pre-post task difference bet ween the score of post-task inqui ry  Y. Sugawara et al. Table 1. Summary of the total significance scores (see text) resulting from a statistical analysis of verbal (semantic) res- ponses to the fragrances of 21 essential oils and linalool as well as its enantiometric isomers, for which semantic question- naire assessments wer e conducted as a function of two behavioral tasks (i .e., mental ari thmetic an d audito ry task). The obtained total significance score for a given aroma in relation to the task assigne d to the participants with the number of participants in parenthesis Odorant Task assigned to the participants (1) Essential oils Basil 5.0 (n = 22) 0.0 (n = 22) Bergamot 2.0 (24) 2.5 (18) Carda m on 0.0 (24) 0.0 (23) Chamom i le 0.5 (21) 1.0 (23) Cinna mon 2.0 (36) 8.5 (41) Clove 0.5 (20) 2.0 (19) Cyp r es s 0.5 (20) 1.0 (18) Geranium 5.5 (21) 2.5 (30) Ho leaf/wood 1.0 (19) 2.5 (18) Juniper 6.5 (17) 6.5 (19) Laven der 0.0 (20) 1.5 (21) Lemon 8.0 (43) 7.0 (41) Li me 6.0 (24) 1.0 (18) Marjoram 2.0 (21) 1.0 (30) Orange 5.0 (22) 1.0 (30) Palmarosa 2.0 (18) 3.5 (21) Peppermi n t 6.0 (20) 3.5 (23) Rosemary 1.0 (20) 0.5 (18) Sanda lwood 2.0 (21) 6.5 (22) Spearmi nt 4.5 (18) 3.0 (18) Ylan gylang 2.0 (19) 5.5 (24) (2) Linalool and its enantiometric isomers Linalool 5.5 (20) 5.0 (22) (RS)-(±)-linalool 4.0 (18) 6.5 (21) (R)-(-)-linalool 4.5 (23) 7.0 (24) (S)-(+)-linalool 3.5 (26) 4.0 (23) and pre-task inquiry (post minus pre) was plotted against the 13 impression descriptors. Figure 1 shows several representative spectrographs. In each of thes e, if the descriptors regarded as significant by t-test have a p ositive value and are shown above the horizontal axis, this signifies a positive (or favorable) correlation between the fragrance of a given aroma and the type of task, according the positive descriptors (i.e., “fresh,” “airy,” “elegant,” “pleasant,” “comfortable,” etc.). If negati ve val ue s ap p e ar b elow the axi s, t hi s su g gest s a n unfavo r ab l e ( ne ga ti ve ) correlation between the fragrance and the type of task in terms of the negative descriptors (i.e., “stale,” “heavy,” 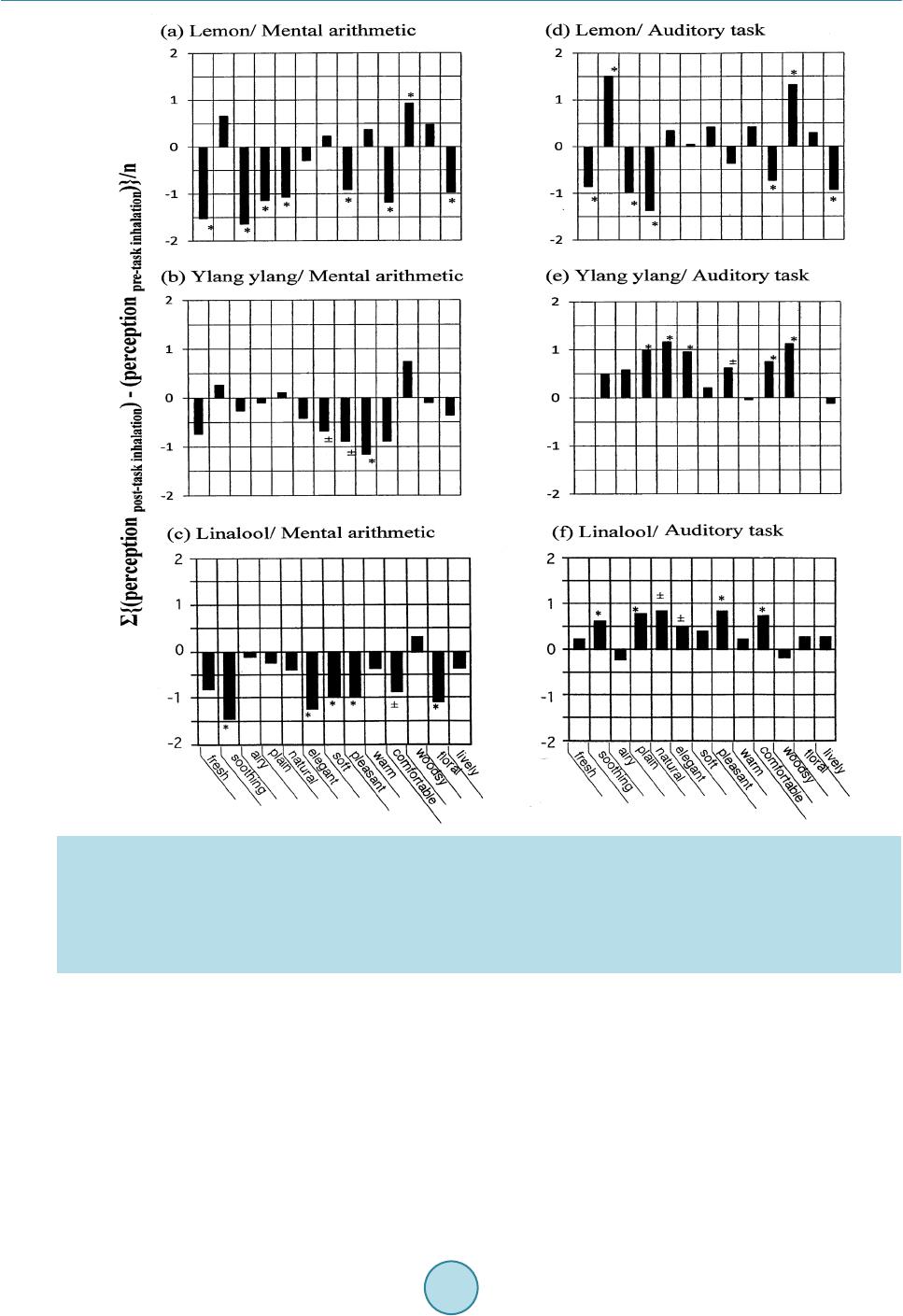 Y. Sugawara et al. Figure 1 . Sensory spect ra of essential oils of lemon and ylangylang as well as linalool as a functi on of two behavio ral tasks. A sensory test was conducted twice before and after the task assigned to the participants, in which aroma perception was e va- luated by 13 impression descriptors consisting of contrasting pairs of adjectives. The pre-post task d ifference in t he score of each of the impression descriptors is plotted on the ordinate as a bar graph. The statisti cal significance evaluated by t-test of each des cript or was marked with a single asterisk (*) if th e pre-post impression difference was regarded significant with p < 0.05, ± if regarded significant with p = 0.05 - 0.1, and unmarked if p ≥ 0.1. The number of participants was (a) 43, (b) 19, (c) 20, (d) 41, (e) 24 and (f) 22. “unrefined,” “unpleasant,” “uncomfortable,” etc.). Figure 1 contains a summary of the responses to inhalation of the fragrance s of essential oils of lemon and ylangylang, and linalool. As seen i n Figure 1, the sensory spectrograph obtained after inhalation of lemo n in assoc iation with the men- tal arithmetic task indicates an unfavorable (downward or negative) correlation between the fragrance and the task ( Figure 1(a)). On the ot her hand , the spectrograph after inhalation o f lemon in associ atio n with the auditory task denotes that half of the significant descriptors were positive and the other half negative (Figure 1(d)). In turn, negative (unfavorable) correlation similar to the spectrograph for lemon/mental arithmetic (Figure 1(a)) was in view in the spectrograph of ylangylang/mental arithmetic (Figure 1(b)). In contrast, positive (fa- vorable) correlation between the fragrance and the task was obvious i n the spectrograph of yla ngylang/auditory  Y. Sugawara et al. task (Figure 1(e)). This means that the sensor y attribute aft er inhalatio n of the fragrance of ylangylang produced opposite (contrasting) si gn s d ur in g t he t wo b e ha vio r al ta sk s in terms of the sensory evaluation spectrum (Figure 1(b) and Figure 1(e)). One was representing downward spectrograph in relation to the mental arithmetic task and the other representing upward spectrograph in association with the auditory task. In the Figure, it was ap- parent that similar opposing effects for the two behavioral tasks were found for linalool (Figure 1(c) and Figure 1(f)). These imply that differe nt behavioral ta sks (or different “extraneous conditio ns”) might influe nce the per- ceived odor quality of a given aroma after inhalation. In other words, that the finer nuances were visible in each spectrum when observed as a function of the different behavioral tasks indicated that this method of sensory profiling could be practical for assessing odor perception in participants. However, none of the spectra reached statistical significance among the 50 cases contained in Table 1, in- cludi ng the ca ses sho wn in Figure 1. I n our expe rimental desig n, in each spectrograph (as shown in Figure 1), the statistical significance of each descriptor was marked and scored as follows: * (asterisk) and significance score of 1 if the difference was significant such that p < 0 .05; ± and a significance score of 0.5 if the difference was significant such that p = 0.05 - 0.1; and no symbol and a significance score of 0 if the difference was not significant suc h that p ≥ 0.1. The sum of these scores provided a total significance score = Σ13i = 1 (significance score of descriptor)i. Total significance scores were calculated by simple addition of the statistical significance scores marked on the individual descriptors in the relevant spectrum. In Figures 1(a)-(f), we obtained total sig- nificance scores of 8.0 for lemon/mental arithmetic, 7.0 for lemon/auditory task, 2.0 for ylangylang/mental arithmetic, 5.5 for ylangylang/auditor y task, 5.5 for linaloo/mental arithmetic, and 5.0 for linalool/auditor y task. Table 1 shows a summary of the obtained total significance scores for the 21 essential oils as well as linalool and its enantiomers in relation to the two behavioral tasks ( mental arithmetic and audito ry task). Here the total significance scores were a convenient index for evaluating whether the relevant spectrographs were statistically significant. This issue ha s b e en t he s ub j ec t o f o ur st ud ies (S ugawar a, 2008; Sugawara et al., 2009a; Yamagata & Su gawara, 2014). W e finally employed a sign test with n = 13, corresponding to the number of descriptors used in our sensor y test. W e found tha t, for a sign test with n = 13 , the re sulting se nsor y spectr a would be signi fica nt (at p < 0.05) when the number of descriptors regarded as significant at a probability value of p < 0.05 according to the t-test was gr eate r t han 1 0 ( out o f 13 desc rip tor s). In contr ast, whe n thi s val ue was l ess t han 3 , t he n ull h y- pothesis could be rejected. As demonstrated in Table 1, we found no sensory spectra with total significance scores of > 10 amon g the 50 cases examined, although 8.5, which was derived from the spectrograph of cinnamon/auditory task, was the largest value. Regardless, the resulting sensory spectra warranted particular interest (Figure 1). 4. Sensory Profiles of Enantiometric Isomers of Linalool: Discrimination between Enantiomers in Terms of the Sensory Evaluation Spectrum as a Function of Behavioral Task Without regard to our statistical defects asso ciated with the sig nificance of the sensor y spectra mentioned in the previous section, in thi s sectio n, we describe our research attempts on odor discrimination of enantiomers of li- nalool, quantified with the sensory evaluation spectrum and observed as a function of behavioural task (Suga- wara, 2001, 2008; Sugawara et al., 2000, 2013). Optically active linalools [(R)-(–)-, (S)-(+)- and (RS)-(±)-forms] (Figure 2) were obtained by repeated flash column chromatography on silica gel (solvent: hexane/ethyl acetate, 9:1, v/v) of lavender oil, coriander oil and commercial linalool (see Sugawara et al., 2000 for details). For example, lavender oil (2.0 g) was subjected to flash chromatography on silica gel to yield linalool (439 mg), and its spectral (EI-MS, IR and 1H-NMR), chro- matographic (analytical thin-layer chromatography; TLC, Merck 60 GF254 silica gel plate), and gas liquid chromatography (GLC; CP-cycl odextrin-β-236M-19) behaviour was compared with those of authentic speci- mens (kindly supplied by Dr. Y. Hiraga of Hiroshima University, Japan). The linalool was identified as (R)-(−)-linalool based on co-GLC ana lysi s with a n authe nti c (R)-form and was found to have a specific rotation of [α]D = −15.1˚, a 97.0% purity on GLC and a 21.95% total yield. Using the sa me method, (S)-(+)-linalool was obtained from coriander oil, with a 32.9% total yield, a content of the (S)-form of 88.3% and (R)-form 11.7% on GLC, and [α]D = +17.4˚. Similarly, (RS )-(±)-linalool with a 74.5% total yield, a co ntent of the (S)-form of 49.1% and (R)-form 50.9% on GLC, and [α]D = 0˚ was re -purified from commercial linalool. 