Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2009, 2, 245-253 doi: 10.4236/jbise.2009.24038 Published Online August 2009 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online August 2009 in SciRes. http://www.scirp.org/journal/jbise Preparation and properties of cast polyurethane elastomers with molecularly uniform hard segments based on 2,4-toluene diisocyanate and 3,5-dimethyl-thioltoluenediamine Xiao-Dong Chen*1,2, Nan-Qiao Zhou1, Hai Zhang2 1National Engineering Research Center of Novel Equipment for Polymer Processing, The Key Laboratory of Polymer Processing Engineering Ministry of Education, South China University of Technology, Guangzhou, 510640, China; 2GuangZhou SCUT Bestry Technology Joint-stock Co., Ltd., South China University of Technology, Guangzhou, 510640, China Email: cxdzlgzhnlg2003@163.com Received 16 December 2008; revised 2 March 2009; accepted 5 March 2009. ABSTRACT A series of three cast polyurethane elastomers were prepared from 2,4-toluene diisocyanate (TDI) and 3,5-dimethyl-thioltoluenediamine (D MTDA) chain extender, with polyethylene adi- pate (PEA), polyoxytetramethylene glycol (PTMG) and polycaprolactone (PCL) soft seg- ments. The polyol molecular weights em- ployed was 2000g/mol. The polyurethane elastomers were characterized by an elec- tronmechanical universal testing machine, an Akron abrasion loss tester, a LX-A Shore du- rometer, a rebound resilience equipment and a Dynamic- Mechanical analyzer. In addition, fractured surface of the polyurethane elas- tomers was investigated by a field emission scanning electron microscopy (SEM). The test results showed the PCL based elastomer ex- hibits the excellent tear and stress-strain properties that polyester based elastomers offer, while retaining superior compression set and resilience similar to polyether based elas- tomers. The static and dynamic properties of the PCL based elastomer were more suitable for dynamic applications. The SEM micro- graphs of all polyurethane samples indicated the existing of the microphase separation structure. Particles of the dispersed phase formed by the hard phase and crystalline part of the soft phase grows bigger with the in- creasing crystallinity of the soft segments. The hard domains are irregular shapes and with the sizes of a few micrometers. Keywords: Soft Segment; Structure; Cast Polyure- thane Elastomer; Properties 1. INTRODUCTION In the recent decades, polyurethane elastomers have been successfully employed in a growing variety of uses and applications, due to their broad range of outstanding properties [1,2,3,4,5,6,7,8,9,10,11,12]. The polyurethane elastomers are composed of short, alternating polydis- perse blocks of soft and hard segments. The soft seg- ments with a low glass transition temperature are formed generally from polyethers or polyesters, generally of mo- lecular weight 400-5000. The rigid, polar hard segments with a high glass transition temperature are based on diisocyanates and low-molecular-weight chain extenders [6,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Because there exists a degree of thermodynamic im- miscibility between the hard urethane segments and the soft polyol segments, polyurethane elastomers exhibit microphase separation, which could result in a structure that can be considered as hard segment domains dis- persed in a soft segment matrix [6,13,21,25,27,28,29,30, 31,32]. The resultant two-phase micro-domain structure exhibited by polyurethane elastomers is responsible for their superior mechanical properties. Usually, micro- phase separation is incomplete and the hard and soft segment phases still contain certain amounts of the other segment. The mean domain size increases from 10 to 20 nm as the hard segment content increases and the shapes of hard domains are in the form of spheres 5-20 nm, or long needles 5 nm thick and 50-300 nm long [6,14]. The two-phase micro-domain structure is greatly in- fluenced by the molecular structure of the diisocyanate, polyol, and chain extender [13,14,33,34], by the ratio of hard segment and soft segment components [35], by the average segment length employed (including molecular weight distribution) [13], by the crosslinking density [18], and by the thermal history of the material [36]. Processing