Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 676-683 doi:10.4236/msa.2011.26093 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using as-Grown Antimony Oxide Microstructures Aslam Jamal*, Mohammed Muzibur Rahman, Mohammed Faisal, Sher Bahadar Khan Centre for Advanced Materials and Nano-Engineering (CAMNE) and Department of Chemistry, Faculty of Sciences and Arts, Na- jran University, Najran, Kingdom of Saudi Arabia. Email: draslamjamal@gmail.com Received December 7th, 2010; revised December 30th, 2010; accepted May 18th, 2011. ABSTRACT Flower shaped antimony oxide (Sb2O3) microstructures were synthesized in a large quantity via simple solution method using aqueous mixtures of antimony chloride and hexamethylene diamine (HMDA). The morphological characteriza- tions were done by field emission scanning electron microscopy (FESEM), which revealed that the synthesized products possess flower-shaped microstructures. The detailed structural characterizations performed by X-ray diffraction (XRD), Fourier transform infrared spectrophotometer (FT-IR) and Raman spectrophotometer confirmed that the synthesized microstructures are well-crystalline antimony oxide. The Energy dispersive spectroscopy (EDS) shows that the grown products are composed of Sb and O. Optical properties of the synthesized products were characterized by UV-Visible spectrophotometer which exhibits a well defined peak at ~291.0 nm. The photo-catalytic activity of the Sb2O3 micro- structures was evaluated by degradation of acridine orange (AO), which mineralized almost 63.0% in 150 min. The chemical sensing properties of Sb2O3 microstructures was also studied by I-V technique using chloroform as a detecting solvent. The fabricated chloroform sensor demonstrates good sensitivity of 0.1154 µA·cm–2·mM–1, lower-detection limit (~0.1 mM), large-linear dynamic range (LDR, 0.122 mM to 1.22 M) with linearity (R = 0.7898) in short response time (10.0 sec). Keywords: Antimony Oxide Microstructures, XRD, FE-SEM, Photo Degradation, Acridine Orange, Chloroform Chemical Sensing 1. Introduction Developing nano-science and nanotechnology is getting valuable attention in terms of research and development at the present time. In the field of nanotechnology, the antimony oxide nano-crystalline particles emerges as a challenging prospect due to having amazing preparation, characterization, catalytic degradation, and fabrication as well as their wide range of other applications. The syn- thesis of nano-materials has received remarkable atten- tion in view of the size, shape, structural arrangement, properties and potential applications in advance level. Generally, Sb2O3 is used as conductive materials, high- efficiency flame-retardant synergist in polymers, dyes, pigments, paints, adhesives, and industrial coating mate- rials [1-3]. Sb2O3 nano-rods, nano-particles, nano-tubes, nano-belts [4-6] and hollow spheres [7] have been fabri- cated by various conventional routes and techniques. Micro-structured antimony oxides can be prepared by various general methods including vapor-solid route [8], hydrothermal synthesis [9,10], vapor condensation [10, 11], sol-gel [12], X-ray radiation–oxidization route [13], and gas condensation [14] techniques. However, scientist are quite interested on controlled growth, mechanism, characteristics, and advanced applications of the Sb2O3 microstructure [15]. During the past decades, many re- searchers have put focus on searching for a direct and effective method to solve this problem [16,17]. Recently, the opt-electronic and surface properties of Sb2O3 micro- structures have been extensively studied [2,3]. Here we further investigate their property as a photo-catalyst and chemi-sensor. Organic dyes such as acridine orange are the common pollutants and affect the environment due to its hazard- ous and carcinogenic nature. Thus detoxification of these organic pollutants needs an urgent and effective process.  Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using 677 as-Grown Antimony Oxide Microstructures Several methods have been used but photo-catalytic deg- radation is one of the superlative and attractive substi- tutes for the degradation of these organic pollutants. Therefore, Sb2O3 has been proposed as a catalyst for the detoxification of organic dye in the presence of UV ra- diation. Organic solvents are also one of the hot environmental problems and badly effect the environment due to their toxicity. One of these organic solvents is chloroform which cause cardiac or respiratory arrest [18] and depress the central nervous system [19]. Thus it is very important organic compound to have details study and develop de- vices or sensors for the detection and quantification of chloroform using microstructure materials. In general, metal oxides are being extensively utilized due to their unique surface activities imparted by huge surface areas, which can make them ideal sensing elements as chemi- sensors. The detection of chloroform in liquid phase by I-V technique using antimony oxide surface is developed for the first time. In the present investigation we have made an attempt to develop a photo-catalyst and chemi-sensor and for this purpose antimony oxide (Sb2O3) microstructures were synthesized. Sb2O3 microstructures were characterized by UV, FT-IR, Raman spectroscopy, XRD, FE-SEM and EDS. These microstructures were applied for catalytic application and sensing application and demonstrated good degradation and sensing properties. 2. Experimental 2.1. Materials and Methods Analytical grade antimony chloride, hexamethylene dia- mine, ammonium hydroxide, acridine orange, and chlo- roform were purchased from Sigma-Aldrich and used as received. The solution was made in double distilled water before performing any reaction. For the synthesis of an- timony oxide microstructure, the solutions were prepared by adding antimony chloride (0.5 M, 50.0 mL) and hexamethylene diamine (0.5 M, 50 mL) in distill water in equimolecular proportion. The pH (9.3) was adjusted by adding drop wise ammonium hydroxide and then mag- netically stirred for 6 hour at 60.0˚C. After cooling, white precipitates were obtained and washed with acetone for two to three