 Journal of Power and Energy Engineering, 2015, 3, 185-193 Published Online April 2015 in SciRes. http://www.scirp.org/journal/jpee http://dx.doi.org/10.4236/jpee.2015.34026 How to cite this paper: Yakub, M.I., et al. (2015) Pyrolysis of Oil Palm Residues in a Fixed Bed Tubular Reactor. Journal of Power and Energy Engineering, 3, 185-193. http://dx.doi.org/10.4236/jpee.2015.34026 Pyrolysis of Oil Palm Residues in a Fixed Bed Tubular Reactor Mohammed Isah Yakub1, Abakr Yousif Abdalla1, Kabir Kazi Feroz2, Yusuf Suzana3, Alshareef Ibraheem4, Soh Aik Chin4 1Energy, Fuel and Power Technology Research Division, School of Engineering, The University of Nottingham Malaysia Campus, Selangor Darul Ehsan, Malaysia 2Department of Chemical Engineering and Applied Chemistry, Aston University, Birmingham, UK 3Depart ment of Chemical Engineering, Universiti Teknology Petronas (UTP), Tronoh, Malaysia 4Crops for the Future (CFF), The University of Nottingham Malaysia Campus, Selangor Darul Ehsan, Malaysia Email: kebx3iye@nottingham.edu.my, Yousif.Abakr@nottingham.edu.my, f.kabir@aston.ac.uk Received November 2014 Abstract Searching for alternative energy sources continues to grow in recent times due to the fear of energy insecurity in the near future and environmental and sociopolitical issues associated with the use of fossil fuel. Among the renewable energy sources, biomass is the only source that has carbon in its building blocks which can be processed to liquid fuel. In this study, pyrolysis of oil palm residues (trunk, frond and empty fruit bunch) was carried out in a fixed bed tubular reactor under nitrogen atmosphere at 30 mL/min, 30˚C/min heating rate and 600˚C rea cti on temperature. Pyrolysis products (bio-oil, bio-char and non-condensable gas) were characterized. Water content, acidity (pH), higher heating value (HHV) and oxygen content of the bio-oil varied between 39.28 - 43 wt%, 2.92 - 3.20, 19.29 - 21.92 MJ/kg and 58.47 - 59.85 wt% respectively. Low pH, highwater and oxygen contents in the oil make it unsuitable for being used as fuel and therefore require up- grading. Scanning electron microscopy and ultimate analysis of the bio-char suggests that it is a porous material and consists mainly carbon between 82.22 - 84.96 wt% and has HHV in the range of 25.98 - 27. 65 MJ/kg. This may be used as solid biofuel, adsorbent and source of carbon. High percentage of hydrogen (H2) and carbon monoxide (CO) were observed in the non-condensable gas which may be processed to transportation fuel via Fisher-Tropsch process. Oil palm residues represent good source of renewable energy when all the pyrolysis products are efficiently uti- lized. Keywords Oil Palm Residue, Pyr olysi s, Bio-Oil , Bio-Char, Non-Condensable Gas 1. Introduction Energy demand continues to grow with increasing population and industrialization which has put heavy depen- dence on the fossil fuel. Recently, the demand for fossil oil stands at about 94.4 million barrel per day [1] which  M. I. Yakub et al. may continue to rise. This will definitely put more pressure on the known reserves and may lead to energy crises in future. Another consequence with the use of fossil fuel is emission of greenhouse gas that has impacted nega- tively on our environment. Several measures have been suggested but the cost of treating the greenhouse