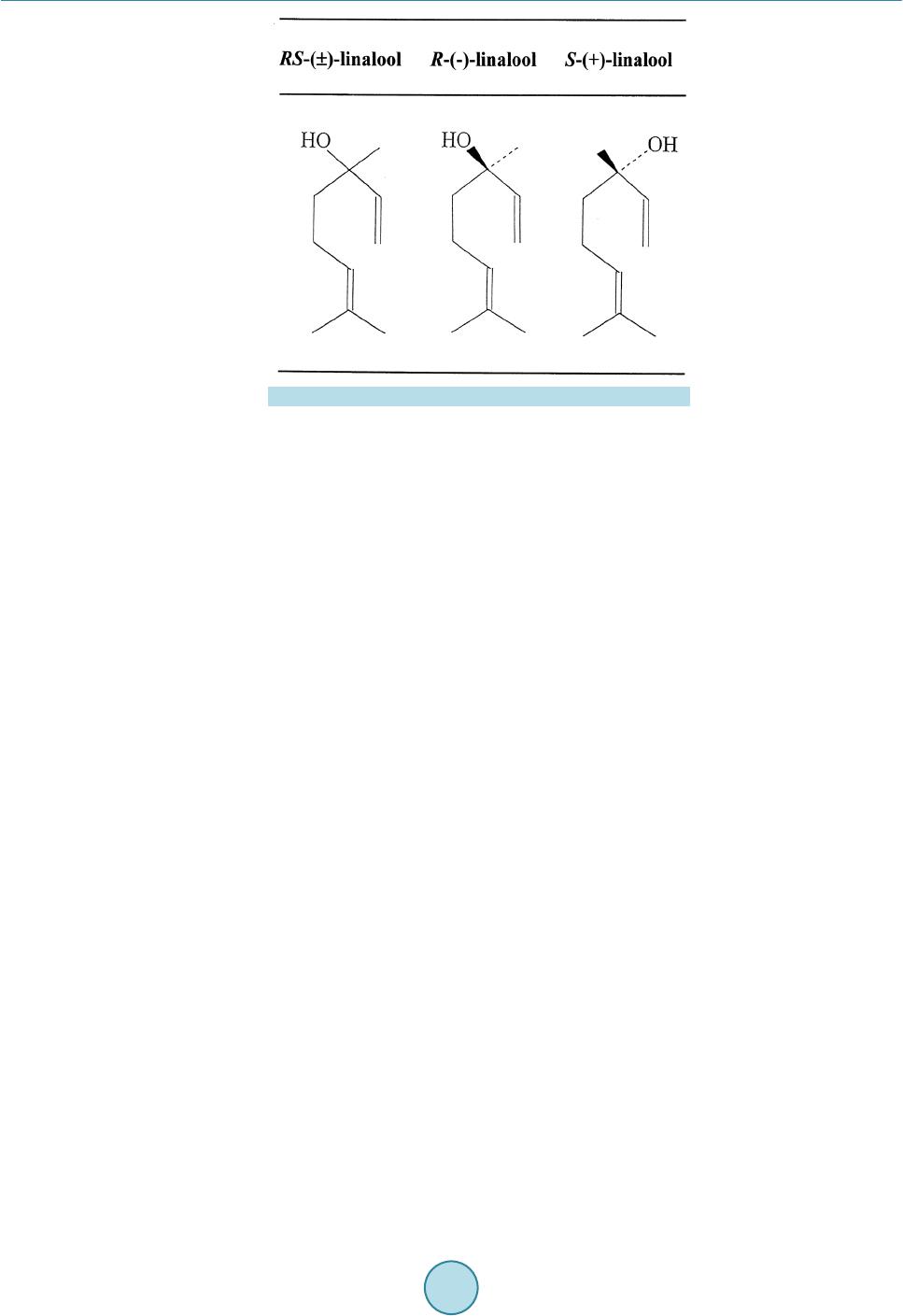 Y. Sugawara et al. Figure 2. Formula of enantiometric isomers of linalool. We use d 2 0 mg/mL soluti o ns o f the ena ntio mer s for senso r y pro filing b eca use t his co nce ntr atio n was r ate d as at least level 3 (“weak odour but the odour can be readily detected”) by the judges when they were presented with 50, 20, 10, 1 and 0.1 mg/mL solutions. The sensory spectra obtained using the optically active linalools in combination with the two behavioral tasks are summarized in Figure 3. Since (RS)-(±)-linalool was purified from commercial linalool and identified as a racemic mixture of the (R)-form (50.9%) and (S)-form (49.1%) with [α]D = 0˚, we used (RS)-(±)-linalool as a reference. The sensory evaluation spectra for purified linalool (Figure 3( a) and Figure 3(d)) showed practically the same task-dependence of perception as the commercial linalool (Figure 1(c) and Figure 1(f)). The total significance score of purified linalool was 4.0 for the mental arithmetic task (Figure 3(a)) and 6.5 for the auditory task (Figure 3(d)). This was in good agreement with the scores obtained using the commercial linalool, which were 5.5 with mental arithmetic (Figure 1(c)) and 5.0 with the auditor y task (Figure 1(f)). Figure 3 shows the sensory evaluation spectra from participants inhaling either (R)-(–)- or (S)-(+)-forms of linalool while undertaking the mental arithmetic or auditory tasks. (RS)-(±)-linalool was used as a reference. After mental ar ithmetic ( Figur e s 3(a)-(c), left hand side) the spectra showed negative values, indicating that the perceived odour quality of (R)-(–)- and (S)-(+)-linalools and the racemic mixture of the (R)- and (S)-forms was less favourable after the task. After the auditory task (Figures 3(d)-(f), right hand side) the spectra showed mostly positive values. Thus, the spectra were completely reversed after the auditory task compared with the ment al arithmetic task. Furthermore, a closer examination of the spectra obtained for the auditory task made it obvious that the sensory spectrum of (R)-(–)-linalool was identical to that of the reference (RS)-(±)-linalool, but not to that o f (S)-(+)-linalool, for whic h ha lf o f the si gnif ica nt d escr ipt ors were po siti ve a nd the ot her hal f nega- tive. It should be noted afresh that none o f the spe ctra sho wn in Figure 3 reached statistical significance, because the total significance scores were all ≤10 (Table 1). Ho wever, given t hese find ings and t he fact tha t Ohlof f and Klein (1962) suggest ed ( +) - and (–)-linalool are petitgra in-li ke and la ve nd er -like, respe cti vely, it is interes tin g to speculate that different enantiomers of linalool may evoke distinct odour perception in a task-depe nde nt ma nne r . 5. Multi-Channel Skin Thermometer Study: Assessing the Statistical Significance of the Sensory Evaluation Spectra Here, we describe the results of our multi-channel skin thermometer stud y. Because we considered a variety of strategies for addressing statistical defects associated with the significance of the sensory spectra. Conclusively, we found that mul t i-cha nnel skin ther mo mete r data might co mplement the s tatistical d efects of the r elevant se n- sory spectra. Emotional excitement or apprehension is known to induce a slight increase in skin temperature (Elam & Wallin, 198 7; Oka et al., 2001, 2008; Yamakoshi et al., 2007; Ziegler & Cash, 1938). As the perception of and 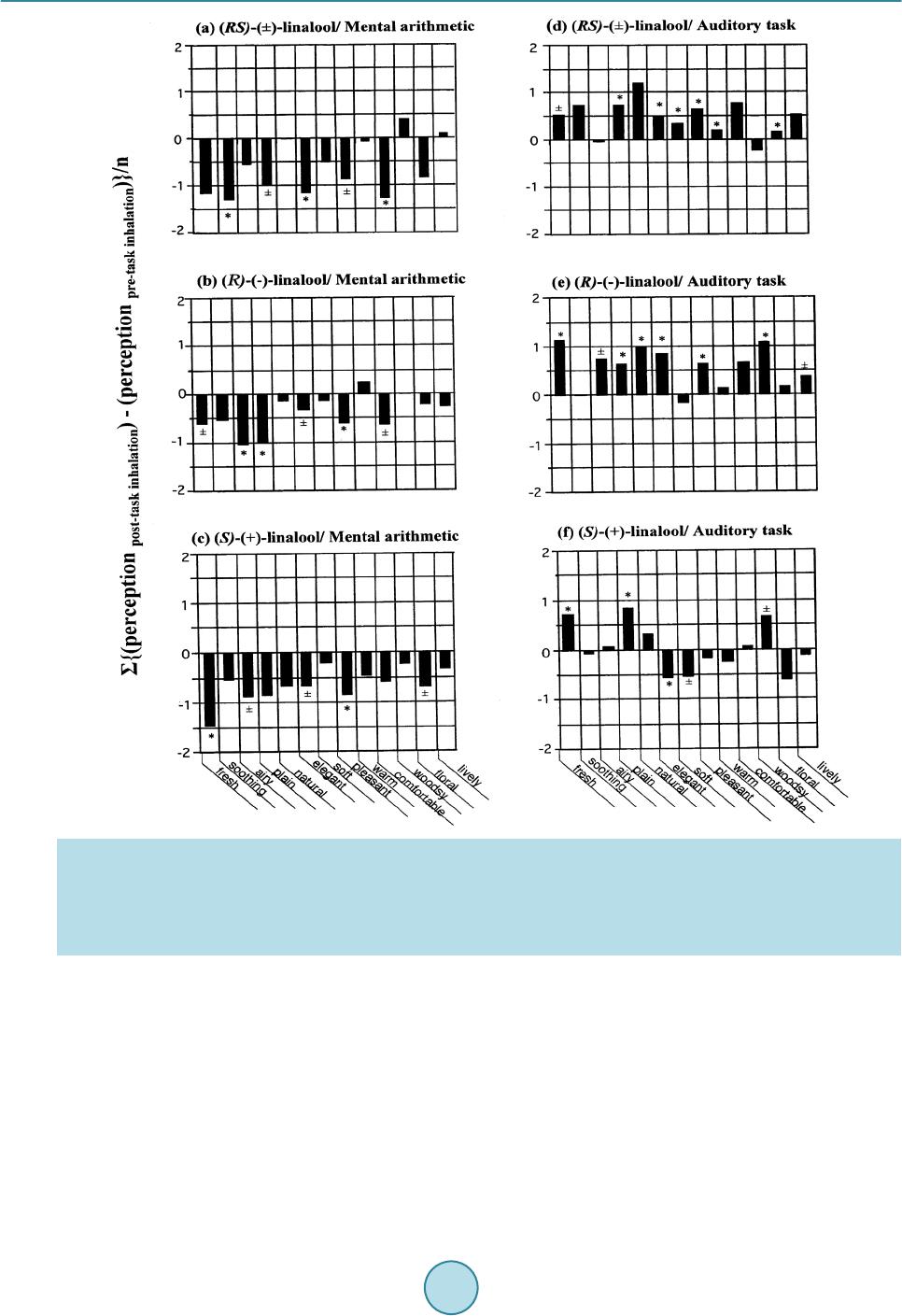 Y. Sugawara et al. Figure 3. Sensory spectra of enantiomers of linalool [(RS)-(±)-, (R)-(−)- and (S)-(+)-forms] as a function of two behavioral tasks. Redrawn from Sugawara et al . (2013). (a) (RS)-(±)-linalool, (b) (R)-(−)-linaloo l and (c) (S)-(+)-linalool when the sub- jects undertaking mental arithmetic; and (d) (RS)-(±)-linalool, (e) (R)-(−)-linalool and (f) (S)-(+)-linalool while undertaking the auditory task. Concentration of enantiomer was every 20 mg/mL (in diethyl phthalate), which was loaded and moistened in an inhalator (300 mL vol.) by applying 200 μL of each solution. The number of participants was (a) 18; (b) 23; (c) 26 ; (d) 21; (e) 24 and (f) 23. response to odors are intimately related to both emotional expression and genesis of emotion, skin temperature changes can serve as an effective index for the study of the relationship between odor and its emotional and physiological responses in humans. Multi-channel skin thermometer measurements were cond ucted in a climatic chamber maintained at 20˚C and 60% relative h umidity. A total o f 415 pa rticipants completed the st udy. Partic ipants were all fe male students at Prefectural University of Hiroshima, ranging in age from 18 to 22 years. The procedure was first explained to each participant, who were then encouraged to relax and allowed to rest quietly for 5 min before the test began. None of the participants were suffering from any chronic diseases, nasal congestion, or upper respiratory tract infections, and no par ticipants were taking an y medication, re medies, or contrace ptive pills. To a void the poten- tial confounding effects of hormonal profile and physiological status, female participants did not participate in  Y. Sugawara et al. the study during menses. Ambient noise was kept at a low level (30 - 40 dBA) during the experiments . We recorded skin temperature (fingertip) using a thermometer (Anritsu AM-7052; Anritsu Meter Co., Tokyo, Japan) equipped with a multi-channel data collector. Thin surface thermistors (2 × 10 mm) were used as sensors. They were attached with adhesive tape to the tips of all fingers on the left hand and to the palm at the base of the first finger of t he left hand. Various parts of the body have different temperatures that undergo circadian fluctuations. Thus, we conducted a pilot experiment in which the thermistors were affixed to the left cheek, left earlobe, forehead, tip of the left first finger, left pal m (at the base of the first finger ), and back of the left hand (at the base of the first finger). We used an odorless blank as a negative control. The skin temperature curve obtained from the tip of the left first finger and left palm showed a small but significant increase after odor inhalation, while practically no change was observed at the other measurement points (Sugawara & Kawasaki, 2000). Therefore, we developed a mul- ti-channel thermometric technique by which skin temperature could be measured from the fingertips and palm of the left ha nd (Satoh & Sugawara, 2003; Sugawara et al. , 2006, 2008, 2009a, 2013). All data were stored on a personal computer at a sampling rate of 15 s via an A/D converter connected to the multi-channel thermometer. This allowed summation of the data from each channel every 15 s so that a single temperature curve could be obtained for the six measurement points. The experimental protocol was as follows: (1) inhalation o f the odorle ss blank (total inhalatio n: 3 min; pr esence of the odo rless blank: 30 s), ( 2) inhalation of the essential oil before the task (total: 5 min, presence of the fragrance: 1 min), (3) 5 min of the task, (4) post-task inhalation of essential oil (total: 5 min; presence of the fragrance: 1 min), and (5) inhalation of the odorless blank (total: 3 min; presence of the odorless blank: 30 s). For each experimental run, we constructed a temperature profile (bar chart) by integrating the temperature curves (per minute) with each section of the skin temperature measurement protocol (mean temperatures at 1-minute intervals). We were then able to calculate the net skin temperature change between the presentation of the odorless blank and the presentation of the target fragrance: (TMMAodor − TMMAo)/TMMAo, where TMMAodor is the mean minute -based average temperature during odor presentation, and TMMAo is the intensity during the presen- tation of the odorless blank. Figure 4 shows a representative sample of the skin tempera ture result s from particip a nts who inhaled le mon before and after the auditory task. As shown in Figure 4(a) (individual curves) and Figure 4(b) (summation), the skin temperature curves showed a small but considerable change during odor presentation. However, there was considerable individual variation between trials in an experimental run. Therefore, for each trial, the minute- based mean average temperature was calculated for the different odor presentation periods (Figure 4(c)). On t he basis of the minute-based te mperature profile, the net change in skin temperature for each experi mental run was calculated according to the formula described above. This method produced the results shown in Figure 4(d) and Figure 4(e). In Figure 4(d), the cases showing an increase in skin temperature after the task are plotted in the left diagram, while those showing a decrease are represented in the right diagram. Figure 4(e) shows the overall mean values of the net temperature changes between pre- and post-task inhalations. Similarly, Figure 5 shows an exa mple of the skin temperature results from participants who inhaled palmaro- sa either in relation to mental arithmetic (Figure 5(a)) or the auditory task (Figure 5(b)). As shown in Figure 5(a) and Figure 5(b), for each trial, cases with an increase in skin temperature after the task are plotted in the left diagram, while those showing a decrease are represented in the middle diagram. A bar graph on the right side of Figure 5 contains the overall (summarized) mean values of the net intensity changes between pre- and post-task inhalations. Figure 5(a) and Figure 5(b) clearly demonstrate that the temperature response to palma- rosa occurred in opposite directions for the two behavioral tasks: we observed a significant increase in skin temperature between pre- and post-task inhalations for mental arith metic (at p < 0.01): t0 = 3.555 ≥ td.f. (d .f. = 10, p = 0.01) = 3.106 (Figure 5(a)), and a tendency for skin temperature to decrease for the auditory task, al- though this change did not rea c h statistical significance (Figure 5(b)). Similar to palmarosa, the temperature response to inhalation of lemon occurred in opposite directions for the two behavioral tasks: we observed a significant decrease in skin temperature between the pre- and post-task i n- halations for mental arit hmetic ( at p < 0.05): t0 = 2.585 ≥td.f. (d. f. = 12, p = 0.05) = 2.179 , and a sig nific ant in- crease in skin temperature for the auditory task (at p < 0.05): t0 = 2.655 ≥td.f. (d.f. = 13, p = 0.05) = 2.145, as sho wn in Figure 4. Also, we observed a significant increase in skin temperature for basil/mental arithmetic (at p < 0.05): t0 = 2.173 ≥td.f. (d.f. = 16, p = 0 . 