Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 700-705 doi:10.4236/msa.2011.26096 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Equilibrium State of Anatase to Rutile Transformation for Titanium Dioxide Film Prepared by Ultrasonic Spray Pyrolysis Technique Ghassem Kavei1,2, Auppatham Nakaruk2, Charles Chris Sorrell2 1Materials and Energy Research Centre, Tehran, Iran; 2School of Materials Science and Engineering University of New South Wales, Sydney, Australia. Email: g-kavei@merc.ac.ir, prof.kavei@hotmail.com Received November 26th, 2010; revised December 25th, 2010; accepted May 9th, 2011. ABSTRACT Titanium dioxide thin films were deposited on (0001) -quartz substrate by spray pyrolysis method. The method which an aerosol of Titanium Butoxide, generated ultrasonically, was sprayed on the substrate at temperature of 400˚C, kept at this temperature for periods of 3, 13, 19 and 39 hours. The developed films at a crystal phase correspond to the TiO2 anatase and rutile phases. Their surface roughness increased by annealing the samples at 600, 800 and 1000˚C. De- posited film annealed at 1000˚C showed preferable orientation in (110) direction. The crystal evolution and crystallo- graphic properties of this material was studied by Lotgering method, X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM). The study revealed that the deposition process was nearly close to the classical Chemical Vapour Deposition (CVD) technique that is generally employed to produce films with smooth surface and good crystalline properties with a thickness of about 1 µm, as measured by Focused Ion Beam. Keywords: Titanium Dioxide, Ultrasonic Spray Pyrolysis, Annealing Treatment, Phase Transformation, Focused Ion Beam (FIB) 1. Introduction In the past decade, considerable efforts were made to synthesize self-cleaning materials, especially titanium dioxide (TiO2 or titania). Basically, for self-cleaning ap- plication, titania can be used in various forms such as powder, [1] thin film [2-4] and thick films [5]. However, thin and thick films are most commonly used due to their flexible and applicable properties. Furthermore, titania film can be employed as gas sensors [6] purifier of envi- ronmental pollutants [7], photovoltaics and photocataly- sis [8-10]. Therefore, plenty of literature reviews had paid attention to thin or thick film coating methods such as magnetron sputtering [11], Pulsed Laser Deposition (PLD) [12], Chemical Vapour Deposition (CVD) [13], sol-gel [6], gel oxidation [14], screen printing [15], an- odic oxidation [16], and electrophoretic deposition [17]. Titanium oxide films are prepared by an ultrasonic nebu- lization and pyrolysis technique developed by [18,19]. For self-cleaning applications, a titania film with small band gap and large surface area (small grain size) is re- quired. However, it is known that anatase phase will pro- vide small grain size, but the band gap of the product is greater than that of rutile. On the other hand, rutile phase has smaller band gap but