gas is high and may not be economical feasible [2] [3] particularly in the present time of declining crude oil price. The dwindling crude oil price may not be far from a deliberate political move to discourage the develop- ment of renewable energy. If we must keep our environment safe, viable for agricultural production and avoid energy crises in future, the time to act is now and the development of alternative renewable energy is inevitable. The use of lignocellulosic biomass (non-food materials) such as forest residues, agro-wastes, energy grasses, aquatic plants and algae etc for biofuel production continue to gain tremendous attention as they are evenly dis- tributed across the globe and have also eliminated initial public perception of food insecurity associated with first generation biofuels which were produced from food materials [4]-[6]. In addition, these materials have low level of sulfur, nitrogen and ash content which make them relatively environmental friendly. Malaysia is one of the countries in the world that is in to agricultural enterprises. This sector generates million tons biomass an- nually which create disposal problem. In 2009, about 243.6 million tons of biomass was generated. This value is projected to be 312 million tons in 2020 in which biomass from palm oil industry constitutes largest portion. [7]-[11]. In order for this sector to remain attractive, efforts are geared towards utilization of the biomass for sustainable energy production [9] [12] [13]. The objective of this paper was to carry out pyrolysis of oil palm re- sidues to bio-oil, bio-char and syngas and characterization of the products. 2. Experiment 2.1. Materials Oil palm residues such as trunk (OPT), fronds (OPF) and empty fruit bunch (OPEFB) chopped 6 to 8 cm were collected from Crops for the Future (CFF) field Research Centre and transported in plastic bags to research building of University of Nottingham Malaysia campus. Samples from the OPT were taken from first 10 ft from bottom (OPT/10ft), between 10 ft and 20 ft (OPT/20ft) and between 20 and 30 ft (OPT/30ft) while fronds were grouped to stem only (OPFS) and fronds total (OPFT) which consist of stem and leaves. The materials were oven dried at 105˚C for moisture content determination according to BS EN 14774-1 standard [14]. The dried materials were then shredded in a rotor beater mill RetschR to particle sizes between 0.2 - 2 mm and stored in air tight plastic bags. 2.2. Feedstock Characterization Volatile matter and ash content on dry basis were determined according BS EN 15,148 [15] and BS EN 14,775 [16] respectively. Fixed carbon was computed by subtracting the percentage compositions of ash and volatile matter from the bone dry sample mass. Elemental composition of the ash was analyzed using energy dispersive X-ray (EDX). Higher heating value (HHV) was determined using oxygen bomb calorimeter Parr 6100 following BS EN 14,918 [17]. Elemental compositions of the biomass were determined using CHNS analyzer, LECO Corporation USA. Thermo gravimetric study was carried out in thermo gravimetric analyzer (TGA) Perkin El- mer Simultaneous thermal analyzer (STA) 6000 in nitrogen atmosphere, flow rate 20 mL/min at temperature be- tween 300 - 1000 K and heating rate of 10 K/min. Structural composition of the biomass was determined ac- cording to NREL/TP-510-42618 [18]. 