05) = 2.1 10, and j uniper/mental a rithmetic (at p < 0.01): t0 = 3.587 ≥td.f. (d.f. = 11,  Y. Sugawara et al. Figure 4. The observed skin temperature changes following inhalation of lemon in association with the auditory task. The numbers assigned to the graph represent the sensor spots on the left hand: 1, the tip of the thumb; 2, the tip of the first finger; 3, the tip of the second finger; 4, the tip of the third finger; 5, the tip of the fourth finger, and 6, the palm. There were 2 0 par- ticipants for lemon/auditory task. p = 0.01) = 3.055. Figure 6 shows a representative example of our attempts to combine the obtained fingerti p skin temperature results with the associated sensory evaluation spectra. Figure 6 is a summary of the verbal (sensor y evaluation spectrum) and non-verbal (skin temperature changes) responses of i nhali ng the fra grance s of le mon, palmarosa, basi l, and j uniper. In the Figure , the perceived sensory attributes are displayed according to the sensory evalua- tion spectrum and the associated changes in skin temperature, both as a function of the two behavioral tasks (mental arith metic and the auditory) For the data in Figure 6, the prac tical p otentialitie s of the s kin te mper ature d ata for the e va luation o f stati stic- al significance might complement any statistical defects associated with the sensory spectrographs, even if the sensory spectra were not statistically significant. Moreover, the subtle differences between the two behavioral tasks could be viewed in terms of verbal (sensory spectrum) and non-verbal (fingertip skin temperature) changes as a function of the two behavioral tasks. In other words, the combination of non-verbal with verbal data might be highly informative, not only for studying the psychophysiological responses of essential oil fragrances, but also for examining the relationship between odor and its olfacto ry discr imination and responses i n humans. 6. Verbal (Sensory Evaluation Spectrum) and Non-Verbal (Skin Temperature Change) Endpoints of Inhaling Essential Oils Fragrances as a Function of Behavioral Task As our skin temperature measurements were proceeded, we narrowed our focus to involve only the following 12  Y. Sugawara et al. Figure 5. Skin temperature changes following inhalation of palmarosa as a function of two b ehavioral t asks. We calculated the net intensity change in skin temperature (see text) between pre- and post-task inhalations with respect to the odorless blank and the target fragrance in each trial. In the line segment grap h, changes in net intensity are connect ed by a solid line for each par ticipan t in each experimental trial. Cases i n which we observed upward skin temperature changes are plotted on the left, while those with a downward tendency are shown in the middle panels. The summarized mean values of net intensi- ty changes obtained from pre- and post-task inhalations are depicted in a bar graph on the right. (a) There were 11 partici- pants for pal marosa/mental arithmetic; and (b) 18 for palmarosa/auditory task. essential oils: basil, bergamot, cardamom, cinnamon, juniper, lemon, orange, palmarosa, peppermint, sandal- wood, spearmint and ylangylang. We also used a multi-channel skin thermometer to measure changes associated with the other essential oils (bergamot, cardamom, cinnamon, orange, peppermint, sandalwood, spearmint and ylangyla ng) without mentione d in the pre vious s ectio n. Figure 7 sho ws a summary of the obtained verbal (sen- sory evaluation spectrum) and non-verbal (skin te mpera ture cha nges) data in relation to t he two different beha- vioral tasks. As cle arly sho wn in Figure 7, the inhalation of t he essential o ils of bergamot, cardamom, cinnamon, orange, peppermint, sandalwood, spearmint, and ylangylang seemed to induce a decrease in skin temperature for the two behavioral tasks. Meantime, we observed a significant decrease in skin temperature between pre- and post-task inhalations for sandalwood/auditory task (at p < 0.05): t0 = 2.318 ≥ td.f. (d.f. = 19, p = 0.05) = 2.086 and spear- mint/mental ar ithmetic (at p < 0.01): t0 = 4.153 ≥ td.f. (d .f. = 16, p = 0.01) = 2.898. In this way, the verbal and non-verbal performance associated with the 12 target odors were unified in rela- tion to the two behavioral tasks. The unified 24 odor-task combinations (the 12 target odors in relation to the two tasks) are summarized in Figure 6 and Figure 7. Based o n the relationship between verbal and non-verbal responses of the 12 target odors during the two tasks, we made an attempt to classify the obtained 24 odor-task combinations into three categories: 1) the first category (Figure 6(a)), which included lemon and palmarosa, elicited task-dependent dual changes in fingertip skin temperature; 2) the second category (Figure 6(b)), which included b asil and juniper, not only elicited an increase in skin temperature, but also the increased sensory spec- tra i n a non-task d epende nt fashio n; and 3) the third c ategory (Figure 7), which included bergamot, cardamom,  Y. Sugawara et al. (a) (b) Figure 6. S ummary of th e verbal and no n-verbal responses following inhalation of essential oils of lemon, palmarosa, basil, and juni per. Figu re shows senso ry evaluation spectra and skin temperatu re changes as a function of two behavioral tasks. (a) lemon and palmarosa; and (b) basil and juniper. See Table 1 and the text for more information about the participants in the sensory profiling study and the skin temperature study, respectively. cinnamon, orange, peppermint, sandalwood, spearmint, and ylangylang, which elicited a decrease in skin tem- perature regardless of the behavioral task assigned to the participants. Lemon and palmarosa, which we assigned to the first category in our criteria, produced task-dependent res- ponses. As previously mentioned, lemon produced opposing effects for the two behavioral tasks: a significant decrease in skin temperature between pre- and post-task inhalations for mental arithmetic (at p < 0.05), and a significant increase in skin temperature for the auditory task (at p < 0.05). Palmarosa elicited similar task-de- pendent character istics to le mon in terms of fingertip ski n temperature. Essential oils of basil and j uniper, which we assigned to the second category, elicited increases in skin temperature as well as an increase in the sensory spectra for the two tasks, both in a non-task depende nt fashion. Co ntrary to this, essen tial oils of bergamot, car- damom, cinnamon, orange, peppermint, sandalwood, spearmint, and ylangylang, which were placed in the third category, decreased skin temperature for the two tasks in a non-task dependent fashion. However, the latter half of the group (bergamot, peppermint, sandalwood, and ylangylang) were associated with contrasting directions of change in the sensory spectra for the two tasks. With respect to the essential oils belonging to the third category in our criteria (bergamot, cardamom, cinna- mon, orange, peppermint, sandalwood, spearmint, and ylangylang), a more detailed inspection of the obtained sensory spectrographs led us to classify these further into two groups: those associated with contrasting sensory spectra in a task-dep end ent fashion (i. e. , one produced an increase and the other produced a decrease); and those (the rest) associated with undistinguished sensory spectra. Essential oils of bergamot, peppermint, sandalwood, and ylangylang appeared to the former, whereas cardamom, cinnamon, orange, and spearmint constituted the latter.  