the resultant grains are larger compared to anatase. Having this point in mind, one can conclude that an intermediate phase between anatase and rutile probably benefits from both small band gap and moderate grain size and is advantageous for self-cleaning materials. It is well known that titanium dioxide consists of three phases: brookite, anatase, and rutile [20]. Generally, anatase will transform into rutile at ~600˚C, [21]. How- ever, in thin film, the anatase temperature transformation to rutile can rise up to 900˚C [22]. This study has been designed to confirm the formation of a film with mixed phase of anatase and rutile struc- tures at 400˚C. This was verified by inspecting the varia- tion of effective parameters (such as time, substrate and annealing temperature) on the samples. 2. Methodology Ultrasonic spray pyrolysis was used to provide fully-  Equilibrium State of Anatase to Rutile Transformation for Titanium Dioxide 701 Film Prepared by Ultrasonic Spray Pyrolysis Technique dense anatase and rutile thin films. The precursor mate- rial (A starting solution) was prepared from titanium Bu- toxide ([Ti(OCH2CH2CH2CH3)4], Reagent Grade 97 wt%, Sigma-Aldrich) dissolved in methanol (Reagent Plus ≥ 99 wt%, Sigma-Aldrich) at a titanium concentration of 0.5 M. The aerosol as a mist was produced by means of a generic ultrasonic generator of 1.7 MHz frequency. Com- pressed air at pressure of 1.3 atm was admitted through a shape tube (∩ shape, 12 cm in diameter) to align mist molecules and direct molecules toward substrate. This causes the molecules to be deposited uniformly on the substrate. The substrate was (0001) -quartz (1 × 20 × 20 mm) heated at a constant temperature up to 400˚C for 3 - 34 hours during the deposition process using a com- mercial hotplate (SEM heater equipped with thermo- digital controller). Deposited TiO2 films were annealed in air at 600˚C, 800˚C, and 1000˚C for 7 h, at a heating rate of 5˚C per minute, followed by a natural cooling process overnight. TiO2 films were characterised by the following tech- niques: 1) Mineralogy and phase analysis of the films was examined using laser Raman microspectroscopy with a 514 nm Ar laser integrated with an optical micro- scope (Renishaw in via), 2) the structures were defined via glancing angle X-ray diffraction (GAXRD, angle of incidence 1˚, penetration depth < 300 nm, Phillips X’pert Materials Research Diffraction), and standard X-ray powder diffraction (XRD, 20˚ - 80˚ 2θ, speed 0.02˚ 2θ/s, step 0.01˚ 2θ, scans done in situ, Phillips X’pert Multi- purpose X-ray Diffraction System) (MPD-Shurr) with 45 kV and 40 mA cathode voltage and current, respectively. As a result, CuKα line radiation (λ = 1.5405 Å) was ob- tained. 3) Surface morphology was evaluated by Field Emission Scanning Electron Microscopy (FESEM) using Hitachi (S4500X) model. 