2.3. Pyrolysis and Pyrolysis Products Characterization Pyrolysis was carried out in a fixed bed tubular reactor of length 102 cm and diameter 10.5 cm (Figure 1) under nitrogen atmosphere of 30 mL/mi n and reaction temperature of 600˚C. This condition has been established in our previous studies [19]. The reactor was heated electrically at heating rate of 30˚C/min. 30 g of biomass sam- ple (2 - 4 mm particle size) was used in each experiment. The reaction time was kept at 15 min (±2 min) or until no significant amount of non-condensable gas was observed. The pyrolysis vapor was condensed in a condenser connected to chiller with cooling water at 4˚C and the oil was collected for further analysis. Non-condensable gas was passed through a gas scrubber and the dried gas composition was analyzed. Bio-char was collected at end of each experiment after the reactor temperature cool to room temperature and further analyzed. 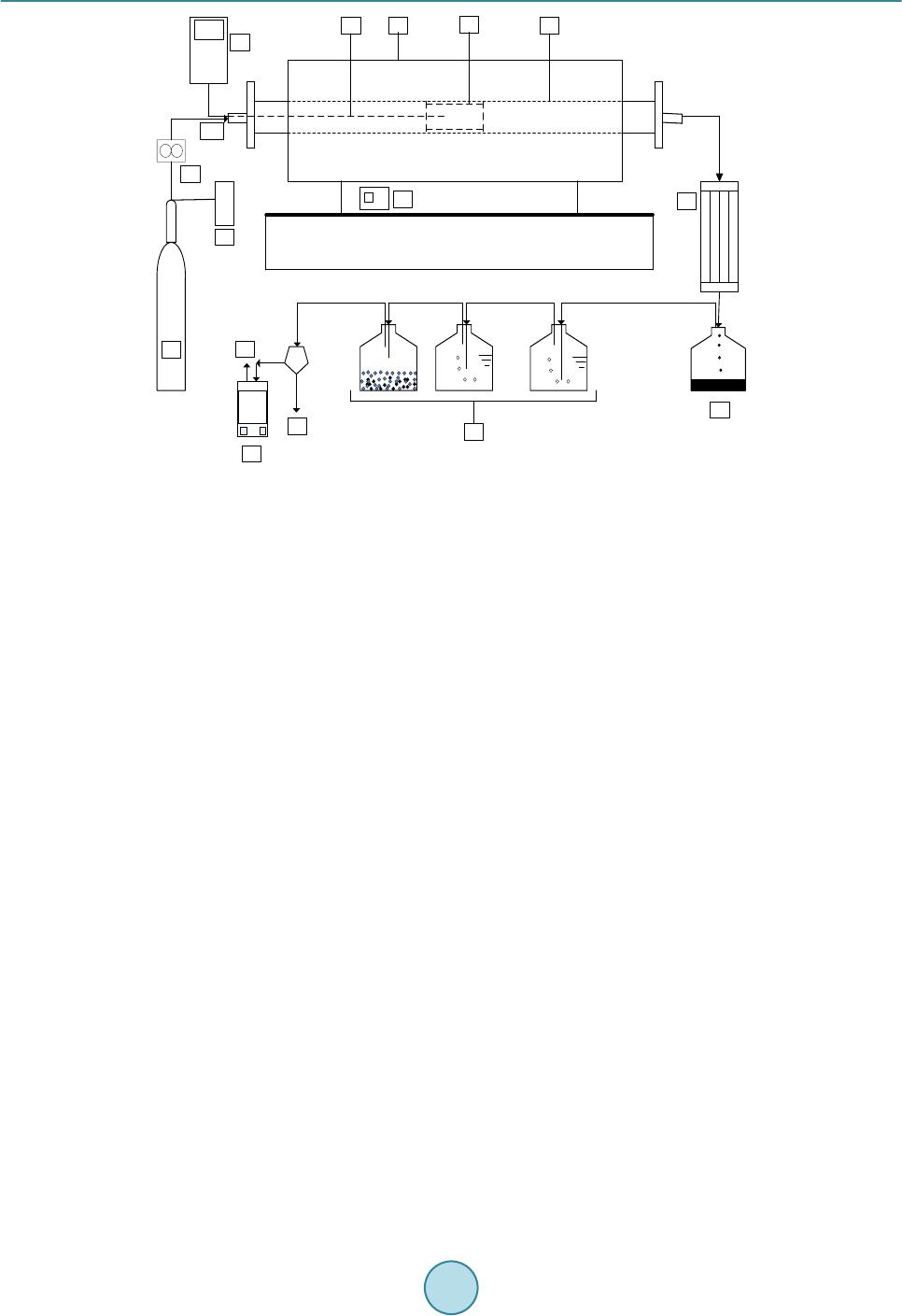 M. I. Yakub et al. Figure 1. Fixed bed Pyrolysis System. (A) Nitrogen cylinder, (B) pressure gauge, (C) nitrogen flow meter, (D) digital thermometer, (E) thermocouple, (F) tubular furnace, (G) packed bed, (H) alumina tube, (I) furnace control button, (J) condenser, (K) oil collector, (L) gas scrubber, (M) gas analyzer, (N) non-condensable gas discharge. After each production, characterization of bio-oil was carried out within 24 hours. The oil was first filtered using PTFE syringe filter of 0.45 µm pore size and 13 mm diameter. Higher heating value was determined using oxygen bomb calorimeter Parr 6100 according to ASTM D240-09 [20]. Walk LAB microcomputer pH meter TI9000 was used to determine the pH. Density and viscosity were determined using Anton Paar density meter DMA 4500 M and Brookfield DV-E viscometer respectively. Bio-oil water content was determined using Karl Fischer V20 volumetric titrator Mettler Toledo for water content according to ASTM E 203-01 [21] [22]. Ele- mental compositions of the oil were determined using the procedure outlined above under the feedstock charac- terizatio n. Composition of non-condensable pyrolysis product was determined using Dräger X-am 5000 gas analy- zer. Proximate and ultimate analyses of bio-char were carried out following the same procedures used for the feedstock characterization. Scanning electron microscope (SEM) FEI Quanta 400 FE-SEM was used to evaluate surface characteristics of the bio-char. 3. Results and Discussion 3.1. Feedstock Characteristics Proximate, ultimate and structural analyses results are shown in Table 1. Moisture content in the samples varied from 8.44 to 77.52 wt% with OPEFB having the least moisture content while the highest percentage was rec- orded in the OPT/30ft. Moisture content is an important parameter as it affects biomass quality, storage and handling especially if it is to be converted via thermo chemical process. During pyrolysis, the initial moisture in the biomass takes up heat from the system and vaporizes and later condenses in the bio-oil which leads to lower value of HHV. Volatile matter on dry basis were found to be 82, 82.05, 80.11, 80.88, 79.69 and 75.01 wt% for OPT/10ft, OPT/20ft, OPT/30ft, OPFS, OPFT and OPEFB respectively. The ash content and heating values were between 2.07 and 6.51 wt%, and 16.07 and 18.60 MJ/kg. The