Y. Sugawara et al. Figure 7. Summary of the verbal an d non-verbal responses following inhalation of bergamot, card amom, cinnamon , orange, peppermint, sandalwood, spearmint, and ylangylang essential oils in terms of the sensory evaluation spectra and skin tem- perature changes as a function of two behavioral tasks. The circumstances are identi cal to thos e shown in Fig ure 6. In our research, the verbal tests always antedated the non-verbal tests because the verbal tests were used to screen the essential oils that would be used in the no n-verb al inve stiga tions. As for information about the number  Y. Sugawara et al. of the participants in the former antedated verbal tests, see Table 1. As to the number of participants in the latter non-verbal tests who completed the mental arithmetic task while we conducted skin temp erature measurements was 17 for basil, 14 for bergamot, 18 for cardamom, 18 for cinnamon, 12 for juniper, 13 for lemon, 20 for orange, 11 for palmarosa, 18 for peppermint, 20 for sandalwood, 17 for spearmint, and 18 for ylangylang. The number of participants who completed the auditory task while we conducted skin temperature measurements was 19 for basil, 12 for bergamot, 20 for cardamom, 18 for cinnamon, 20 for juniper, 14 for lemon, 20 for orange, 18 for palmarosa, 20 for peppermint, 20 for sandalwood, 18 for spearmint, and 20 for ylangylang. 7. Discussion Sensory analysis can involve a variety of tools and tests for participative or objective evaluation of sensory properties. Among these, descriptive sensory analysis employs a group of trai ned ind ividuals (ge nerall y 6 - 12) who identif y and quanti f y specific sensory attributes (Dra ke, 2004, 2007; Lawless & Heymann, 1988; Meilgaard et al., 1999). In contra st, our senso ry ana lysis i nvolve d untr ained indi vidual s as pa nelist s, re sulting i n var iation s in the level o f interest in the sensor y target, sensitivity to t he stimuli, and susceptibilit y to fatigue. As described elsewhere (Sugawara, 2008; Sugawara et al., 2009a; Yamagata & Suga wara, 2014), this was evident from our questionnaire, whereby the standard deviation of the responses was remarkably large when compared with the value of the mean for the 13 descriptors studied. Considering the large standard deviation, these means were sta- tistically non-significant. Regard l ess, sensory spectra c an represent specific se nsory attributes at a level of quality t hat is equal to that of the traditional (conventional) descriptive sensory analyses (Sugawara, 2008; Sugawara et al., 2009a, 2013; Ya- maga ta & Sugawara, 2014). While the traditional analysis involves groups of trained individuals who identify and quanti fy specific se nsory attributes with the goal o f repr oducibilit y and consiste ncy, we believe t hat our o b- tained sensory spectra exhibited satisfactory-to-good agreement both in terms of rep r oducibility and consistency, as well as the shape and characteristics of the total significance scores (i.e., the sum of the statistical scores for each descriptor in a given sensory evaluation spectrum: 1.0 for items denoted with *; 0.5 for items denoted with ±; 0.0 for items that are unmarked). Additionally, we substantiated the number of panelists required for our study, and showed that this number was similar to that required in conventional descriptive sensory analysis, even if usi ng u nt rained (inexperienced) individuals on the panel. In this c onte xt, we e mp l oy e d sensory spectra as a measure (sensory profiling) of the perceived odor quality in participants after inhalation of a given aroma on the basis of the task-dependent sensory questionnaire assess- ments in this research. There are roughly two types of sensory spectra. The first type includes descriptors regarded as significant by a t-test with a positive value; these were shown above the horizontal axis. The other type includes descriptors that had negative values; these appeared below the horizontal axis. The former spectra denote positive (or favorable) correlation between the fragrance of a given aroma and the type of task using descriptors like “fresh,” “airy,” “elegant,” “pleasant,” “comfortable,” among others, while the latter spectro graphs s uggest an unfavor able (neg- ative) correlation between the fragrance and the type of task in terms of the setting descriptors. The spectra derived from lemon/mental arithmetic (Figure 1(a)) and ylangylang/mental arithmetic (Figure 1(b)) as well as linaloo l/mental a rithmetic (Figure 1(c) ) seemed to be examples showing unfavo ra ble ( negat ive ) correlation between the fragrance and the type of task assigned to the partic ipants. On the other hand, the spectra resulting from ylangylang/auditory task (Figure 1(e)) and linalool/auditory task (Figure 1(f)) were seen in fa- vorable (positive) type. Besides, there was a type of spectrograph like lemon/auditory task (Figure 1(d)), wherein half of the significant descriptors were positive and the other half negative. Peculiarly, ylangylang pro- duced opposite signs for the two behavioral tasks in terms of sensory spectrograph: an unfavorable (negative) type in associatio n with the m e ntal ar ithmetic ta sk (Figure 1(b)) and a favorable (positive ) typ e in relation to the auditor y task (Figure 1(e)). Similar task-dependent results wer e found in inhali ng the fragr ances of esse ntial oils of bergamot, peppermint and sandalwood (Figure 7) as well as linalool (Fig ure 1( c) a nd Figure 1(f)). Equally, (R)-(–)-linalool in association with the auditory task was found to produce positive (favorable) val- ues in the sensory spectrum (Figure 3(e)), and this effect was quite similar to that o f (RS)-(±)-linalool (Figure 3(d)) but contrasted with that of (S)-(+)-linaloo l (Figure 3(f)). In contrast, (R)-(–)-linalool in association with mental ar ithmetic (Figure 3(b)) produced nega tive ( unfavourable)valu es in the spectrum (that is, perceived od our quality), and this feature closely resembled the effects of (S)-(+)-linalool (Figure 3(c)) and (RS)-(±)-linalool (Figure 3(a)). Taken together with the previous report that the aromas of (+)- a nd ( –)-li nalo ol a re p etitgrain-like  Y. Sugawara et al. and lavender-like, respectively (Ohloff & Klein, 1962), our findings suggest that the enantio mers of linalool are significantly different odorants and also that t he p e rception of them is task-dependent. Thus, the resulting sensory spectra (Figure 1 and Figure 3) warranted particular interest. However, the changes of the spectra listed in Table 1 including the cases in Figure 1 and Figure 3 were insignificant. Be- cause we did not find any sensory spectra with a total significance score of > 10 among our 50-case studies. We expect that this is due to extraordinary deviation in both the pre- and post-task descriptor scores (Sugawara, 2008; Sugawara et al., 2009a; Yamagat a & Sugawa ra, 2014). Along with the line o f this thi nkin g, we considered a variety of strategies for addressing statistical defects as- sociated with the significance of the sensory spectra. As we have mentioned, we conclusivel y found that multi- channel skin thermometer data might complement the statistical defects of t he relevant sensory spectra. T he dia- gra ms shown i n Figure 6 demonstrate that the practical potentialities o f the skin temperature data for the evalu- ation of statistical significance might complement any statistical defects associated with the sensory spectro- graphs, even if the sensory spectra were not statistically significant. Moreover, the subtle differences between the two behavioral tasks could be viewed in terms of verbal (sensory spectrum) and non-verbal (fingertip skin temperature) expre ssions as a function of the two behavioral tasks. Throughout the fingertip skin temperature mea surements, we concentrated our focus to involve only the fol- lowing 12 essential oils (basil, bergamot, cardamom, cinnamon, juniper, lemon, orange, palmarosa, peppermint, sandalwood, spearmint and ylangylang). Based on the relationship between verbal and non-verbal responses of the 12 target odors during the two tasks (mental arithmetic and the auditory), we tentatively classified our 24 odor-task combinations into three categories: (1) the first category (Figure 6(a)) included lemon and palmarosa because they elicited opposite changes in skin temperature for the two behavioral tasks; (2) the second category (Figure 6(b)) contained basil and juniper, as these were associated with increases in skin temperature for the two behavioral tasks in a non-task dependent fashion; and (3) the other essential oils were placed in the third category (Figure 7), since inhalation of these essential oils was associated with decreases in skin temperature for the two tasks in a non-task dependent fashion. Moreover, it was apparent that the 24 odor-task combinations could be divided into two groups in terms of skin temperature changes between pre- and post-task inhalations: those that elicited an increase (Figure 6(b)); and those that led to a decrease (Figure 7). Emotional excitement or apprehension is known to induce a slight increase in human skin temperature (E lam & Wallin, 1987; Oka et al., 2001, 2008; Yamakoshi et al., 2007; Ziegler & Cash, 1938), although the mechanisms by which odorants induce such changes are unknown, and what’s more the complicated factors influence cutaneous vascular responses with respect to skin temperature changes (Abramson & Ferris 1940; Allwood et a l., 1959; Arnott & Macfie, 1948; Elam & Wallin, 198 7; Harris et al., 1952; Gaskell, 1956; Kellerova & Delius 1969; Roddie, 1983; Roddie et al. 1957; Rowell, 1981; Vallbo et al., 1979). However, if feelings of excitement or apprehension induce slight increases in skin temperature (Elam & Wallin, 1987; Oka et al., 2001, 2008; Yamakoshi et al., 2007; Ziegler & Cash, 1938), it is reasonable to as- sume that our target odors can be regarded as having either distressing/agitating sentiments when associated with i nc re as e s in sk in t e mp er a t ure ( Figure 6(b)) o r r el axi ng/ sed a tin g senti me nt s when associated with decreases in skin temperature (Figure 7). Mehrabian and Russell (1974) constructed a set of verbal texts describing different situations, and a scale for rating these texts (the Semantic Differential Scale). They asserted that pleasure and arousal are the principal di- mensions of affective response to the environment. Here, pleasure can be defined as the degree to which one has favorable feelings towards a situation, while arousal is defined as the degree to which one feels excited in the situation. When this method was applied to materials describing common events, Bensafi et al. (2002) demo n- strated that the first two factors accounting for most of the variance in descriptions were pleasure and arousal. Perception of e motional sti mu li is th us associa ted with e xpli cit re actions, such a s verba l resp onses, a nd to i mpli- cit output, suc h as variations in the autonomic nervous system (Greenwald et al., 1989; Lang et al., 1998). Based on Bensafi et al. (2002) we speculated that: (1) the sensory spectra can be used as an index of plea- santness (or unpleasantness); and (2) the overall mean values of the net skin temperature changes between pre- and post-task inhalatio ns can b e regarded as an index of arousal state. Specifically, upward sensory spectra can be considered to represent favorable (pleasant) feelings towards a situation while participants inhale the fra- grance of a given aroma, whereas downward sensory spectra can represent unfavorable (unpleasant) feelings towards a situation. Likewise, an increase in skin temperature (i.e., the overall mean values of the net skin tem- perature changes between pre- and post-task inhalations) could designate a distressed/agitated state of the par-  Y. Sugawara et al. ticipant as well as distre ssing/agitati ng properties of a given aroma, while a decrease could denote a relaxed/se- dated state and relaxing/sedating properties of a target odor. Along this line of thinking, it seemed that administration of basil and juniper, which were members of the second category in our criteria (Figure 6(b)), could bring about favorable/pleasant feelings in terms of non-task dependent up ward sensory spec tra and lead distressin g/agitatin g sentime nts associated with non-task d epende nt increases in skin temperature. In this context, ho wever, group membership of berga mot, cardamom, cinna mon, orange, peppermint, sandalwood, spearmint, and ylangylang as these being ass igned t o the third c ategor y in our criteria (F igure 7) would be confusing. T his is why the essential oils b el onging to t he thir d c ategory in our cr it e- ria could be divided into two groups: those (bergamot, peppermint, sandalwood, and ylangylang) associated with contr asti ng senso ry spectra in a task-dependent fashion (one was upward and the other was downward); and the remainder (cardamom, cinnamon, orange, and spearmint) that were associated with contrasting sensory spectra in a non-task-dependent fashion. Here we will address the former gro up of essential oils fir st, i.e., those co mprising exactl y half of the esse ntial oils in our third category. This is because Hongratanaworakit and Buchbauer made an intriguing observation during two studies of ylangylang in 2004 and 2006. Hence, we will hereinafter deal with ylangylang as a typical example of this group, for which a characteristic feature could be an obtained sensory spectra that clashes with the results of behavioral tasks in terms of direction: the sensory spectrograph was upward when t he par ticip ants completed the mental arithmetic task and downward when they completed the auditory task. Note that the de- creases in skin temperature associated with the two tasks were identical to those found for the remaining essen- tial oils (cardamom, cinnamon, orange, and spearmint). In Hongratanaworakit and Buchbauer’s first study (2004), the authors characterized the effect of ylangylang after inhalation as harmonizing rather than relaxing/sedating on the basis of their findings that administra tion of ylangyla ng after inhalation led to a decrease in blood pressure and pulse rate. However, they observed increased participative attention and alertness in response to administration of ylangylang. Thus, they generated the con- cept of “harmonization,” which is consistent with otherwise contradictory psychophysiological outcomes. In their subsequent report ( H ongra t a naworakit & Buchbauer, 2006), the authors reported that transdermal absorp- tion of ylangylang caused a decrease in blood pressure and an increase in participative calmness and relaxation compared with a control scenario, and suggested that administration of ylangylang via transdermal absorption has a relaxing/sedating effect under specific experimental conditions. Given their findings, our ylangylang data is intriguing. The inhalation of ylangylang in combination with mental arithmetic produced an unpleasant/unfavorable feeling after inhalation, whereas inhalation combined with the auditory task brought about a pleasant (favorable) feeling. Conversely, ylangylang seemed to have sim- ilar relaxing/sedating properties during both the mental arithmetic and the auditory tasks. It seems that the ob- served effect of ylangylang in our experiment, in combination with mental arithmetic, was in good agreement with the Hongratanaworakit and Buchbauer’s conception of “harmonization” (2004). Given this notion, we searched for other essential oils that had the same profile as ylangylang in terms of task-dependent sensory spectra and fingertip skin temperature changes. The results are shown in Figure 8(a). We identified three o ther essential oils that shared the sensory spectra of ylangylang in terms of net intensity fingertip skin temperature changes. These were bergamot, pepp er mint, and sandalwood. Thus, we reconsidered the remaining half of the essential oils (cardamom, cinnamon, orange, and spearmint) in our third cat egor y, as sho w n in Figure 8(b). T he y all were associated with decreases in skin temperature dur- ing the two behavioral tasks, but we found no common features in their sensory spectra. The total significance score s for the se e sse ntia l o il s were mostly less than 3, so we expected that a null hypothesis could be rejected. A small exception was seen for a cinnamon/auditory task (a total significance score of 8.5) and a spearmint/mental arithmetic (a total significance score of 4.5). Additionally, in the sensory spectrograph for spearmint/mental arithmetic, half of the significant descriptors were positive and the other half negative. Altogether, the essential oils belonging to this group (cardamom, cinnamon, orange, and spearmint) could be regarded as having relax- ing/sedating properties, as shown in Figure 8(b). Lemon and palmarosa were classified as miscellaneous, as these were assigned to the first category in our cri- teria (Figure 6(a)) on the basis that inhalation of lemon and palmarosa produced skin temperature responses in opposite directions for the two behavioral tasks. Based on the above discussion, that a significant increase in skin temperature was elicited by lemon/the auditory task (at p < 0.05) and palmarosa/mental arithmetic (at p < 0.01) could indicate that these essential oils have distressing/agitating properties. However, a significant  Y. Sugawara et al. (a) (b) Figure 8. Reconstruction of the architecture of Figure 7, employing the concept of “harmonization” introduced by Hongra- tanaworakit and Buchbauer to describe the effect of ylangylang (2004). In terms of the sensory spectra and skin temperat ure changes as a function of two behavioral tasks assigned to the participants, (a) includes essential oils showing “harmoniza- tion”; and (b) shows those without “harmonization”. decrease in skin temperature induced by lemon/mental arithmetic (at p < 0.05) and palmarosa/the auditory task (although this change did not reach statistical significance) could be regarded as a reflection of their relaxa- tion/sedation proper tie s. Controversial findings are often reported in aroma research. It is of particular importance to mention the  Y. Sugawara et al. controversy surrounding the lemon fragrance. Support for the hypothesis that the lemon fragrance causes dis- tress/agitation has been provided by the observation that lemon aroma causes an increase in heart rate (Kikuchi et al., 1992; Yamaguchi, 1990). However, the opposite is supported by the findings of a study by Manley (1993), in whi ch the ad ministr ation o f a le mon aro ma through an a ir co nditionin g syste m resulted in a decrease in con- tingent negati ve variatio n (CN V) as well as a decre ase in key entr y erro rs of video disp la y ter minal (VDT ) ope r- ators. In a recent study (Akipinar, 2005) in which the author wa s interested in ident i fying the effects of lemon on cognitive lea rning, atte ntio n, and memory, a lemon aroma increased the atte ntion levels o f students (at p < 0.05), enhanced memory (at p < 0.05), and had positive effects on cognitive l ear ning (a t p < 0.05). Similar