conditions, such as temperature, can also  246 X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 SciRes Copyright © 2009 JBiSE change the domain structure significantly [37]. Some researchers have employed many characterization tech- niques to understand the relationship between the chemical architecture, morphology, and chemical prop- erties [37,38]. It is well known that the size, shape, and structure of the hard-segment and soft-segment domains play a crucial role in determination of macroscopic properties [34,39]. Therefore, static and dynamic proper- ties of polyurethane elastomers can be tailored by select- ing different diisocyanates, polyols and chain extenders, or by simply varying the processing temperature [40]. In this study, a series of polyurethane elastomers based on polyethylene adipate (PEA), polyoxytetrame- thylene glycol (PTMG) and polycaprolactone (PCL) with molecular weight of 2000 as soft segments and hard segments based on the combination of 2,4-toluene diisocyanate and 3,5-dimethyl-thioltoluenediamine. In addition to general mechanical properties, resistance to thermal degradation, abrasion and dynamic properties were investigated, and the micro-phase structure images of samples were observed and captured by a Field Emis- sion Scanning Electron Microscope (FE-SEM). The re- lationship between micro-phase structure and macro- scopic properties was discussed. These key engineering properties are considered essential and the obtained re- sults will provide foundation for the formula and struc- ture design of the compounds for various applications, especially for high-loading dynamic applications. 2. EXPERIMENTAL 2.1. Materials PEA was obtained form JingXing Polyurethane Co., Ltd. (WuXi, China). PTMG was produced by Mitsubi- shi Chemical Co., Ltd, Nippon. PCL was purchased from Dow Chemical, USA. The three polyols should be dehydrated in vacuum at 100~110°C for 2 hours before use and their values were described in detail in Table 1. 2,4-toluene diisocyanate, purchased from Qingdao Yutian Chemical Company, was imported in original package and used as received. The chain extender, 3,5-dimethyl-thioltoluenediamine, was purchased Albe- marle Company and should be purified by dehydrated in vacuum at 80 for 1 night before use. Dib℃utyltin dilaurate (DBTDL) was acquired from Atofina Chemi- cals. 2.2. Preparation of Polyurethane Elastomers Traditionally, polyurethane elastomers can be synthe- sized via a “one-shot” process or prepolymer method. While the one-shot process is the quickest and easiest of the manufacturing techniques, preparation via the pre- polymer method imparts greater control over the chem- istry of the reaction, influencing the structure, mechani- cal properties, reactivity and processability of the fin- ished product [41,42]. In this study, the prepolymer method was used. The first stage involves preparation of a prepolymer from the polyol in excess diisocyanate to produce an isocyanate-terminated molecule. Subsequent reaction of the prepolymer with a diol or diamine chain extender constitutes the second stage, which produces a multi-block copolymer. 2.2.1. First Stage: Preparation of Prepolymer 2,4-toluene diisocyanate (0.83mol, 145g) was added into a 4-necked round bottom-boiling flask equipped with an overhead mechanical stirring unit, a ther- mometer and a vacuum take-off/nitrogen inlet. A polyol (0.37mol, 740g) was melted in an oven and added to TDI with stirring and reacted at 80 °C for 2 h under a nitrogen atmosphere to give a polyurethane prepolymer as a viscous liquid. And the prepolymer was examined for NCO content by using a standard method of n-butyl amine titration. Table 1. Specifications of the three polyols. Polyols Molecular structure of the polyol Hydroxyl value, mgKOH/g Acid value, mgKOH/g Molecular weight PEA HO-[-CH2-CH2-OOC-(CH2)4-COO-)n-CH2-CH2-OH56 ≤0.5 2000 PTMG HO-(-CH2-CH2-CH2-CH2-O-)n-H 56 ≤0.02 2000 PCL HO-[-(CH2)5COO-]m-R-[-OOC(CH2)5-]n-OH 56 ≤0.05 2000 Table 2. Mechanical properties of elastomers based on different backbones. Properties PEA-TDI PCL-TDI PTMG-TDI 100% Modulus, MPa 4.05 3.76 2.3 300% Modulus, MPa 12.15 10.43 3.7 Tensile Strength, MPa 51.15 44.09 29.86 Elongation at break,% 468 438 380 Tear Strength, KN/m 69 61 52 Compression Set, % 5.6 4 