ash consist mainly potassium (K), chlorine (Cl), silicon (Si) and calcium (Ca). Other elements identified include phosphorus (P), aluminum (Al), magnesium (Mg) and traces of sodium (Na) and manganese (Mn) (Table 2). Ultimate analysis revealed that oil palm resi- dues have 45.05 - 60.17 wt% carbon, 5.1 - 6.12 wt% hydrogen, 0.06 - 1.82 wt% nitrogen, 0.04 - 1.63 wt% sulfur and 32.61 - 48.82 wt% oxygen. Similarly, the cellulose, hemicellulose and lignin content were between 28 - 38.77 wt%, 14.85 - 26.28 wt% and 21.42 - 31.07 wt% correspondingly. These characteristics are in agreement  M. I. Yakub et al. Table 1. Characteristics of oil palm residues (average values 3 replicates). aAs received; bdry basis; cby difference. Property Biomass OPT/10ft OPT /20ft OPT/30ft OPFS OPFT OPEFB Proximate analysis (wt%) Moisture contenta 49.27 72.90 77.52 11.71 31.92 8.44 Volatile matterb 82.00 82.05 80.11 80.88 79.69 7 5.01 Fixed carbonc 15.03 15.88 14.36 15.51 13.80 20. 23 Ash contentb 2.97 2.07 5.53 3.61 6.51 4.76 HHV (MJ/kg) 17.73 17.91 17.33 17.55 18.60 16.07 Ultimate analysis (wt%) Carbon 54.22 59. 77 60.17 45.05 50.68 4 5.26 Hydrogen 7.10 7.28 7.93 5.86 6.12 5.13 Nitrogen 0.06 0.06 0.06 0.23 0.90 1.82 Sulfur 0.26 0.28 0.28 0.04 1.63 0.50 Oxygenc 38.36 32.61 31.56 48.82 4 0.67 47.29 Structural composition (wt%) Cellulose 38.73 33.96 31.08 38.77 32.23 29.56 Hemi cellu lose 18. 87 20.77 19.96 19.43 26.28 28.71 Li g n i n 29.20 30.76 31.07 21.42 24.54 1 4.85 Table 2. Ash elemental composition determined by EDX. Each value is an average of 3 repli cates. Biomass Elemental composition (wt%) dry ash basis Na Mg Al Si P S Cl K Ca Mn Others OBEFB - 3.25 1.16 14.21 1.20 2.58 16.73 58.51 2.30 - 0.06 OPFT - 2.20 1.60 36.33 2.25 1.13 6.49 23.86 24.40 1.51 0.23 OPFS - 1.51 0.64 2.72 2.15 3.24 19.75 50.22 19.68 - 0.09 OPT/30ft 0.24 2.43 3.99 3.92 1.08 4.03 14.85 41.12 27.75 - 0.59 OPT/20ft 0.38 4.86 0.86 3.83 2.74 5.64 6.86 47.45 24.96 - 2.42 OPT/10ft 0.56 6.99 1.12 9.08 2.00 7.53 4.52 55.74 11.09 - 1.37 with Thermo gravimetric analysis (TGA) result as shown in Figure 2. DTG showed thermal decomposition of various components in the each oil palm residue. Peak (a) at 478 K may be attributed to decomposition of extractives. While (c) and (d) correspond to decomposition of hemicellu- lose at temperatures 543 K and cellulose at 600 K respectively. Beyond 600 K, Point (e), signifies decomposi- tion of lignin [23]. No noticeable peak was observed in the lignin decomposition region. Consequently, it can be deduced that lignin contents of oil palm residues have uniform thermal decomposition. 3.2. Pyrolysis Products Distribution and Characteristics The pyrolysis products (bio-oil, bio-char and non-condensable) distribution are shown in Table 3. Bio-oil yield recorded was 31.97, 34.26, 31.47, 29.56, 34.23 and 30.23 wt% from OPT/10ft, OPT/20ft, OPT/30ft, OPFT, OPFS and OPEFB respectively with corresponding bio-char of 30.56, 29.67, 34.69, 35.50, 29.43 and 38.45 wt%. The amount of non-condensable gas was computed by subtracting the percentage compositions of bio-oil and bio-char from the bone dry sample mass. The high yield of oil recorded from OPT/20ft and OPFS may be attri- 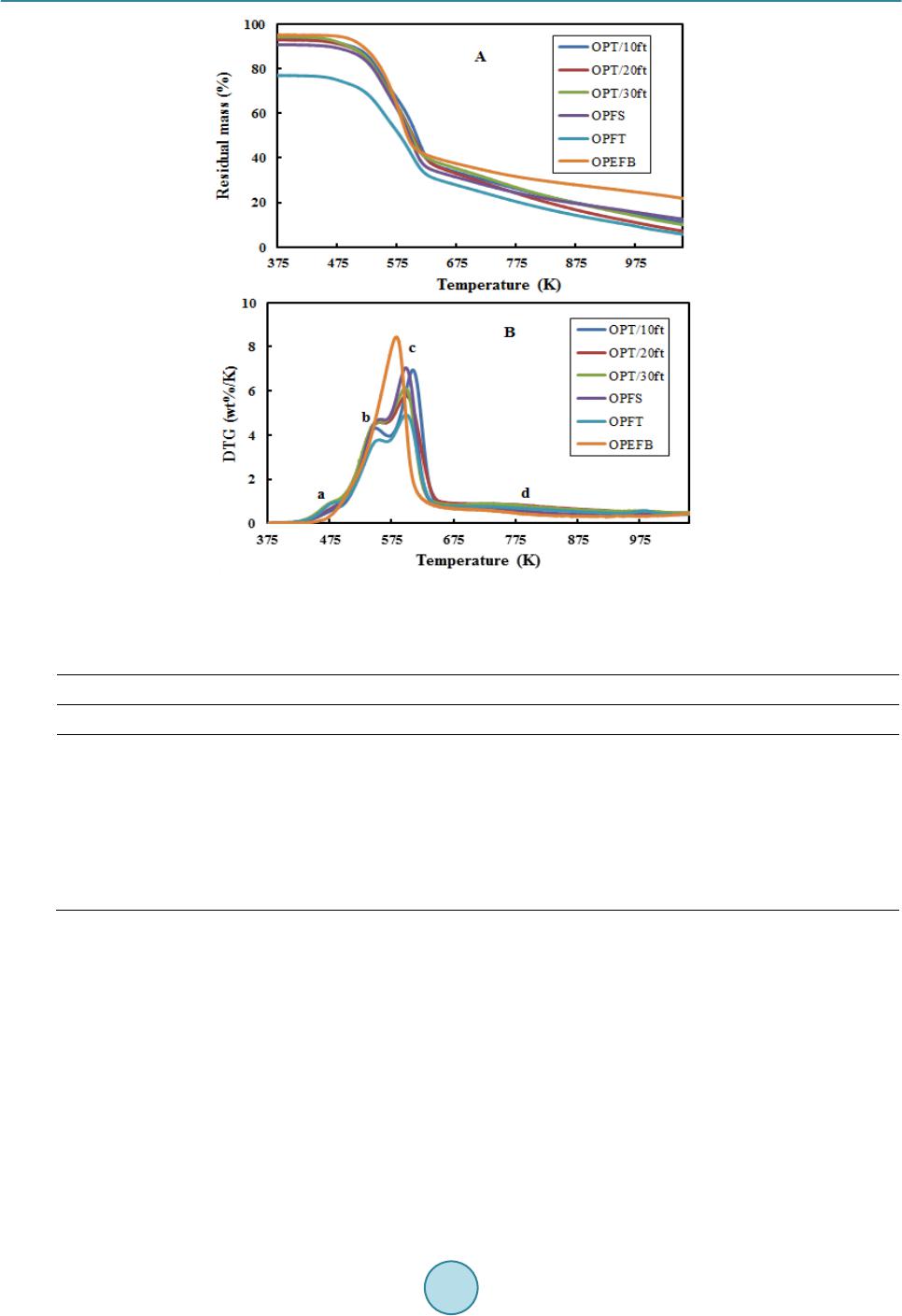 M. I. Yakub et al. Figure 2. Thermo gravimetric analysis of oil palm residues (A) TGA, (B) DTG under nitrogen atmosphere (20 mL/min); heating rate (10 K/min). Table 3. Pyrolysis product distribution from oil palm residue at 600˚C, 30 mL/min N2 flow and 30˚C/min heating rate. Biomass Pyrolysis products distribution (wt%) Oil Gas Char OPT/10ft 31.97 37. 