4) Film thickness was deter- mined using single-beam focused ion beam (FIB) milling (FEI XP200). In this system, a voltage of 30 kV was ap- plied between the sample and probe, leading to a current of 64 pA. Gallium ions (Ga3+) were used to erode a square hole in the film and an image of the cross-section of the layers was viewed at an angle of 45˚. To perform SEM and FIB measurements, samples were coated with ~20 nm thick gold (Au) layer using sputtering technique. 3. Results and Discussion Figure 1 show XRD patterns of titania films deposited on the substrate. Table 1 also summarises the list of peaks from XRD data. It can be seen that as-deposited film at 400˚C consists of anatase and rutile phases of tita- nium dioxide. There is a strong peak of anatase at (101) plan with 2θ = 25.50˚ and a small peak of rutile at (110) with 2θ = 27.58˚. After annealing at 600˚C and 800˚C for 7 h, the XRD patterns suggested that the rutile phase was dominated. At annealing temperature of 1000˚C, anatase was totally transformed into rutile. Roughly speaking, anatase phase transformation into rutile occurs at ~600˚C in titania powder [21]. Transfor- mation temperature rises up to ~900˚C in the case of tita- nia film [22]. Figure 2 shows XRD pattern of samples being deposited in the substrate at 400˚C with various durations, 3 - 24 hours. As depicted in this figure, the patterns of TiO2 films are similar and contain anatase phase. This implies that the nature of rutile phase formed on as-deposited film does not change with temperature du- ration. If a fixed substrate temperature had any direct effect on the phase transformation of the TiO2 film, the rutile phase would elevate after the long duration that the films had been kept at a defined temperature (here is 400˚C) (see Figure 2). Therefore, the possible reason for development of rutile phase in as-deposited film might be a kinetic energy in TiO2 film for phase stabilisation at particular temperature. In other words, the structural transformation is greatly designated for an annealing temperature and is almost independent of annealing du- ration; a period which is not definitely longer than 7 hours as far as titania is concerned (see Figure 1). Figure 3 shows the surface morphology of TiO2 films at different annealing temperatures. Comparing the im- ages of as-deposited film and annealed film at 600˚C revealed that the film contained anatase phase (with small grain size ~100 nm) and rutile phase (with large grain size ~500 nm). Annealing temperature increment densifies rutile phase. These results confirmed by XRD patterns depicted in Figure 1 in which the as-deposited film and annealed film at 600˚C consisted of both anatase and rutile phases. After annealing at 800˚C for 7 h, the surface morphology showed that rutile phase is dominant. When annealed at 1000˚C, the film contained no anatase, implying that anatase had completely turned into rutile with the aver- age grain size of ~500 nm. Since annealing process does not affect the film thick- ness, as reported by, [23] the FIB photograph of the as-deposited film in Figure 4 represents the thickness of all TiO2 films. As illustrated in the figure, thickness of as-deposited film was constantly ~1 m. Furthermore, the grain size of anatase-rutile intermediate phase can be seen in FIB image. More detailed analysis for the ex- perimental results was studied, and basic mechanisms are given for anatase to routile transformation analysis [19]. 