4.4 Rebound, % 32 41 44 Hardness, Shore A 77 77 78  X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 247 SciRes Copyright © 2009 JBiSE 2.2.2. Second Stage: Synthesis of Polyurethane The obtained prepolymer (200g) was heated to 80 ℃ under vacuum (<2 mm Hg). The chain extender (21g) was added to the prepolymer. The resultant mixture was stirred at high speed for 60 seconds. If time permitting, the mixture should be degassed (for 1-2 min) to remove the air introduced by stirring. Then the mixture was poured into a pre-heated mold (110). The bubbles on ℃ the surface can be removed by sweeping it with a burner flame or with a stream of hot air, what can make the bubbles expanded and bursted. The mold was cured in a vented oven at 110 for 30 minutes. The polymer ℃ sheets were demolded and post-cured at an elevated temperature (for 12-16h at 110). The parts were stored ℃ at ambient temperature for 1 month. During this period, secondary chemical reactions should be completely and the microstructure would become established. This is very important for testing the dynamic properties. 2.3. Characteristics The tensile strength and elongation at break were deter- mined with an electronmechanical universal testing ma- chine (INSTRON Co. LTD, Model 5566, USA). The abrasion resistance was performed with an Akron abra- sion loss tester. The hardness was tested with a LX-A Shore durometer according to standard method (ISO 48- 1984). The resilience was measured by a rebound resil- ience equipment (CJ-6A, ShangHai fourth chemical machine factory). The dynamic mechanical analysis was carried out in an air atmosphere by means of a NETZSCH Instrument, Dynamic-Mechanical Analyzer DMA242, on samples of following sizes: 2.0×5.8× 10.0mm. The tests at 10 Hz frequencies, ±2N maximum dynamic stress, ±40μm maximum deformation ampli- tude and the temperature range of -100~150, with a ℃ heating rate of 5/min were accomplished. Fract℃o- graphs were observed with a Field emission scanning electron microscopy (FE-SEM, Philips XL30 ESEM- FEG). Samples were prepared by tearing brittle samples (0.5mm thick) at low temperature by immerging in liq- uefied nitrogen. All samples were coated with a layer of gold or platinum before characterization. 3. RESULTS AND DISCUSSION 3.1. Influence of the Polyol Structure on the Mechanical Properties of Polyurethane Elastomers Table 2 reports a list of some general mechanical prop- erties of a series of the polyurethane elastomers based on PEA, PTMG and PCL as soft segments and hard seg- ments based on the combination of 2,4-toluene diisocy- anate and 3,5-dimethyl-thioltoluenediamine. The PEA based elastomer had better tensile strength and elonga- tion, and much better tear resistance compared to the PTMG based elastomer. However, its compression set and resilience were inferior to the PTMG based elas- tomer. Interestingly, the PCL based elastomer offered very competitive stress-strain properties and tear resis- tance when compared with the PEA based elastomer, while significantly improved compression set and resil- ience over the PEA based elastomer. Its ability to retain elastic properties after prolonged compressive stresses was as good as the PTMG based elastomer, while its resilience performance was close to that of the PTMG based elastomer. From the testing data shown in Table 2, it is clear that the PCL based elastomer possesses more balanced properties. It exhibits the excellent tear and stress-strain properties that polyester based elastomers offer, while retaining superior compression set and resil- ience similar to polyether based elastomers. 3.2. Influence of the Polyol Structure on the Phase Morphology of Polyurethane Elastomers The SEM method was employed to investigate the mor- phology of fractured surface of the polyurethane elastom- ers. As shown in the presented images (Figures 1-3), mi- crophase separation can be observed for all polyurethane samples tested. A continuous phase is visible in SEM micrographs, which is created by the amorphous part of the soft phase and the intermediate phase, i.e., the so-called matrix. Particles of the dispersed phase are encapsulated in that matrix. They are formed by the hard phase and the crystalline part of the soft phase. Three principal thermodynamic factors