47 30.56 OPT/20ft 34.26 36. 07 29.67 OPT/30ft 31.47 33. 84 34.69 OPEFB 29.56 34.94 35.50 OPFS 34.23 3 6.34 29.43 OPFT 30.23 31.32 38.45 buted to lower ash level in the respective biomass sample since high biomass ash normally favor char formation [23]. From the physicochemical characteristics of bio-oil obtained (Table 4). Bio-oil collected in this study from each feedstock was dark brown homogenous liquid. Acidity (pH) of the oil was between 2.92 and 3.20. This is an indication that the oil may contain substantial amount of organic acids [24] [25]. Water content of the oil va- ried from 39.28 - 43.0 wt% which was mainly due to product of dehydration during pyrolysis reaction [26]. Density was between 1.02 and 1.04 g/cm3 which are higher than that of gasoline (0.723 g/cm3) and diesel (0.838 g/cm3) fuels. Viscosity was found to be between 2.06 and 2.41cp. Ultimate analysis revealed that the bio-oil has 32.96 - 35.74 wt% carbon, 6.06 - 6.99 wt% hydrogen, 0.01 - 1.02 wt% nitrogen, 0.01 - 0.99 wt% sulfur and 58.47 - 59.85 wt% oxygen. Low nitrogen and sulfur contents of the oil suggest that bio-oil from oil palm residues can be used as a source of clean energy. Higher heating value of the oil ranged between 19.24 and 21.92 MJ/kg. These values are only  M. I. Yakub et al. about 41% - 47% of higher heating value of gasoline (47 MJ/kg) and 43% - 49% that of diesel (45 MJ/kg). This can be attributed to the high water and oxygen contents in the oil and hence the need for upgrading. The non-condensable gas measured using Dräger X-am 5000 gas analyzer were methane (CH4), hydrogen (H2), carbon monoxide (CO), and traces of hydrogen sulfide (H2S) (Table 4). The presence of H2S may be at- tributed to sulfur in the source biomass. The gas consist mainly CH4, H2 and CO which are due to reforming and cracking and cracking hydrocarbons, and cracking of oxygenated organic compounds respectively in the vola- tiles at high temperature [27] [28]. High composition of H2 and CO in the gas was observed which went beyond the measuring range (0.2 vol %) of the gas analyzer. This signifies that the non-condensable gas can be used as a source of hydrogen for upgrad- ing of bio-oil to meet the fuel standard. In addition, it can also be processed to transportation fuel via Fisher- Tropsch synthesis due to high value H2/CO (vol/vol) [29] [30]. Table 4. Properties of bio-oil, bio-char and non-condensable gas from oil palm residues produced at 600˚C, 30˚C/min, 30 mL/min N2 f l ow. Property Bio-Oil OPT/10ft OPT /20ft OPT/30ft OPFS OPFT OPEFB Appearance Dark brown Physicochemical properties pH 3.20 3.18 3.20 3.08 2.92 3.16 Water content (wt%) 43.00 41.00 42.00 3 9.28 39.78 40.22 HHV (MJ/kg) 19.24 21.76 20.15 21.92 21.22 21.61 Viscosity (cp) at 25˚C 2.06 2.22 2.11 2.41 2.36 2.29 Density (g/cm3) 1.02 1.03 1.03 1.04 1.04 1.03 Ultimate analysis (wt%) Carbon 32.96 33. 