controversies exist for other aromas. For instance, Manley (1993) reported that a ylangylang aroma appeared to possess a stimulating effect, as it caused an increase in CNV magnitude. Hongratanaworakit and Buchbauer (2004, 2006) demonstrated that its effect can be characterized as relaxation/sedation. With respect to sandalwood, Hongratanaworakit, Heuberger, and Buchbauer compared the effects of sandalwood administered via transdermal absorption (Hongratanaworakit et al., 2004) and inhalation (Heuberger et al., 2006) on physio- logical para meters, as well as mental and emotional co nditions, in healthy hu man par ticipants. The authors co n- cluded that the administration of sandalwoodoil via transder mal absorption and in specific experimental condi- tions provoked physiological d eactivation and behavioral ac tivation (Hongratanaworakit et al., 2004), wh il e a d- ministration via inhalation and in different experimental circumstances elevated pulse rate, skin conductance level, and systolic blood pressure (Heuberger et al., 2006). As for pepp ermint, evidence for its sti mulating pro p- erties includes an observation that peppermint aroma caused an increase in electroencephalography (EEG) speed and heart rate during sleep (Badia et al., 1990), an incre as e in C NV ma g nit ud e (Manley, 1993, Torii et al., 1988), a decrease in theta activity (Klemm et al., 1992), and enhanced EEG and behavioral arousal during stage 1 sleep (Carskadon & Herz, 2004). However, arguments against the stimulating properties of peppermint include the finding that peppermint aroma produced a significant decrease in gross speed, net speed, and accuracy in a typ- ing task (Barker et al., 2003); more NREM sleep, less REM sleep, and more slow-wave sleep (Goel & Lao, 2006); and increased alertness, decreased temporal demand, and decreased frustration during simulated driving (Raudenbush et al., 2009). In consideration of the above-mentioned opposing views about the psychophysiological properties of essential oils, and given our findings regarding the subtle nuances of expression between the verbal (psychological) and non-verbal (physiological) effects of essential oil aromas in humans, the described controversies may in part be a reflection of the versatile psychophysiological properties of essential oils. Specifically, essential oils seem to function as neurophysiological stimuli that can cause different perceptions and elicit diverse reactions, depend- ing on the internal and ex traneo us conditions of the par ticipants, as well as manifestatio ns of higher-order olfac- tory processing. The unique features related to early levels of the olfactory system have been extensively studied and docu- mented (Axel, 1995; Buck, 1996; Buck & Axe l , 1991; Li et al., 2008; Lledo et al., 2005; Mombaerts, 1999; Yo- shida & Mori, 2007; Zhang & Firestein, 2002), however, little is known about processes operating upstream. Further research is needed to characterize the mechanisms underlying odor discrimination beyond the early stages of olfactory processing. The limbic system is likely to be involved, as it includes the amygdala, septum, hippocampus, anterior thalamus, and hypothalamus. It plays a vital role in motivation, memory, emotions, and instinctive behaviors (Buckle, 2004; Price & Price, 2007). Thus, it is possible that the elucidation of high- er-order olfactory processing, as well as an improved understanding of the versatile psychophysiological proper- ties of essential oils, especially those associated with task-dependence, may contribute to the resolution of sev- eral controversies often seen in aroma research. To address these issues, further studies of these phenomena should consider external conditions, such as behavioral tasks assigned to participants. Such studies will poten- tially contribute to aroma research and the study of human chemoreception, enabling a deeper understanding of the worki ng principl es of t he hig hly sophist i cated human ol factory sys t em. 8. Conclusion 1) We have attempted to examine the relationship between mood change, odor and its psychophysiological responses, by focusing on the possible verbal (semantic) and non-verbal (skin temperature) changes in humans induced by smelling the fragrances of essential oils as well as linalool and its enantiomers. The obtained find- ings suggest t hat our verbal inspectio ns toget her with non-verbal tests are useful for shedd ing more light on the finer nuances of odor discrimination and psychophysiological responses to odorants i n humans.  Y. Sugawara et al. 2) This article is an overview of our verbal (semantic) research results as well as the non-verbal outcomes over the past decade. Because we believe that this approach has the potential to be highly informative, so that such i nfor mat ion may provide clues to our long-standi ng i ssue how p rec isel y hu mans a nd the ir o lfact or y sys tem can detect and discriminate a great variety of odors and subtle differences in the molecular structures of a given aroma. 3) Sensory evaluation has been a key component in a serie s of our r esearc h activities. I n o ur semantic i nquiry, we asked the participants to complete behavioural tasks during fragrance exposure. We used the Uchida-Krae- pelin test as a mental arithmetic task and listening to environmental (natural) sounds as an auditory task. The sensory test was conducted twice, once before and once after the task. We then represented the perceived sen- sory attributes reported by the participants via a bar graph (sensory spectrograph), whereby the mean of the im- pressions was plo tte d against the seman tic descriptor s. 4) Specifically, in the case of ylangylang spectrum in association with mental arithmetic, there was an unfa- vorable (or negative) correlation between the fragrance and the type of task assigned to the subject. On the other hand, yla ngyl ang spe ctr um i n re lati on to the audit or y tas k w as s hown to b e p ositi ve c orr ela tion b et wee n the fra- grance and the task. Similar results were found for bergamot, peppermint and sandalwood essential oils as well as linalool. This implies that different behavioural tasks influence the perceived odor quality of inhaling odor- ants. Moreo ver, the finer nuances visible in the appearance of each spectrum show how this method of sensory profiling can be practical for assessing odor perception in participants. 5) Eq ually, inhalation of (R)-(–)-linalool or (RS)-(±)-linalool in associatio n with the audit ory task produced a positive impression of odour quality, whereas this effect was not seen for (S)-(+)-linalool. In contrast, admini- stration of (R)-(–)-linalool, (S)-(+)-linalool and (RS)-(±)-linalool in association with mental arithmetic all pro- duced a negative impression of odour quality. This indicates that the enantiomers of linalool have different smells and that the perception of them is task-dependent. 6) T he resulting sensory spectra warranted particular interest. Ho wever, there were no sensory spectra where- in the changes of the spectra were significant among the 50 cases including the 21 essential oils and linalool as well as its enantiomers as a function of the two behavioral task s (mental arithmetic task and the auditory task). 7) Hence, we considered a variety of strategies for addressing statistical defects associated with the signific- ance of the sensory spectra. Conclusively, we found that multi-channel skin thermometer data might comple- ment the sta tistica l defec ts o f the r eleva nt se nsor y spectr a. T hat is, t he pr actica l po tentialiti es o f the skin te mper- ature data for the evaluation of statistical significance might complement any statistical defects associated with the sensory spectrographs, even if the sensory spectra were not statistically significant. 8) Throughout the skin temperature measurements, we concentrated our focus to involve only the following 12 essential oils (basil, bergamot, cardamom, cinnamon, juniper, lemon, orange, palmarosa, peppermint, san- dalwood, spearmint and ylangylang). 9) Based on the relationship between verbal and non-verbal responses of the 12 target odors during the two tasks (mental arith metic and the auditor y), we classified the 24 odor-task combinations i nto three categories: 1) the first category, which included lemon and palmarosa, elicited task-dependent dual changes in fingertip skin temperature; 2) the second category, which included basil and juniper, not only elicited a n increase in skin tem- perature, but also the increased sensory spectra in a non-task dependent fashion; and 3) the third category, which included bergamot, cardamom, cinnamon, orange, peppermint, sandalwood, spearmint, and ylangylang, which elicited a decrease in skin temperature regardless of the behavioral task assigned to the participants. 10) T hus, the subtle differences between the two behavioral tasks while inhaling the fragrances of the above- mentioned 12 target odors could be viewed in terms of verbal (sensory spectrum) and non-verb al (fin gertip skin temperature) expressions as a function of the two behavioral tasks. 