4. Conclusions TiO2 thin films with smooth surface and crystalline structure were obtained by pyrolysing an ultrasonically Copyright © 2011 SciRes. MSA 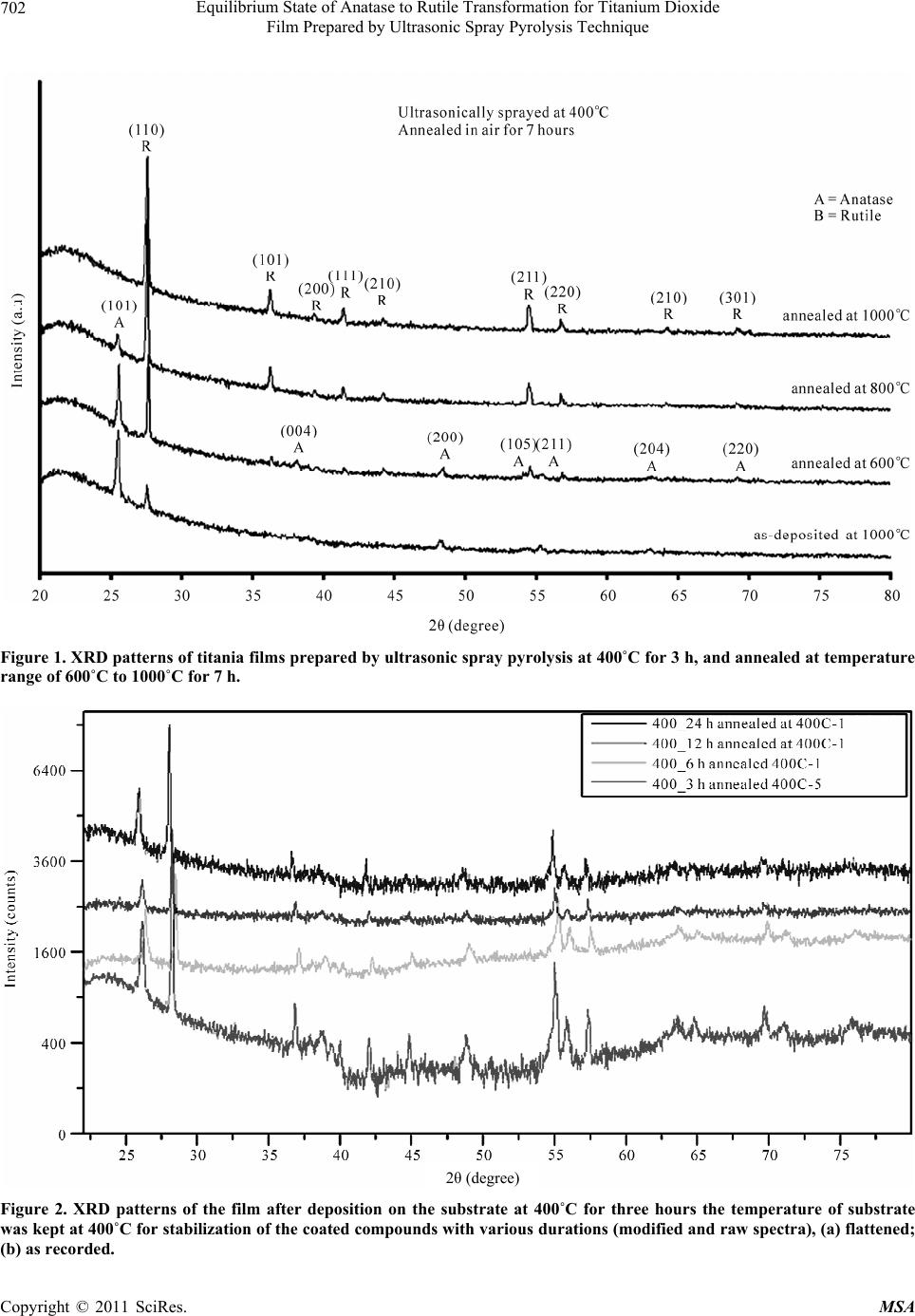 Equilibrium State of Anatase to Rutile Transformation for Titanium Dioxide Film Prepared by Ultrasonic Spray Pyrolysis Technique Copyright © 2011 SciRes. MSA 702 Figure 1. XRD patterns of titania films prepared by ultrasonic spray pyrolysis at 400˚C for 3 h, and annealed at temperature range of 600˚C to 1000˚C for 7 h. 2θ (degree) Figure 2. XRD patterns of the film after deposition on the substrate at 400˚C for three hours the temperature of substrate was kept at 400˚C for stabilization of the coated compounds with various durations (modified and raw spectra), (a) flattened; (b) as recorded.  Equilibrium State of Anatase to Rutile Transformation for Titanium Dioxide 703 Film Prepared by Ultrasonic Spray Pyrolysis Technique Table 1. Comparison between experimental (Film) and reported (Powder) 2θ peak positions from X-ray diffraction data for anatase and rutile. Annealing TiO2 Polymorph Plane None 600˚C 800˚C 1000˚C Literature Reference (101) 25.50 25.52 25.50 25.31 (004) 38.02 37.79 (200) 48.38 48.40 48.05 (105) 54.20 54.21 53.89 (211) 55.34 55.42 55.07 (204) 62.69 (220) 70.30 Anatase (215) 75.05 JCPDS 71-1166 (110) 27.58 27.63 27.57 27.57 27.43 (101) 36.31 36.23 36.23 36.08 (200) 39.41 39.42 39.19 (111) 41.48 41.38 41.41 41.24 (210) 44.25 44.27 44.21 44.04 (211) 54.54 54.46 54.46 54.32 (220) 56.82 56.80 56.82 56.62 (310) 64.29 64.04 (301) 68.25 68.99 Rutile (112) 70.03 69.79 JCPDS 72-1148 As-deposited at 400˚C Annealed at 600˚C Annealed at 800˚C Annealed at 1000˚C 3 μm Figure 3. SEM images of the TiO2 films surface showing morphology of the surfaces at different annealing temperatures. Copyright © 2011 SciRes. MSA  Equilibrium State of Anatase to Rutile Transformation for Titanium Dioxide Film Prepared by Ultrasonic Spray Pyrolysis Technique Copyright © 2011 SciRes. MSA 704 Figure 4. Thickness of as-deposited film (measured to be ~1 m by FIB). generated aerosol of Titanium (IV) Tert-Butoxide. Crys- tal structure analysis, granular morphology and surface roughness of as-deposited films and structure escalation of once-annealed samples, revealed that the growth process is closer to chemical vapour deposition rather than to the typical splashing mechanisms of spray pyro- lysis technique. Substrate temperature maintained at 400˚C. At higher temperatures, evolution of rutile phase was significant. The developed surface showed different magnitudes of the rutile phase intensity depending on annealing temperatures. A crystalline phase of out- standing transition to rutile was occurred when samples were annealed at 1000˚C. 5. Acknowledgements The