80 33.10 3 4.11 34.08 33.96 Hydrogen 6.99 6.22 6.94 6.06 6.10 6.13 Nitrogen 0.01 0.01 0.02 0.11 0.36 1.02 Sulfur 0.19 0.16 0.18 0.01 0.99 0.21 Oxygen* 59.85 59.81 59.76 59.71 58.47 58.68 Proximate analysis (wt%) Bio-char (dry basis) Ash content 11.65 11.53 11.99 12.10 12.59 13.02 Volatile matter 23.16 23.86 23.77 2 3.45 22.95 22.45 Fixed carbon (%)* 65.19 6 4.61 64.24 64.45 64.46 64.53 HHV (MJ/kg) 27.65 27.68 27.16 26.21 26.42 25.98 Ultimate analysis (wt%) Carbon 84.90 84. 10 83.81 8 4.96 83.21 82.22 Hydrogen 2.96 2.80 2.90 2.77 2.97 2.88 Nitrogen 0.09 0.08 0.09 0.08 0.29 0.21 Sulfur 0.02 0.02 0.02 0.08 0.11 0.11 Oxygen* 12.03 13.00 13.18 12.11 13.42 14.58 Non-condensable gas (volume %) nitrogen free basis H2S 0.0016 0.0014 0.0015 0.0 011 0.0018 0.0017 CH4 2.66 2.71 2.68 2.76 2.69 2.59 CO >0.2 >0.2 >0.2 >0.2 >0.2 >0 .2 H2 >0.2 >0.2 >0.2 >0.2 >0.2 >0.2 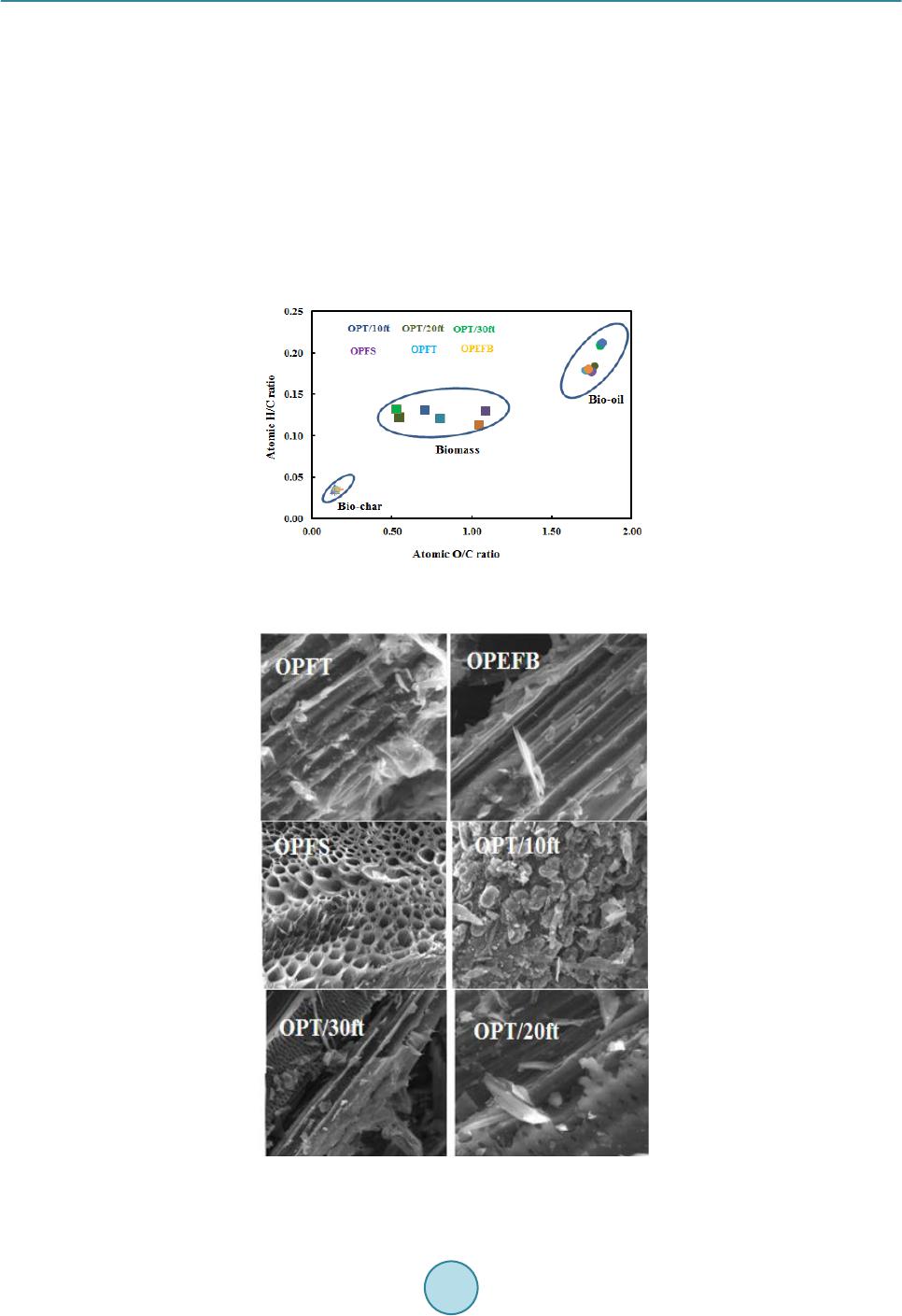 M. I. Yakub et al. Characteristics of the bio-char are also given in Table 4. From the proximate analysis, ash content, volatile matter and HHV were between 11.53 - 13.02 wt%, 22.45 - 23.86 wt% and 25.96 - 27.68 MJ/kg while fixed car- bon ranged between 64.24 and 65.19 wt%. The low volatile content recorded was as a result of removal of vola- tiles during the pyrolysis. Ultimate analysis revealed that the bio-char is predominantly made up of carbon and less hydrogen and oxygen content. Lower composition of nitrogen and sulfur were also observed in the bio-char sample. Atomic ratio of hydrogen/carbon (H/C) and oxygen/carbon (O/C) of bio-char was evaluated and com- pared with that of bio-oil and the source biomass as shown in Van Krevelen diagram in Figure 3. The plot showed distinct regions for bio-char, bio-oil and the source biomass with bio-char appearing at the lower left hand corner, biomass at the middle and bio-oil at top right hand corner. The lower values of H/C and O/C rec- orded in the bio-char samples are indications of loss of hydrogen and oxygen due to hydration, decarboxylation and decarbonylation [30] which lead to enrichment of the bio-char with carbon. SEM evaluation (Figure 4) re- vealed that bio-char samples have porous structures and can be used as adsorbent and in soil amendment. Figure 3. Van Krevelen diagram of oil palm residues and their corresponding bio- oil and bio-char . Figure 4. SEM images of bio-char from oil palm residue. Scanning conditions: HV (20 kV), Mag (2400×), low vacuum and size (40 µm) .  M. I. Yakub et al. 4. Conclusions Pyrolysis of oil palm residues was carried out in a fixed bed tubular reactor under nitrogen atmosphere at 30 mL/min and reaction temperature of 600˚C. Bio-oil, bio-char and non-condensable gas yield were between 29.56 - 34.26 wt%, 29.43 - 38.45 wt% and 31.32 - 37.47 wt% respectively. The bio-oil was acidic (pH: 2.92 - 3.2), highly oxygenated (58.47 - 59.85 wt%) and had high moisture content between 39.28 - 43 wt%. The higher heating value of the oil was between 19.24 - 21.92 MJ/kg. These characteristics suggest that the bio-oil require further upgrading in order to meet fuel quality standard. The bio-char recorded was highly carbonaceous (82.22 - 84.96 wt% carbon) and had higher heating value be- tween 25.96 - 27.68 MJ/kg. Scanning electron microcopy revealed that bio-char is made of porous structure. These properties indicate that bio-char can be used as asolid biofuel, asource of carbon and as an adsorbent. The composition of non-condensable gas was mainly hydrogen and carbon monoxide which can be further processed to transportation fuel. In