contribute to the for- mation of that phase structure: mobility of hard segments, viscosity of the system, and interactions between hard segments [14]. The hard phase and the crystalline part of the soft phase form the so-called domains with irregular shapes and with the sizes of a few micrometers. The size of the domains is mainly dependent on its content of rigid segments and the crystallinity of soft phase, which is clearly visible for the three samples. The soft phase is amorphous in some cases (Figure 2). A small number of tiny particles composed of rigid segments can be ob- served in the fractured surface only. Crystallization of the soft phase causes the domains bigger in case of the PEA based elastomer (Figure 1) and PCL based elas- tomer (Figure 3). The particle size of the dispersed phase grows bigger with the increasing crystallinity of the soft segments. At the same time, the intermediate phase interface of the PCL based elastomer is much smoother than that of the PEA based elastomer due to its lower degree of crystallinity and less regular arrange- ment of soft segments. It should be noticed that there are some scratches and cracks in the surface of the PCL based elastomer (Figure 3). We obtained an explanation hat the PCL based elastomer had been exposed under t 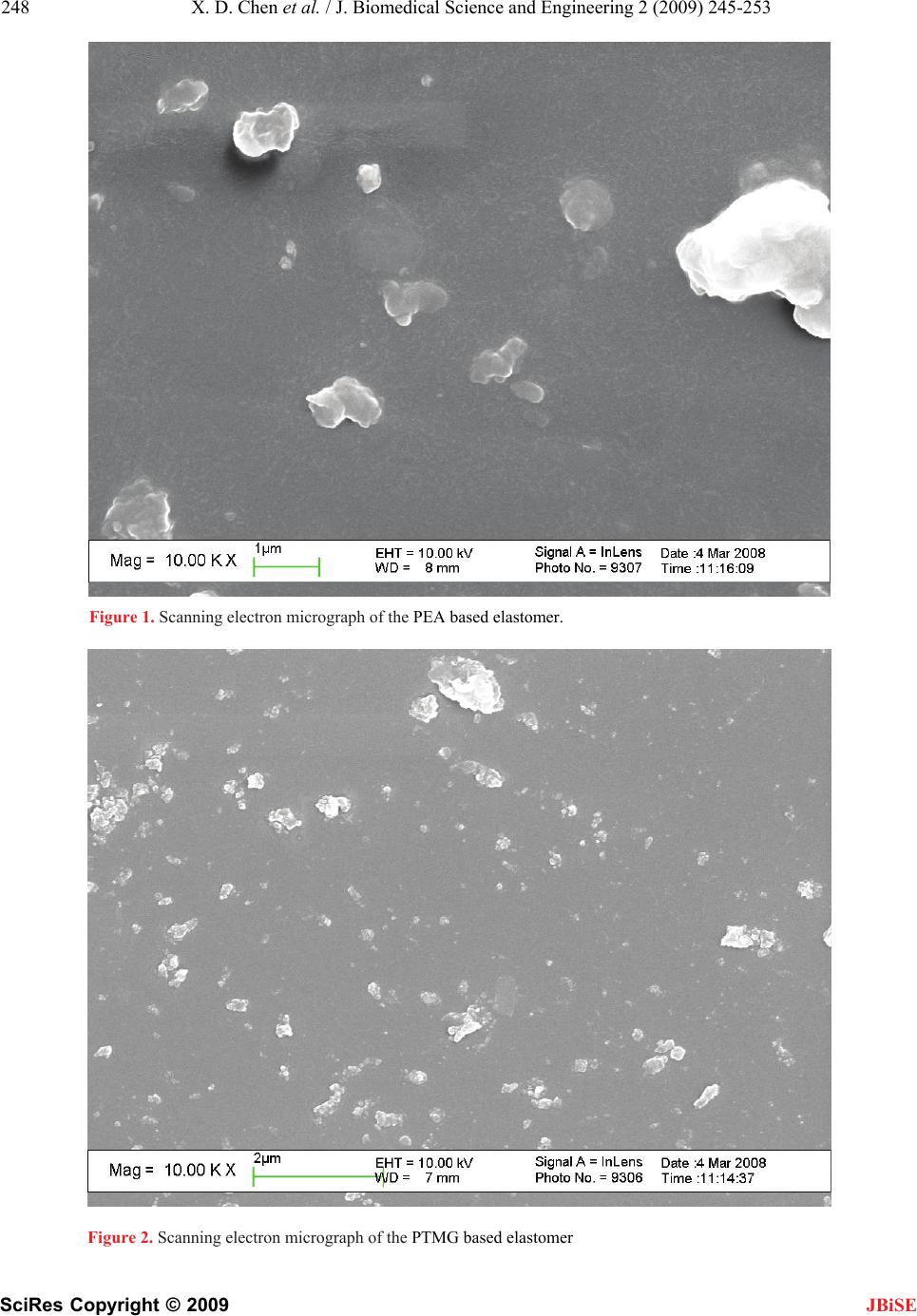 248 X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 SciRes Copyright © 2009 JBiSE Figure 1. Scanning electron micrograph of the PEA based elastomer. Figure 2. Scanning electron micrograph of the PTMG based elastomer 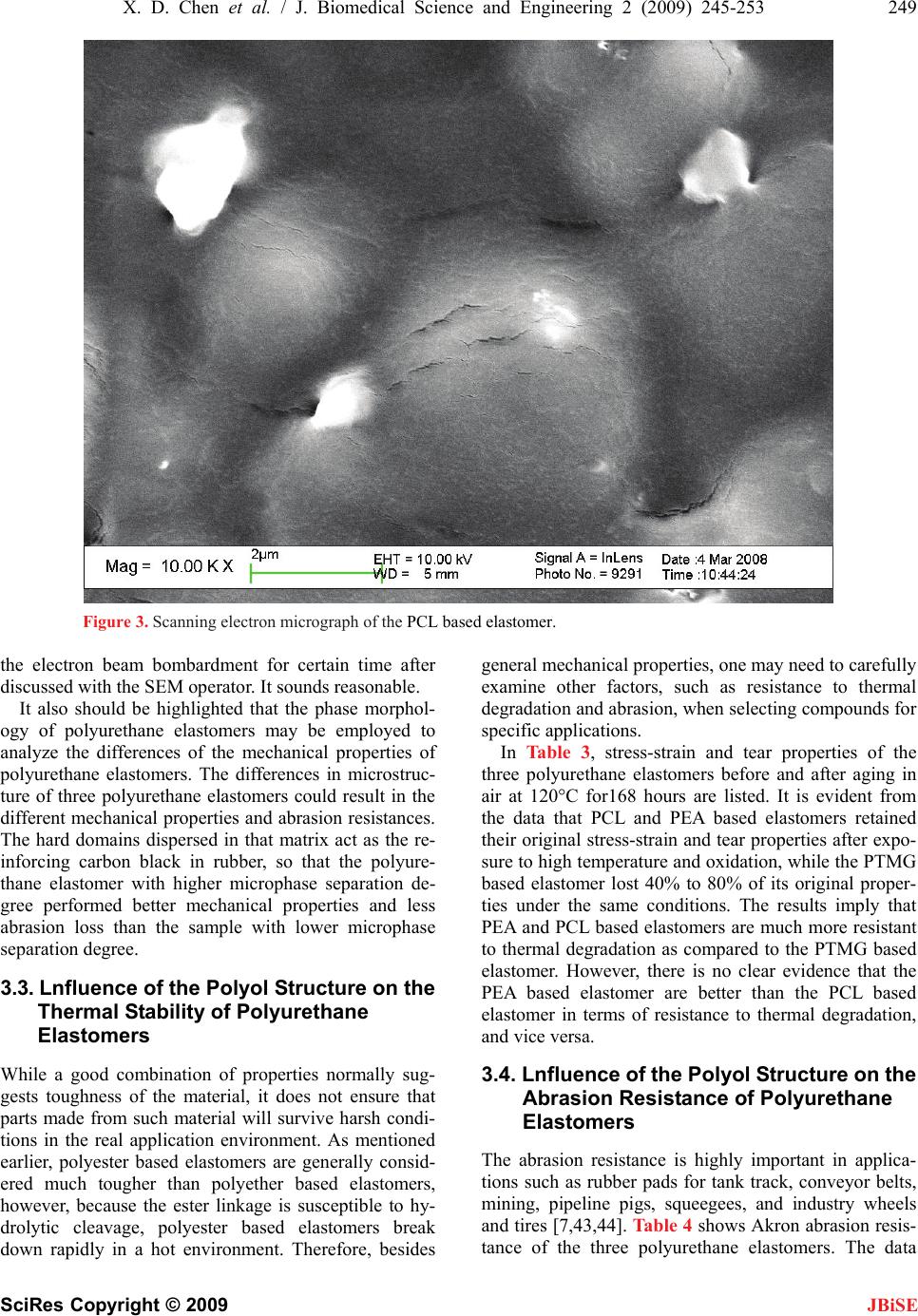 X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 249 SciRes Copyright © 2009 JBiSE Figure 3. Scanning electron micrograph of the PCL based elastomer. the electron beam bombardment for certain time after discussed with the SEM operator. It sounds reasonable. It also should be highlighted that the phase morphol- ogy of polyurethane elastomers may be employed to analyze the differences of the mechanical properties of polyurethane elastomers. The differences in microstruc- ture of three polyurethane elastomers could result in the different mechanical properties and abrasion resistances. The hard domains dispersed in that matrix act as the re- inforcing carbon black in rubber, so that the polyure- thane elastomer with higher microphase separation de- gree performed better mechanical properties and less abrasion loss than the sample with lower microphase separation degree. 3.3. Lnfluence of the Polyol Structure on the Thermal Stability of Polyurethane Elastomers While a good combination of properties normally sug- gests toughness of the material, it does not ensure that parts made from such material will survive harsh condi- tions in the real application environment. As mentioned earlier, polyester based elastomers are generally consid- ered much tougher than polyether based elastomers, however, because the ester linkage is susceptible to hy- drolytic cleavage, polyester based elastomers break down rapidly in a hot environment. Therefore, besides general mechanical properties, one may need to carefully examine other factors, such as resistance to thermal degradation and abrasion, when selecting compounds for specific applications. In Table 3, stress-strain and tear properties of the three polyurethane elastomers before and after aging in air at 120°C for168 hours are listed. It is evident from the data that PCL and PEA based elastomers retained their original stress-strain and tear properties after expo- sure to high temperature and oxidation, while the PTMG based elastomer lost 40% to 80% of its original proper- ties under the same conditions. The results imply that PEA and PCL based elastomers are much more resistant to thermal degradation as compared to the PTMG based elastomer. However, there is no clear evidence that the PEA based elastomer are better than the PCL based elastomer in terms of resistance to thermal degradation, and vice versa. 