11) If feelings of excitement or apprehension induce slight increases in skin temperature, it is reasonable to assume that our target odors can be regarded as having either distressing/agitating sentiments when associated with increases in skin temperature or relaxing/sedating sent iments when associated with decreases in skin tem- perature. YlangYlang could be such a representative example. Decrease s in skin temperature measurements while inhaling the fragrance of ylangylang indicated that inhalation of ylangylang brought about relaxing/sedating sentiments in the participants for both the mental arithmetic and auditory tasks. In contrast, the sensory spectra showed that an unfavorable impression of the odor was associated with mental arithmetic, whereas a favorable impression was associated with the auditory task. 12) Our results of ylangylang were in good agreementwith the conception of “harmonizing” rather than re- laxi ng/sed a t ing, wh ich wa s introduced by Hongra tana woraki t and Buchba uer (2 004 ) to describe the psychophy-  Y. Sugawara et al. siological effects of ylangylang inhalation. When we used the obtained sensory spectra and skin temperature profiles of ylangylang as a reference to evaluate the other tested odorants, we found that bergamot, peppermint and sandalwood could be classified as “harmonizing”. 13) Odorant receptors are encoded by the largest gene family thus far identified, making it a very diverse group of receptors. In our studies, however, the fragrance administered to the participants was well controlled and should activate the same set of receptors regardless of behavioral task. 14) Hence, the observed task-dependence of odorperception and psychophysiological responses likely origi- nates from higher-order processes in the brain. A great deal is known about the initial stages of olfactor y proc- essing, but much less is known about processes beyond the olfactory bulb. Our research over t he past decade in- dicates that further work is needed to characterize the mechanisms of odour discrimination beyond the nasal epitheliu m. References Abramson, D. L., & Ferris, E. B. (1940). Responses of Blood-Vessels in the Resting Hand and Forearm to Various Stimuli. American Hea rt Jou rnal, 19, 541-553. http://dx.doi.org/10.1016/S0002-8703(40)90195-8 Akipinar, B. (2005). The Role of Sense of Smell in Learning and the Effects of Aroma in Cognitive Learning. Pakistan Journal of Social Sci ences, 3, 952-960. Allwood, M. J., Barcroft, H., Hayes, J. P. L. A., & Hirsjarvi, E. A. (1959). The Effect of Mental Arithmetic on the Blood Flow through Normal, Sympathectomized and Hyperhidrotic Hands. The Journal of Physiology, 148, 108-116. http://dx.doi.org/10.1113/jphysiol.1959.sp006276 Arnott, W. M., & Macfie, J. M. (1948 ). Effe ct of Ul nar Nerve Block on Blood Flow in the Reflexly Vasodilated Digit. The Journal of Physiology, 10 7, 233-238. Axel, R. (1995). The Molecul ar Logic of S mell. Scientific American, 273, 13 0-137. http://dx.doi.org/10.1038/scientificamerican1095-154 Badia, P., Wesensten, N., Lammers, W., Culpepper, J., & Harsh, J. (1990). Responsiveness to Olfactory Stimuli Presented in Sleep. Physiology Behavior, 48 , 87-90. Barker, S., Grayhem, R, Koon, J., Perkins, J., Whalen, A., & Raudenbush, B. (2003). Improved Performance on Clerical Tasks Associated with Administration of Peppermint Odor. Per c e ptual Motor Sk il ls , 97, 1007-1010 . http://dx.doi.org/10.2466/pms.2003.97.3.1007 Bensafi, M., Rouby, C., Farget, V., Bertrans, B., Vigouroux, M., & Holley, A. (2002). Autonomic Nervous System Res- ponses to Odours: The Role of Pleasantness and Arousal. Chemical Senses, 27, 703-709. http://dx.doi.org/10.1093/chemse/27.8.703 Billot, M., & Wells, F. (1975). Perfumery Technology: Art, Science, Industry. New York: John Wiley. Buchbauer, G., & Jirovetz, L. (1994). Aromatherapy-Use of Fragrances and Essential Oils as Medicaments. Flavour and Fragrance Journal, 9, 217 -222. http://dx.doi.org/10.1002/ffj.2730090503 Buck, L. (1996). Information Coding in the Vertebrate Olfactory System. Annua l Review of Neu roscience, 19, 517-544. http://dx.doi.org/10.1146/annurev.ne.19.030196.002505 Bu ck, L. , & Axel, R. (1991). A Novel Mu ltigen e Family May En cod e Odorant Recepto rs: A Mol ecular Basi s for Odor Rec- ognition. Cell, 65, 175-187. http://dx.doi.org/10.1016/0092-8674(91)90418-X Buckle, J. (1999). Use of Aromatherapy as a Complementary Treatment for Chronic P ain. Alternative Therapies in Health and Medicine, 5, 42-51. Buckle, J. (2004). Clinical Aromatherapy: Essential Oils in Practice (2nd ed.). New York: Elsevier. Carskadon, M. A., & Herz, R. S. (2004). Minimal Olfactory P erception during Sleep: Wh y Odor Alarms Will Not Work for Humans. Sleep, 27, 402-405. Coombs, C. H. (1964). T he or y of Data. New York: John Wiley. Drake, M. A. (2004). Defining Dairy Flavors. Journ al of Dai ry Scienc e , 87, 777-784. http://dx.doi.org/10.3168/jds.S0022-0302(04)73221-X Drake, M. A. (2007). Sensory Analysis of Dairy Foods. Journal of Dairy Science, 90, 4925-4937. http://dx.doi.org/10.3168/jds.2007-0332 Edge, J. (2003). A Pilot Study Addressing the Effect of Aromather apy Massage on Mo o d, Anxiety and Relaxation in Adult Mental Health. Complementary Therapies in Nursing and Midwifery, 9, 90-97. http://dx.doi.org/10.1016/S1353-6117(02)00104-X Elam, M., & Wallin, B. G. (1987). Skin Blood Flow Responses to Mental St ress in Man Dep end on Body Temperature. Acta  Y. Sugawara et al. Physiologica Scandinavica, 12 9, 429-431. http://dx.doi.org/10.1111/j.1365-201X.1987.tb10609.x Firestein, S. (1991). A Noseful of Odor Receptors. Trends in Neurosciences, 14, 270-272. http://dx.doi.org/10.1016/0166-2236(91)90135-H Friedman, L., & Miller, J. G. (1971). Odor Incongruity and Chiralit y. Science, 172, 1044-1046. http://dx.doi.org/10.1126/science.172.3987.1044 Gaskell, P. (1956). Are There S ympathetic V asodilat or Nerves to the Vessels of the Hand? The Journal of Physiology, 131, 647-656. http://dx.doi.org/10.1113/jphysiol.1956.sp005489 Goel, N., & Lao, R. P. (2006). Sleep Changes Var y Odor Percept io n in Young Adults. Bi ol o gi c al Psy c hol ogy , 71, 341 -349. http://dx.doi.org/10.1016/j.biopsycho.2005.07.004 Greenwald , M. K., Cook, E. W., & Lang, P. J. (1989). Affective Judgment and P sychophysiological Response: Dimensional Covariation in th e Evaluation of Pictorial Stimuli. Journal of Psychophysiology, 3, 51 -64. Guilford, J. P. (1954). Psychometric Methods. New York: McGraw-Hill. Harris, R., Martin, A. J., & Williams, H. S. (1952). Correlation of Skin Temperature and Circulato ry Changes in Muscle and Subcutaneous Tissues of the Hand during Trunk Heating. Clinical Science, 11, 429-440. Heuberger , E., Ho ngratan aworakit, T., & Buchb auer, G. (2006). East Indian Sandalwood and α-Santalol Odor Increase P hy- siological and Self-Rated Arousal in Humans. Plan ta Medica, 72, 792-800. http://dx.doi.org/10.1055/s-2006-941544 Holmes, C., Hopkins, V., Hensford, C., MacLaughlin, V., Wilkinson, D., & Rosenvinge, H. (2002). Lavender Oil as a Treatment for Agitated Behaviour in Severe Dementia: A Placebo Controlled Study. International Journal of Geriatric Psychiatry, 17, 305-308. http://dx.doi.org/10.1002/gps.593 Hongratanaworakit, T., Heuber ger, E., & Buchbauer, G. (2004). Evaluation of the Effects of East Indian Sandalwood Oil and α-Santalol on Humans after Transdermal Absorption. Planta Medica, 70, 3-7. http://dx.doi.org/10.1055/s-2004-8154 46 Hongratanaworakit, T., & Buchbauer, G. (2004). Evaluation of the Harmon izi ng Ef fect of Ylang-Ylang Oil on Humans after Inhalation. Planta Me dica, 70, 632-636. http://dx.doi.org/10.1055/s-2004-827186 Hongratanaworakit, T., & Buchbauer, G. (2006). Relaxing Effect of Ylangylang Oil on Humans after Transdermal Absorp- tion. Phytothera py Research, 20, 758-763. http://dx.doi.org/10.1002/ptr.1950 Kellerova, E., & Delius, W. (1969). Unterschiede der vasomotorischen reaktivitat im muskel- und akralen hautgefassgebiet der oberen und unteren extremitaten. Zeitschrift fur Kreislaufforschung, 58, 917-925. Kikuchi, A., Yamaguchi, H., Tanida, M., Abe, T., & Uenoyama, S. (1992). Effect of Odours on Card iac Response P atterns and Subjective States in a React ion Time Task. Tohoku Psychologica Folia, 51, 74-82. Klemm, W. R., Lutes, S. D., Hendrix, D. V., & Warrenburg, S. (1992). Topographical EEG Maps of Human Responses to Odors. Chemical Senses, 17, 347-361. http://dx.doi.org/10.1093/chemse/17.3.347 Kling, J. W., & Riggs, L. A. (1972). Experimental Ps ychology. New York: Holt, Reinhart and Winston. Kyle, G. (2006). Evaluating the Effectiveness of Aromatherapy in Reducing Levels of Anxiety in Palliative Care Patients: Result s of a Pilot Study. Complementary Therapies in Clinical Practice, 12, 148-155. http://dx.doi.org/10.1016/j.ctcp.2005.11.003 Lang, P. J., Bradley, M. M. , & Hamm, A. O. (1998). Emotion and Motivation: Measuring Affective P erception. Journal of Clinical Neurophysiology, 15, 397-408. http://dx.doi.org/10.1097/00004691-19980 9000-00 004 Lawless, H. T., & Heymann, H. (1988). Sensory Evaluation of Food: Practices and Principals. New York: Chapman and Hall. Leitereg, T. J., Guadagni, D. G., Harris, J., Mon, T. R., & Teranishi, R. (1971). Chemical and Sensory Data Supporting the Difference between the Odors of the Enantiomeric Carvones. Journal of Agricultural and Food Chemistry, 19, 785 -78 7. http://dx.doi.org/10.1021/jf60176a035 Li, W., Howard, J. D., Parrish, T. B., & Gottfried, J. A. (2008). Aversive Learning Enhances Perceptual and Cortical Dis- crimination of Indiscriminable Odor Cues. Science, 319, 1842-1845. http://dx.doi.org/10.1126/science.1152837 Lin kert, R. (1932). A Technique for the Measurement of Attitudes. Archives of Psychology, 140, 44-53. Lledo, P.