addition, it can also serve as a source of hydrogen for upgrading of the bio-oil. This study has shown that oil palm residues are good sources of renewable energy which can be sustained when all the pyrolysis products are efficiently utilized. Acknowledgements This research was supported by the Crops for the Future (CFF) and the University of Nottingham under grant BioP1 -005. References [1] International Energy Agency (2015) Oil Market Report (OMR). http://omrpublic.iea.org/demandsearch.asp [2] Mo hammed, Y. Optimization and Sensitivity Analysis of Post-Combustion Carbon Capture Using Dea Solvent in a Coal Fired Power Plant. International Journal of Advances in Engineering & Technology, 7, 1681-1690. [3] Yakub, M.I., Moh amed, S. and Danlad i, S.U. Technical and Economic Considerations of Post-Combustion Carbon Capture in a Coal Fired Power Plant. International Journal of Advances in Engineering & Technology, 7, 1549-15 81 . [4] Margeo t, A., H ahn-Hager dal, B. and Edlund, M. (2009) New Improvements for Lignocellulosic Ethanol. Cu rrent Opi- nion in Biotechnology, 20, 372-380. http://dx.doi.org/10.1016/j.copbio.2009.05.009 [5] Ni gam, P .S. and Singh, A. (20 11) Production of Liquid Biofuels from Renewable Resources. Progress in Energy and Combustion Science, 37, 52-68. http://dx.doi.org/10.1016/j.pecs.2010.01.003 [6] Sri rangan , K., Akawi , L., Moo-Young, M. and Chou, C.P . (20 12) Towards Sustainable Production of Clean Energy Carriers from Biomass Resources. Applied Energy, 100, 172 -186. http://dx.doi.org/10.1016/j.apenergy.2012.05.012 [7] Su laiman, F., Abdullah, N., Gerhau ser, H. and Shariff, A. (2011) An Outlook of Malaysian Energy, Oil Palm Industry and Its Utilization of Wastes as Useful Resources. Biomass and Bioenergy, 35, 3775-3786 . [8] Ahmad , S., K adir , M . Z.A.A. and Shafie, S. (2011) Current Perspective of the Renewable Energy Development in Ma- laysia. Renewable and Sustainable Energy Reviews, 15, 897-904. http://dx.doi.org/10.1016/j.rser.2010.11.009 [9] Ng, W.P . Q., Lam, H.L., Ng, F.Y., Kamal, M. and Lim, J.H. E. (2012) Waste-to-Wealth: Green Potential from Palm Biomass in Malaysia. Journal of Cleaner Production, 34, 57-65. http://dx.doi.org/10.1016/j.jclepro.2012.04.004 [10] U mar , M.S., Jennin gs, P. and Urmee, T. (2013) Strengthening the Palm Oil Biomass Renewable Energy Industry in Malaysia. Renewable Energy, 60, 107 -115. http://dx.doi.org/10.1016/j.renene.2013.04.010 [11] Ahma d, S. and Tahar, R.M. (20 14) Selection of Renewable Energy Sources for Sustainable Development of Electricity Generation System Using Analytic Hierarchy Process: A Case of Malaysia. Renewable Energy, 63, 458 -466. http://dx.doi.org/10.1016/j.renene.2013.10.001 [12] Shafie, S.M., Mah lia, T.M.I., Masju k i, H.H. and Andriyana, A. (2011) Current Energy Usage and Sustainable Energy in Malaysia: A Review. Renewable and Sustainable Energy Reviews, 15, 4370-43 77. http://dx.doi.org/10.1016/j.rser.2011.07.113 [13] Chiew, Y. L. and Shimada, S. (2013) Current State and Environmental Impact Assessment for Utilizing Oil Palm Emp- ty Fruit Bunches for Fuel , Fiber and Fertilizer—A Case Study of Malaysia. Biomass and Bioenergy, 51, 109-124. http://dx.doi.org/10.1016/j.biombioe.2013.01.012 [14] BS EN 14774-1 (20 09) Solid Biofuels. Determination of Moisture Content. Oven Dry Method. Total