3.4. Lnfluence of the Polyol Structure on the Abrasion Resistance of Polyurethane Elastomers The abrasion resistance is highly important in applica- tions such as rubber pads for tank track, conveyor belts, mining, pipeline pigs, squeegees, and industry wheels and tires [7,43,44]. Table 4 shows Akron abrasion resis- tance of the three polyurethane elastomers. The data 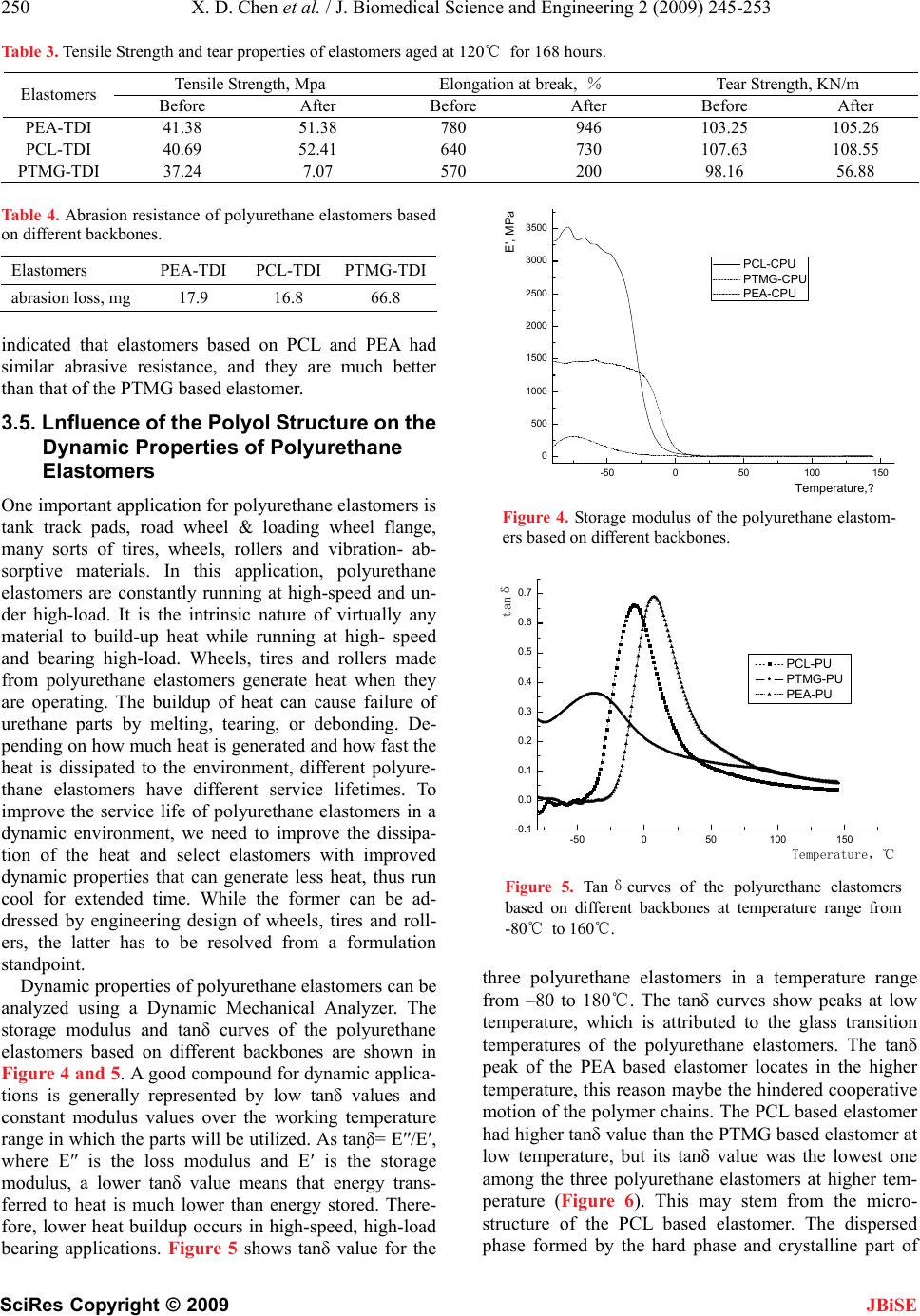 250 X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 SciRes Copyright © 2009 JBiSE Table 3. Tensile Strength and tear properties of elastomers aged at 120℃ for 168 hours. Tensile Strength, Mpa Elongation at break, % Tear Strength, KN/m Elastomers Before After Before After Before After PEA-TDI 41.38 51.38 780 946 103.25 105.26 PCL-TDI 40.69 52.41 640 730 107.63 108.55 PTMG-TDI 37.24 7.07 570 200 98.16 56.88 Table 4. Abrasion resistance of polyurethane elastomers based on different backbones. Elastomers PEA-TDIPCL-TDI PTMG-TDI abrasion loss, mg 17.9 16.8 66.8 indicated that elastomers based on PCL and PEA had similar abrasive resistance, and they are much better than that of the PTMG based elastomer. 3.5. Lnfluence of the Polyol Structure on the Dynamic Properties of Polyurethane Elastomers One important application for polyurethane elastomers is tank track pads, road wheel & loading wheel flange, many sorts of tires, wheels, rollers and vibration- ab- sorptive materials. In this application, polyurethane elastomers are constantly running at high-speed and un- der high-load. It is the intrinsic nature of virtually any material to build-up heat while running at high- speed and bearing high-load. Wheels, tires and rollers made from polyurethane elastomers generate heat when they are operating. The buildup of heat can cause failure of urethane parts by melting, tearing, or debonding. De- pending on how much heat is generated and how fast the heat is dissipated to the environment, different polyure- thane elastomers have different service lifetimes. To improve the service life of polyurethane elastomers in a dynamic environment, we need to improve the dissipa- tion of the heat and select elastomers with improved dynamic properties that can generate less heat, thus run cool for extended time. While the former can be ad- dressed by engineering design of wheels, tires and roll- ers, the latter has to be resolved from a formulation standpoint. Dynamic properties of polyurethane elastomers can be analyzed using a Dynamic Mechanical Analyzer. The storage modulus and tanδ curves of the polyurethane elastomers based on different backbones are shown in Figure 4 and 5. A good compound for dynamic applica- tions is generally represented by low tanδ values and constant modulus values over the working temperature range in which the parts will be utilized. As tanδ= E″/E′, where E″ is the loss modulus and E′ is the storage modulus, a lower tanδ value means that energy trans- ferred to heat is much lower than energy stored. There- fore, lower heat buildup occurs in high-speed, high-load bearing applications. Figure 5 shows tanδ value for the -50050100 150 0 500 1000 1500 2000 2500 3000 3500 Temperature,? E', MPa PCL-CPU PTMG-CPU PEA-CPU Figure 4. Storage modulus of the polyurethane elastom- ers based on different backbones. -50050100 150 -0.1 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 tanδ Temperature,℃ PCL-PU PTMG-PU PEA-PU Figure 5. Tanδcurves of the polyurethane elastomers based on different backbones at temperature range from -80℃ to 160℃. three polyurethane elastomers in a temperature range from –80 to 180. The tanδ curves show peaks at low ℃ temperature, which is attributed to the glass transition temperatures of the polyurethane elastomers. The tanδ peak of the PEA based elastomer locates in the higher temperature, this reason maybe the hindered cooperative motion of the polymer chains. The PCL based elastomer had higher tanδ value than the PTMG based elastomer at low temperature, but its tanδ value was the lowest one among the three polyurethane elastomers at higher tem- perature (Figure 6). This may stem from the micro- structure of the PCL based elastomer. The dispersed phase formed by the hard phase and crystalline part of  X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 251 SciRes Copyright © 2009 JBiSE 50 60 70 80 90100 0.06 0.09 0.12 0.15 0.18 tanδ Temperature,℃ PCL-PU PTMG-PU PEA-PU Figure 6. Tan δ curves of the polyurethane elastomers based on different backbones at operating temperature zone. the soft phase reinforced the elastomer, and the strong interactions caused by lower degree of