first author thanks the Ministry of Science, Research & Technology of Iran for giving him the chance to sab- batical leave. Also, the authors are grateful for the finan- cial support of Austral Brick Co. Pty. Ltd., which al- lowed these and other researches in this category to be undertaken. REFERENCES [1] D. Dvoranová, V. Brezová, M. Mazúr and M. A. Malati, “Investigations of Metal-Doped Titanium Dioxide Photo- catalysts,” Applied Catalysis B: Environmental, Vol. 37, No. 2, 2002, pp. 91-105. doi:10.1016/S0926-3373(01)00335-6 [2] T. Miyagi, M. Kamei, T. Ogawa, T. Mitsuhashi, A. Ya- mazaki and T. Sato, “Pulse Mode Effects on Crystalliza- tion Temperature of Titanium Dioxide Films in Pulse Magnetron Sputtering,” Thin Solid Films, Vol. 442, No. 1-2, 2003, pp. 32-35. doi:10.1016/S0040-6090(03)00934-9 [3] A. Mills, G. Hill, S. Bhopal, I. P. Parkin and S. A. O’Neill, “Thick Titanium Dioxide Films for Semicon- ductor Photocatalysis,” Journal of Photochemistry and Photobiology A, Vol. 160, No. 3, 2003, pp. 185-194. doi:10.1016/S1010-6030(03)00206-5 [4] T. Watanabe, A. Nakajima, R. Wang, M. Minabe, S. Koi- zumi, A. Fujishima and K. Hashimoto, “Photocatalytic Activity and Photoinduced Hydrophilicity of Titanium Dioxide Coated Glass,” Thin Solid Films, Vol. 351, No. 1-2, 1999, pp. 260-263. doi:10.1016/S0040-6090(99)00205-9 [5] T. Nonami, H. Hase and K. Funakoshi, “Apatite-Coated Titanium Dioxide Photocatalyst for Air Purification,” Catalysis Today, Vol. 96, No. 3, 2004, pp. 113-118. doi:10.1016/j.cattod.2004.06.112 [6] C. Garzella, E. Comini, E. Tempesti, C. Frigeri and G. Sberveglieri, “TiO2 Thin Films by a Novel Sol-Gel Proc- essing for Gas Sensor Applications,” Sensors and Actua- tors B, Vol. 68, No. 1-3, 2000, pp. 189-196. doi:10.1016/S0925-4005(00)00428-7 [7] H. Ichiura, T. Kitaoka and H. Tanaka, “Removal of In- door Pollutants under UV Irradiation by a Composite TiO2-Zeolite Sheet Prepared Using a Papermaking Tech- nique,” Chemosphere, Vol. 50, No. 1, 2003, pp. 79-83. doi:10.1016/S0045-6535(02)00604-5 [8] T. Bak, J. Nowotny, M. Rekas and C. C. Sorrell, “Photo- electrochemical Hydrogen Generation from Water Using Solar Energy,” International Journal of Hydrogen Energy, Vol. 27, No. 10, 2002, pp. 991-1022. doi:10.1016/S0360-3199(02)00022-8 [9] T. Bak, J. Nowotny, M. Rekas and C. C. Sorrell, “Photo- Electrochemical Properties of the TiO2-Pt System in Aqueous Solutions,” International Journal of Hydrogen Energy, Vol. 27, No. 1, 2002, pp. 19-26. doi:10.1016/S0360-3199(01)00090-8 [10] J. Nowotny, C. C. Sorrell, L. R. Sheppard and T. Bak, “Solar-Hydrogen: Environmentally Safe Fuel for the Fu- ture,” International Journal of Hydrogen Energy, Vol. 30, No. 5, 2005, pp. 521-544. doi:10.1016/j.ijhydene.2004.06.012 [11] T. Miyagi, M. Kamei, T. Ogawa, T. Mitsuhashi, A. Ya- mazaki and T. Sato, “Pulse Mode Effects on Crystalliza- tion Temperature of Titanium Dioxide Films in Pulse Magnetron Sputtering,” Thin Solid Films, Vol. 442, No. 1-2, 2003, pp. 32-35. doi:10.1016/S0040-6090(03)00934-9 [12] Y. Suda, H. Kawasaki, T. Ueda and T. Ohshima, “Prepara- tion of High Quality Nitrogen Doped TiO2 Thin Film as a Photocatalyst Using a Pulsed Laser Deposition Method,” Thin Solid Films, Vol. 453-454, 2004, pp. 162-166. doi:10.1016/j.tsf.2003.11.185 [13] H. Sun, C. Wang, S. Pang, X. Li, Y. Tao, H. Tang and M. Liu, “Photocatalytic TiO2 Films Prepared by Chemical Vapor Deposition at Atmosphere Pressure,” Journal of Non-Crystalline Solids, Vol. 354, No. 12-13, 2008, pp. 1440-1443. doi:10.1016/j.jnoncrysol.2007.01.108 [14] H. Z. Abdullah and C. C. Sorrell, “Preparation and Charac- terisation of TiO2 Thick Films by Gel Oxidation,” Materi- als Science Forum, Vol. 561-565, 2007, pp. 2167-2170. [15] I. Seigo, P. Chen, P. Comte, M. K. Nazeeruddin, P. Liska, P. Péchy and M. Grätzel, “Fabrication of Screen-Printing Pastes from TiO2 Powders for Dye-Sensitized Solar Cells,” Progress in Photovoltaics: Research and Applica-  Equilibrium State of Anatase to Rutile Transformation for Titanium Dioxide 705 Film Prepared by Ultrasonic Spray Pyrolysis Technique tions, Vol. 15, No. 7, 2007, pp. 603-609. doi:10.1002/pip.768 [16] H. Z. Abdullah and C. C. Sorrell, “Preparation and Char- acterisation of TiO2 Thick Films Fabricated by Anodic Oxidation,” Materials Science Forum, Vol. 561-565, 2007, pp. 2159-2162. [17] H. Z. Abdullah and C. C. Sorrell, “Preparation and Char- acterisation of TiO2 Thick Films Fabricated by Electro- phoretic Deposition,” Materials Science Forum, Vol. 561-565, 2007, pp. 2163-2166. [18] W. Xu, R. Kershaw, K. Dwight and A. Wold, “Prepara- tion and Characterization of TiO2 Films by a Novel Spray Pyrolysis Method,” Materials Research Bulletin, Vol. 25, No. 11, 1990, pp. 1385-1392. doi:10.1016/0025-5408(90)90221-M [19] A. Nakaruk, D. Ragazzon and C. C. Sorrell, “Ana- tase-Rutile Transformation through High-Temperature Annealing of Titania Films Produced by Ultrasonic Spray Pyrolysis,” Thin Solid Films, Vol. 518, No. 14, 2010, pp. 3735-3742. doi:10.1016/j.tsf.2009.10.109 [20] D. Reyes-Coronado, G. Rodríguez-Gattorno, M. E. Espinosa-Pesqueira, C. Cab, R. de Coss and G. Oskam, “Phase-Pure TiO2 Nanoparticles: Anatase, Brookite and Rutile,” Nanotechnology, Vol. 19, No. 14, 2008, pp. 145605-145614. doi:10.1088/0957-4484/19/14/145605 [21] L. E. Depero, P. Bonzi, M. Zocchi, C. Casale and G. De Michele, “Study of the Anatase-Rutile Transformation in TiO2 Powders Obtained by Laser-Induced Synthesis,” Journal of Materials Research, Vol. 8, No. 10, 1993, pp. 2709-2715. doi:10.1557/JMR.1993.2709 [22] D. J. Kim, S. H. Hahn, S. H. Oh and E. J. Kim, “Influence of Calcination Temperature on Structural and Optical Properties of TiO2 Thin Films Prepared by Sol-Gel Dip Coating,” Materials Letters, Vol. 57, No. 2, 2002, pp. 355-360. doi:10.1016/S0167-577X(02)00790-5 [23] N. Martin, C. Rousselot, D. Rondot, F. Palmino and R. Mercier, “Microstructure Modification of Amorphous Ti- tanium Oxide Thin Films during Annealing Treatment,” Thin Solid Films, Vol. 300, No. 1-2, 1997, pp. 113-121. Copyright © 2011 SciRes. MSA |