Moisture. British Standards Institution, London. [15] BS EN 14774-1 (20 09) Solid Biofuels. Determination of Moisture Content. Oven Dry Method. Total Moisture. British  M. I. Yakub et al. Standards Institution, London. [16] BS EN 15148 (20 09) Solid Biofu els . Determination of the Content of Volatile Matter. British Standards Institution, London. [17] BS EN 14775 (2009 ) Solid Biofuels. Determination of Ash Content. British Standards Institution, London. [18] Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton , D. and Crocker, D. (2012 ) Determination of Struc- tural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure. Nat ional Renewable Laboratory, NREL/ TP -510 -42618, US A. [19] Moh ammed, I.Y., Kab ir, F.K., Yusuf, S. and Alshareef, I. (2014) Pyrolysis of Napier Grass. Poster Presentation at Uni- versity of Nottingham Postgraduate Week 2014. [20] ASTM D240 (20 09 ) Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorime- ter. ASTM International, West Conshohocken. [21] ASTM E203 (2001) Standard Test Method for Water Using Volumetric Karl Fischer Titration. ASTM International, West Conshohocken. [22] Moh ammed, I.Y., Kazi, F.K., Abakr, Y. A., Yusu f, S. and Razzaque, M.A. (2015) Novel Method for the Determination of Water Content and Higher Heating Value of Pyrolysis Oil. BioResou rces, 10, 2681 -2690 . [23] Moh ammed, I.Y., Abakr, Y.A., Kabir, F. and Yusuf, S. (2015) Effect of Aqueous Pretreatment on Pyrolysis Characte- ristics of Napier Grass. Journal of Engineering Science & Technology, 10, in Press. [24] Ab u Bak ar, M.S. an d Titiloye, J.O. (2013) Catalytic Pyrolysis of Rice Husk for Bio-Oil Production. Journal of Analyti- cal and Applied Pyrolysis, 103, 362-368 . http://dx.doi.org/10.1016/j.jaap.2012.09.005 [25] Fan, Y., C ai, Y., Li, X., Yin, H., Yu, N., Zhang, R. and Zhao, W. (2014) Rape Straw as a Source of Bio-Oil Via Va- cuum Pyrolysis: Optimization of Bio-Oil Yield Using Orthogonal Design Method and Characterization of Bio-Oil. Journal of Analytical and Applied Pyrolysis, 106 , 63-70 . http://dx.doi.org/10.1016/j.jaap.2013.12.011 [26] Zhang, Q., Chang, J., Wa ng , T. and Xu, Y. (2007) Review of Biomass Pyrolysis Oil Properties and Upgrading Re- search. Energy Conversion and Management, 48, 87-92. http://dx.doi.org/10.1016/j.enconman.2006.05.010 [27] Amutio, M., Lopez, G., Artetx e, M., E lo r di , G. , Olazar, M. and Bilbao, J. (20 12 ) Influence of Temperature on Biomass Pyrolysis in a Conical Spouted Bed Reactor. Res ources , Conservation and Recycling, 59, 23-31. http://dx.doi.org/10.1016/j.resconrec.2011.04.002 [28] Jahirul, M.I., Rasul, M.G., Chowdhury, A.A. and Ashwath, N. (2012) Biofuels Production through Biomass Pyroly- sis—A Technological Review. En ergies, 5, 4952 -50 01. http://dx.doi.org/10.3390/en5124952 [29] Fern ández, Y. and Menéndez, J.A. (2011) Influence of Feed Characteristics on the Microwave-Assisted Pyrolysis Used to Produce Syngas from Biomass Wastes. Journal of Analytical and Applied Pyrolysis, 91, 316-322. http://dx.doi.org/10.1016/j.jaap.2011.03.010 [30] Kim, P., Johnson, A., Edmunds, C.W., Rado sevich, M., Vo gt, F., Rials, T.G. and Labbe, N. (20 11 ) Surface Functional- ity and Carbon Structures in Lignocellulosic-Derived Bio-Chars Produced by Fast Pyrolysis. Energy and Fuel, 25, 4693-4703. http://dx.doi.org/10.1021/ef200915s
|