soft segment crystallinity and hydrogen bonds enable the motion of the polymer chains more synchronous with the load, which makes the heat build up lower at operating tem- perature. It is very important for dynamic application accompanied by heat build up that could possibly weaken materials, thus causing failure. Obviously, the PEA based elastomer might not be the best choice for dynamic applications if similar grades of PTMG and PCL based elastomers are readily available. As for PCL and PTMG based elastomers, though the PCL based elastomer has higher tanδ value at low temperature, some engineers believe that it is the tanδ value at higher temperature that really matters. The higher tanδ value at low temperature implies that a wheel made from the PCL based elastomer will build up heat faster than a wheel made from the PTMG based elastomer when the wheel is cold. However, as the temperature increases, tanδ value decreases. During use, the temperature of the wheel will stabilize at the temperature where heat gener- ated is equal to the heat dissipated, and that will be the operating temperature of the wheel most of the time. This temperature for the PCL based elastomer wheel might be slightly higher than that of the PTMG based elastomer, depending on the engineering design of the wheels. On the other hand, the storage modulus of the PCL based elastomer is the highest one among the three elastomers, this is very helpful for high load application. However, considering the enhanced mechanical strength and resistance to thermal degradation of the PCL based elastomer over that of the PTMG based elastomer, the PCL based elastomer will perform better than the PTMG based elastomer in the field. 4. CONCLUSIONS Three polyurethane elastomers based on different soft segments were prepared and their properties were com- pared side by side. The PCL based elastomer exhibits the excellent tear and stress-strain properties that polyester based elastom- ers offer, while retaining superior compression set and resilience similar to polyether based elastomers. The SEM results of all polyurethane samples showed the existing of the microphase separation structure. Par- ticles of the dispersed phase formed by the hard phase and crystalline part of the soft phase grows bigger with the increasing crystallinity of the soft segments. The hard domains are irregular shapes and with the sizes of a few micrometers. As polyester based polyurethane elastomers, PEA and PCL based elastomers are much more resistant to ther- mal degradation as compared to the PTMG based elas- tomer. Polyurethane elastomers based on PCL and PEA had similar abrasive resistance, and they are much better than that of the PTMG based elastomer. The tanδ value at operating temperature zone of the PCL based elastomer is lower than those of the PEA and PTMG based elastomers. And the PCL based elastomer had higher tanδ value than the PTMG based elastomer at the temperature around zero centidegree. Based on the time-temperature superposition principle, a conclusion can be made that the PCL based elastomer exhibits good wet skid resistance, low rolling resistance and out- standing dynamic application properties[45,46,47]. In a word, The PCL based elastomer possesses more balanced properties. It is a favorable choice for applica- tions where a combination of engineering properties is desired. 5. ACKNOWLEDGEMENTS This research is funded by the Polyurethane Department of GuangZhou SCUT Bestry Technology Joint-stock Co. Ltd. The authors are grateful to the teacher of National Engineering Research Center of Novel Equipment for Polymer Processing for their helpful advice. REFERENCES [1] S. R. Pajtas, (1990) Polyurethane non-pneumatic tire technology, Development and testing history, in SAE In- ternational Congress and Exposition. SAE, Warrendale, PA, USA: Detroit, MI, USA. [2] L. Stokes and S. Pajtas, (1989) New non-pneumatic poly- urethane tire based on innovative technology, Elastomer- ics, 121, 19-23. [3] T. M. Madkour and R. A. Azzam, (2002) Use of blowing catalysts for integral skin polyurethane applications in a controlled molecular architectural environment: Synthe- sis and impact on ultimate physical properties, Journal of Polymer Science Part A: Polymer Chemistry, 40, 2526- 