-M., Gheusi, G., & Vincent, J.-D. (2005). Information Processing in the Mammalian Ol factory System. Psysiologi- cal Reviews, 85, 281-317. http://dx.doi.org/10.1152/physrev.00008.2004 Lor ig, T. S., & S chwartz, G. E. (1988). Brain and Odor: I. Alteration of Human E E G b y Odor Administration. Psychophysi- ology, 16, 281-284. Manley, C. H. (1993). Psychophysiol og i c a l Effect of Odor. Critical Reviews in Food Science and Nutrition, 33, 57-62. http://dx.doi.org/10.1080/10408399309527612 Mehrabian, A., & Russ ell, J. A. (1974). An Approach to Environmental Psychology. Cambridge, MA: MIT Press.  Y. Sugawara et al. Meilgaard, M. C., Giville, G. V., & Carr, B. T. (1999). Sensory Evaluation of Techniques (3rd ed.). Boca Raton, FL: CRC Press. http://dx.doi.org/10.1201/9781439832271 Mombaerts, P. (1999). Molecular Biology of Odorant Receptors in Vertebrates. Annual Review of Neuro science, 2 2, 487 -509. http://dx.doi.org/10.1146/annurev.neuro.22.1.487 Morris, E. T. (1984) . Fragrance. New York: Scribners. Ohloff, G., & Klein, E. (1962). Die absolute konfiguration des linaloolsdurchverknuepfungmitdempinansystem. Tetrahedron, 18, 37-42. Oka, T., Hayashida, S., Kaneda, Y., Takenaga, M., Tamagawa, Y., Tsuji, S., & Hatanaka, A. (20 08). Green Odor Attenuates a Cold Pressor Test-Induced Cardiovascular Response in Healthy Adults. BioPsychoSocial Medicine, 2, 2. http://dx.doi.org/10.1186/1751-0759-2-2 Oka, T., Oka, K., & Hori, T. (2001).Mechanisms and Mediators of Psycholo gical Stress-Induced Rise in Core Temperature. Psychosomatic Medicine, 63, 476-486. http://dx.doi.org/10.1097/00006842-200105000-00018 Perry, N., & Perry, E. (2006). Aromatherapy in the Management of Psychiatr ic Disorders: Clinical and Neurophamacologi- cal Perspectives. CNS Drugs, 20, 257-280. http://dx.doi.org/10.2165/00023210-200620040-00001 Price, S., & Price, L. (2007). Aromatherapy for Health Professionals (3rd ed.). London: Elsevier. Raudenbush, B., Grayhem, R., Sears, T., & Wilson, I. (2009). Effects of Peppermint and Cinnamon Odor Administration on Simulated Driving Alertness, Mood and Workload. North American Journal of Psychology, 11, 245-256. Roddie, I. C. (1983). Circulation to Skin and Adipose Tissue. In J. T. Shepard, & F. M. Abbound (Eds.), Handbook of Phy- siology. The Cardiovascular System (Vol. II, Part 1, Chapter 10, pp. 285-317). Bethesda, MD: American Physiological Society,. Roddie, I. C., Shepherd, J. T., & Whelan, R. F. (1957). The Contribution of Constrictor and Dilator Nerves to the Skin Vaso- dilatation during Body Heating. The Journal of Physiology, 136, 489-497. http://dx.doi.org/10.1113/jphysiol.1957.sp005775 Roderick, W. R. (1966). Current Ideas on the Chemical Basis of Olfaction. Journal of Chemical Education, 43, 510-520. http://dx.doi.org/10.1021/ed043p510 Rowell, L. B. (1981). Active Neurogenic Vasodilation in Man . In P. M Van Houtte, & I. Leusen (Eds.), Vasodilation (pp. 1-17). New York: Raven Press . Russell, G. F., & Hills, J. I. (1971). Odor Difference between Enantiomeric Isomers. Science, 172, 1043-1044. http://dx.doi.org/10.1126/science.172.3987.1043 Satoh, T., & Sugawara, Y. (2003). Effects on Humans Elicited by Inhaling the Fragrance of Essential Oils: Sensory Test, Multi-Channel Thermometric Study and Forehead Surface Potential Wave Measurement on Basil and Peppermint. Ana- lytica l Sciences, 19, 13 9-146. http://dx.doi.org/10.2116/analsci.19.139 Smallwood, J., Brown, R., Coulter, F., Irvine, E., & Copland, C. (2001). Aromatherapy and Behaviour Disturbances in De- mentia: A Randomized Controlled Trial. International Journal of Geriatric Psychiatry, 16, 1010-1013. http://dx.doi.org/10.1002/gps.473 Stevens, S . S. (19 51). Handbook of Experimental Psychology. New York: John Wiley. Sugawara, Y. (2001). Odor Distinctiveness between Enantiomers of Linalool. Current Topics in Analytical Chemistry, 2, 201-210. Sugawar a, Y. (2008) . Sensory Profiling for Verbal Responses in Perceived Odor Q uality in Humans. In L. N. Pi ccard (Ed.), Biol ogical Psychology: New R es earch (Chapter 4, pp. 145-165). New York: Nova Science Publishers. Sugawara, Y., & Kawasa ki, M . (20 00) . Skin Temperature Ch an ge Eli cited by Inhalation of the Fragrance of Essential Oils in Relation to Mental Work: Multi-Channel Thermometric Study on Basil and Peppermint. Journal of Home Economics of Japan, 51, 675-681. Sugawara, Y., Fukui, H., Shigeoka, C., Hasegawa, R., & Okimoto, A. (2006). Multichannel Thermometric Study of Skin Temperature Chan ges in Humans While Inhaling Essential Oils. Flavour and Fragrance Journal, 21, 416-422. http://dx.doi.org/10.1002/ffj.1683 Sugawara, Y., Hara, C., Aoki, T., Sugimoto, N., & Masujima, T. (2000). Odor Distinctiveness between Enantiomers of Li- nalool: Difference in Perception and Resp onses El icited by Sen sory Test and Foreh ead Sur face Poten tial Wave. Chemical Senses, 25, 77-84. http://dx.doi.org/10.1093/chemse/25.1.77 Sugawara, Y., Hara, C., Tamura, K., Fujii, T., Nakamura, K., Masujima, T., & Aoki, T. (1998a). Sedative Effect on Humans of Inhalation of Essential Oil of Linalool: Sensory Evaluation and Physiological Measurements Using Optically Active Lin alools. Analytica Chimica Acta, 365, 29 3-299. http://dx.doi.org/10.1016/S0003-2670(97) 00639 -9 Sugawara, Y., Hino, Y., Kawasaki, M., Hara, C., Tamura, K., Sugimoto, N., Yamanishi, U., Miyauchi, M., Masujima, T., &  Y. Sugawara et al. Aoki, T. (1999). Alteration of Perceived Fragrance of Essential Oils in Relation to Type of Wo rk: A Simple Screening Test for Efficacy of Aroma. Chemical Senses, 24, 415-421. http://dx.doi.org/10.1093/chemse/24.4.415 Sugawara, Y., Mar uyama, S., Nakag awa, N., Yamada, E., Seto, M., Sumihiro, S., Aoi, N., Nishimoto, M., Kobayashi, Y., & Hirano, M. (2008). Verbal and Non-Verbal Responses to Odorants in Humans While Inhaling the Fragrance of Pepper- mint and Spearmint Essential Oils and Linalool. International Journal of Essential Oil Therapeutics, 2, 111-121. Sugawara, Y., Nishimoto, M., Kobayashi, Y., Hasegawa, R., Okimoto, A., & Aoki, T. (2003). Repeatability of the Measure Required for Perceptional Changes of the Fragrance of Essential Oils Was Tested in Terms of Sensory Evaluation Spec- trums. Bull. Fac. Hum an L if e Env i r on. Sc i . Hiroshima Women ’ s Univ. , 9, 21-36. Sugawara, Y., Shigetho, A., Yoneda, M., Tuchiya, T., Matumura, T., & Hirano, M. (2013). Relationship between Mood Change, Odour and Its Physiological Effects in Humans While Inhaling the Fragrances of Essential Oils as Well as Lina- lool and Its Enantiomers. Molecules, 18, 3312-3338. http://dx.doi.org/10.3390/molecules18033312 Sugawara, Y., Sugimoto, C., & Minabe, S. (2009b). Sensory Evaluation of the Deodorizing Efficacy of a Titanium Oxide -Type Deodorant. Journal of Home Economics of Japan, 60, 353-362. Sugawara, Y., Sugimoto, C., Minabe, S., Iura, Y., Okazaki, M., Nakagawa, N., Seto, M., Maruyama, S., Hirano, M., & Ki- tayama, I. (2009a). Use of Human Senses as Sen sors. Sensors, 9, 3184-3204. http://dx.doi.org/10.3390/s90503184 Sugawara, Y., Tomota, T., & Tamura, K. (1998b). Perceived Fragrance of Essential Oils in Relation to Type of Work. Jour- nal of Home Economics of Japan, 49, 1281-1290. Torgerson, W. S. (1958). Theory and Methods of Scaling. New York: John Wiley. Torii, S., Fukuda, H., Kanemoto, H., Miyauchi, R., Hamauzu, Y., & Kawasaki, M. (1988). Contingent Negative Variation and the Psycholo gical Effec ts of Odour. In S. van Toller , & G. H. Dodd (Eds.), Perfumery: The Psychology and Biology of Fragrance (pp. 10 7-120). New York: Chapman Hall. http://dx.doi.org/10.1007/978-94-017-2558-3_6 Vallbo, A. B., Hagbarth, K.-E., Torebjork, H. E., & Wallin, B. G. (1979). Somatosensory, Proprioceptive and Sympathetic Activity in Human Peripheral Nerves. Phys iological Reviews, 59, 919-957. Valnet, J. (1982). The Practice of Aromatherapy. Saffron Walden, MA: C W Daniel Company. Yamagata, Y. , & Su gawara, Y. (2014). Senso ry Evaluat io n Spectr um Metho d as a Descrip ti ve Sensory Anal ysis. Psychology, 5, 1591-1610. http://dx.doi.org/10.4236/psych.2014.514170 Yamaguchi, H. (1990). Effect of Odor on Heart Rate. In M. Indo (Ed.), The Psychophysiological Effects of Odor, Aroma- chology (p. 168). To kyo: K o r yo . Yamakoshi, T., Yamakoshi, K., Tanaka, S., Nogawa, M., Shibata, M., Sawada, Y., Rolfe, P., & Hirose, Y. (2007). A Pre- liminary Study on Driver’s St ress Index Using a New Method Bas ed on Differen ti al Ski n Temperatu re M easur ement . 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon , 22-26 August 2007, 722-725. http://dx.doi.org/10.1109/iembs.2007.4352392 Yoshida, I., & Mori, K. (2007).Odorant Category Profile Selectivity of Olfactory Cortex Neurons. The Journal of Neuros- cience, 27, 9105-9114. http://dx.doi.org/10.1523/JNEUROSCI.2720-07.2007 Zhang, X., & Firestein, S. (2002).The Olfactory Receptor Gene Superfamily of the Mouse. Nature Neurosci ence, 5, 124-13 3. Ziegler, L. H., & Cash, P. T. (1938). A Study of the Influence of Emotions and Affects on the Surface Temperature of the Human Body. The American Journal of Psychiatry, 95, 67 7-696. http://dx.doi.org/10.1176/ajp.95.3.677
|