2536. [4] R. W. Fuest, (2002) Castable polyurethane elastomers-  252 X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 SciRes Copyright © 2009 JBiSE Serving demanding engineering applications, Rubber World, 227(39). [5] C. Hooks, (2005) PU tyre runs cool, smooth, Urethanes Technology, 22(27). [6] M. Furukawa, K. Ken, and S. Kugumiya, et al. (2008) Microphase separation of bulk and ultrathin films of polyurethane elastomers, Macromolecular Symposia, 267, 9-15. [7] I. R. Sare, J. I. Mardel, and A. J. Hill, (2001) Wear-re- sistant metallic and elastomeric materials in the mining and mineral processing industries: An overview, Wear, 250-251, 1-10. [8] Y. Jiang, Y. Shao, and Q. Zhang (1998) Preparation of tire chain of hot-plasticity type polyurethane elastomer, Fine Chemicals, 15, 57-59. [9] Z. Wirpsza, (1993) Polyurethanes: chemistry, technology, and applications, Ellis Horwood, London. [10] V. L. Covolan, R. D. Ponzio and F. Chiellini, et al., (2004) Polyurethane based materials for the production of bio- medical materials, Macromolecular Symposia, 218, 273-282. [11] S. Kutay, T. Tincer, and N. Hasirci, (1990) Polyurethanes as biomedical materials, British Polymer Journal, 23, 267-272. [12] S. M. Clift, (1991) Understanding the dynamic properties of polyurethane cast elastomers, Journal of Elastomers and Plastics, 23, 66-84. [13] A. Eceiza, M. D. Martin, and K. Caba, et al., (2008) Thermoplastic polyurethane elastomers based on poly- carbonate diols with different soft segment molecular weight and chemical structure: Mechanical and thermal properties, Polymer Engineering and Science, 48, 297-306. [14] P. P. Barbara, P. Król, and S. Pikus, (2008) Supramolecu- lar structure of crosslinked polyurethane elastomers based on well-defined prepolymers, Journal of Applied Polymer Science, 110, 3292-3299. [15] H. J. Kogelnik, H. H. Huang, and M. Barnes, et al., (1991) Comparison of the dynamic properties of solid polyurethane elastomers, Journal of Elastomers and Plas- tics, 23, 314-344. [16] G. Oertel, (1985) Polyurethane Handbook: Chemistry- Raw Materials-Processing-Application-Properties, 2ed edition, Hanser Publishers, New York. [17] R. A. Assink, (1977) The study of domain structure in polyurethanes by nuclear magnetic resonance, Journal of Polymer Science: Polymer Physics Edition, 15, 59-69. [18] R. A. Assink and G. L. Wilkes, (1981) Study of domain structure in linear and crosslinked polyurethanes using pulsed proton NMR, Journal of Applied Polymer Science, 26, 3689-3698. [19] J. N. Gorce, J. W. Hellgeth, and T. C. Ward, (1993) Me- chanical hysteresis of a polyether polyurethane thermo- plastic elastomer, Polymer Engineering & Science, 33, 1170-1176. [20] D. J. Martin, G. F. Meijs, and G. M. Renwick, et al., (1996) Effect of soft-segment CH2/O ratio on morphol- ogy and properties of a series of polyurethane elastomers, Journal of Applied Polymer Science, 60, 557-571. [21] D. J. Martin, G. F. Meijs, and G. M. Renwick, et al., (1996) The effect of average soft segment length on morphology and properties of a series of polyurethane elastomers, I. Characterization of the series, Journal of Applied Polymer Science, 62, 1377-1386. [22] D. J. Martin, G. F. Meijs, and P. A. Gunatillake, et al., (1997) The effect of average soft segment length on morphology and properties of a series of polyurethane elastomers, II. SAXS-DSC annealing study, Journal of Applied Polymer Science, 64, 803-817. [23] H. D. Kim, J. H. Huh, and E. Y. Kim, (1998) Compari- son of properties of thermoplastic polyurethane elastom- ers with two different soft segments, Journal of Applied Polymer Science, 69, 1349-1355. [24] K. S. Chen, T. L. Yu, and Y. H. Tseng, (1999) Effect of polyester zigzag structure on the phase segregation of polyester-based polyurethanes, Journal of polymer sci- ence, Part A, Polymer chemistry, 37, 2095-2104. [25] E. G. Bajsic, V. Rek, and A. Sendijarevic, et al., (2000) DSC study of morphological changes in segmented polyurethane elastomers. Journal of Elastomers and Plas- tics, 32, 162-182. [26] B. C. Chun, T. K. Cho, and Y. C. Chung, (2007) Block- ing of soft segments with different chain lengths and its impact on the shape memory property of polyurethane copolymer, Journal of Applied Polymer Science, 103, 1435-1441. [27] P. Król, B. Pilch-Pitera, (2007) Phase structure and ther- mal stability of crosslinked polyurethane elastomers based on well-defined prepolymers, Journal of Applied Polymer Science, 104, 1464-1474. [28] K. Ken, S. Nakamura and, M. Furukawa, (2008) Effect of side groups of polymer glycol on micro- phase-separated structure and mechanical properties of polyurethane elastomers, Journal of Polymer Science Part B: Polymer Physics, 46, 2054-2063. [29] H. B. Zhang, Y. D. Chen, and Y. C. Zhang, et al., (2008) Synthesis and characterization of polyurethane elastom- ers, Journal of Elastomers and Plastics, 40, 161-177. [30] M. Furukawa, Y. Hamada, and K. Ken, (2003) Aggrega- tion structure and mechanical properties of functionally graded polyurethane elastomers, Journal of Polymer Science Part B: Polymer Physics, 41, 2355-2364. [31] H. Goering, H. Krüger, and M. Bauer, (2000) Multimodal polymer networks: Design and characterisation of nano- heterogeneous PU elastomers, Macromolecular Materials and Engineering, 278, 23-35. [32] C. D. Eisenbach and W. Gronski, (1983) Hydrogen bonding and phase separation in segmented polyurethane elastomers as studied by 13C NMR magic angle spinning and FT-IR spectroscopy, Die Makromolekulare Chemie, Rapid Communications, 4, 707-713. [33] K. Madhavan and B. S. R. Reddy, (2006) Synthesis and characterization of poly (dimethylsiloxane-urethane) elastomers: Effect of hard segments of polyurethane on morphological and mechanical properties, Journal of Polymer Science Part A: Polymer Chemistry, 44, 2980-2989. [34] C. D. Eisenbach, T. Heinemann, and A. Ribbe, et al., (1992) Chain architecture and molecular self-organiza- tion of polyurethanes: Perspectives for thermoplastic  X. D. Chen et al. / J. Biomedical Science and Engineering 2 (2009) 245-253 253 SciRes Copyright © 2009 JBiSE elastomers, Die Angewandte Makromolekulare Chemie, 202, 221-241. [35] H. S. Xia, M. Song, and Z. Y. Zhang, et al., (2007) Mi- crophase separation, stress relaxation, and creep behavior of polyurethane nanocomposites, Journal of Applied Polymer Science, 103, 2992-3002. [36] H. C. Jung, S. J. Kang, and W. N. Kim, et al., (2000) Properties of crosslinked polyurethanes synthesized from 4, 4prime-diphenylmethane diisocyanate and polyester polyol, Journal of Applied Polymer Science, 78, 624- 630. [37] L. Wu, X. Luo, and X. D. Wang, (2006) Influence of processing conditions on dual-phase continuous blend system of thermoplastic polyurethane with ethylene- propylene-diene monomer elastomer, Journal of Applied Polymer Science, 102, 5472-5482. [38] C. P. Christenson, M. A. Harthcock, and M. D. Meadows, et al., (1986) Model MDI/butanediol polyurethanes: Mo- lecular structure, morphology, physical and mechanical properties, Journal of Polymer Science Part B: Polymer Physics, 24, 1401-1439. [39] R. R. Lagasse, (1977) Domain structure and time-de- pendent properties of a crosslinked urethane elastomer, Journal of Applied Polymer Science, 21, 2489-2503. [40] C. J. Ong and R. Saxon, (1976) Viscoelastic properties and heat generation in urethane elastomers. Journal of Applied Polymer Science, 20, 1695-1710. [41] T. O. Ahn, I. S. Choi, H. M. Jeong, et al., (1993) Ther- mal and mechanical properties of thermoplastic polyure- thane elastomers from different polymerization methods, Polymer International, 31, 329-333. [42] S. Yamasaki, D. Nishiguchi, and K. Ken, et al., (2007) Effects of polymerization method on structure and prop- erties of thermoplastic polyurethanes, Journal of Polymer Science Part B: Polymer Physics, 45, 800-814. [43] A. I. Hoodbhoy, (1976) Designing with plastics-cast solid-polyurethane industrial tires, Plastics Engineering, 32, 37-38. [44] I. S. Megna, (1986) Polyurethanes for roll covering, Rub- ber World, 194, 20. [45] R. Bond, G. F. Morton, and L. H. Krol, (1984) A tailor- made polymer for tyre applications, Polymer, 25, 132-140. [46] A. Lechtenboehmer, H. G. Moneypenny, and F. A. Mersch, (1990) Review of Polymer Interfaces in Tyre Technology, British Polymer Journal, 22, 265-301. [47] A. M. Shanmugharaj and A. K. Bhowmick, (2003) Dy- namic mechanical properties of styrene-butadiene rubber vulcanizate filled with electron beam modified surface- treated dual-phase filler. Journal of Applied Polymer Science, 88, 2992-3004. |