times and dried at room temperature. The as-grown microstructure antimony oxide does reveal better photo catalytic activity and chemical sensing property. For this purpose, photo-degradation of AO is investigated as model dye compound that results in a better chemical action on antimony oxide microstructure. Chloroform was used as model compound for sensor application. Structural characterizations of the as-grown materials were investigated using field emission scanning electron microscope (FE-SEM; JSM-7600F, Japan), x-ray diffraction (XRD; X’Pert Explorer, PANalytical diffractometer) data was executed with Cu-Kα1 radiation. Fourier transforms infrared spectrometer (FT-IR; Perkin Elmer) spectrum was recorded in KBr dispersion in the range of 400 to 4000 cm–1. UV/visible spectrum were recorded at 291 nm (Perkin Elmer-Lambda 950-UV- visible spectrometer). Raman-scattering spectrum was measured at room temperature with the Ar+ laser line as an excitation source. The chemical sensing of Sb2O3 electrodes have been primarily investigated by I-V tech- nique, where chloroform is used as a target compound. A thin-film was fabricated on electrode substrate by Sb2O3 micro-materials with conducting binder for fabricating the sensor substrate. 2.2. Photo-Catalytic Experiments Photo-degradation of AO was examined by optical ab- sorption spectroscopy. The catalytic reaction was carried out in a 250.0 mL beaker, which contain 150.0 ml of AO dye solution (0.03 mM) and 150.0 mg of catalyst. Prior to irradiation, the solution was stirred and bubbled with oxygen for at least 15 min in the dark to allow equilib- rium of the system so that loss of compound due to the adsorption can be taken into account. The suspension was continuously purged with oxygen bubbling through- out the experiment. Irradiation was carried out using 250W high pressure Mercury lamps. Samples (5.0 ml) were collected before and at regular intervals during the irradiation and acridine orange solution were separated from the photo-catalyst by centrifugation before analysis. The degradation was monitored by measuring the ab- sorbance using UV-visible spectrophotometer (Lambda 950). The absorbance of AO (0.03 mM) was followed at 491.0 nm wavelength. 2.3. Fabrication of Gold Electrode Using Sb2O3 Gold electrode (surface area, 0.0216 cm2) is coated with as-grown Sb2O3 using butyl carbitol acetate (BCA) and ethyl acetate (EA) as a coating agent. Then it is kept in the oven at 60.0˚C for 3.0 h until the film is completely dried. 0.1 M phosphate buffer solution at pH 7.0 is made by mixing 0.2 M Na2HPO4 and 0.2 M NaH2PO4 solution in 100.0 mL de-ionize water. 2.4. Detection of Chloroform Using I-V Technique A cell is constructed consisting of microstructure coated gold electrode as a working electrode and Pd wire is used as counter electrode. Chloroform solution is diluted at different concentrations in DI water and used as a target Copyright © 2011 SciRes. MSA 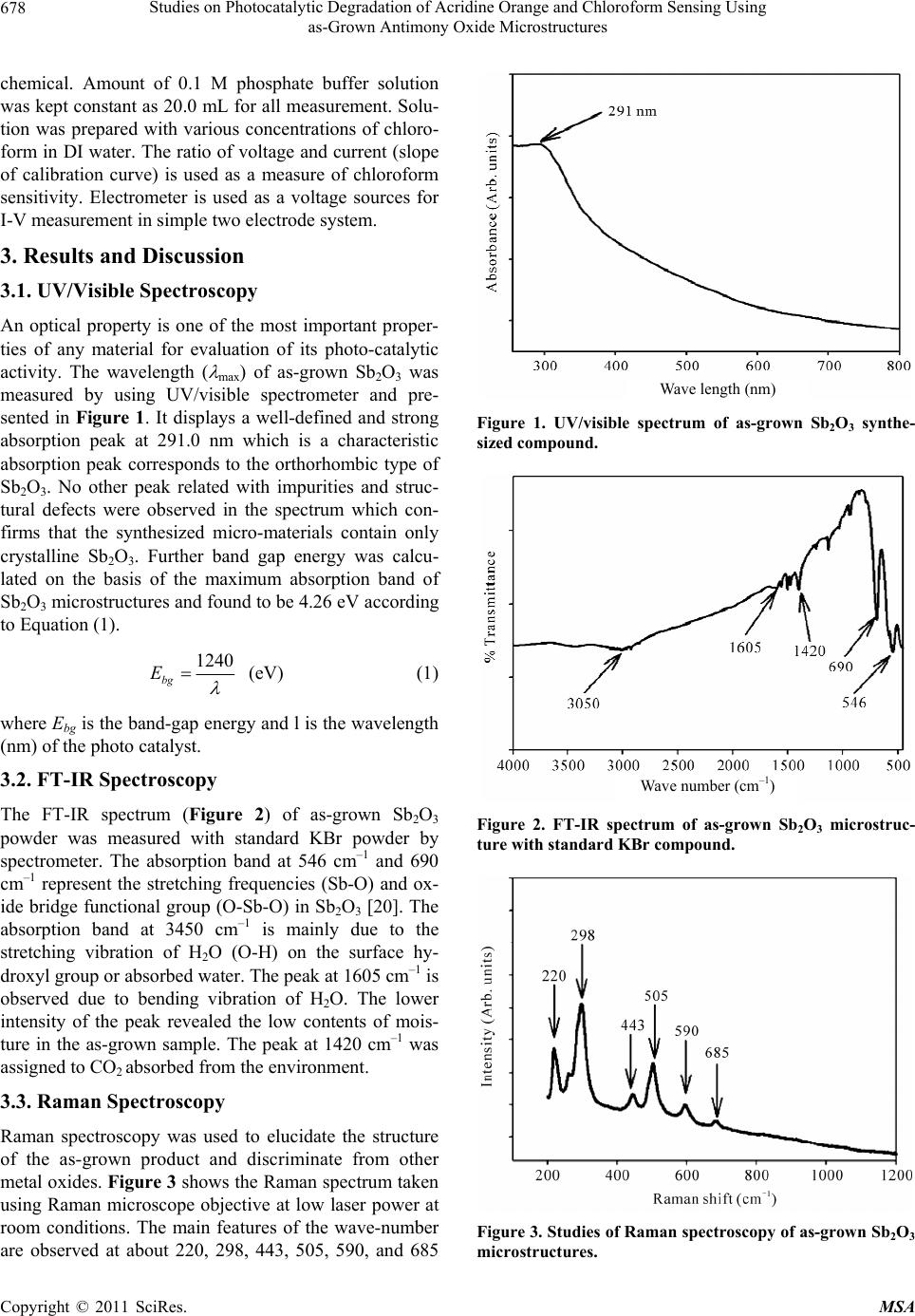 Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using 678 as-Grown Antimony Oxide Microstructures chemical. Amount of 0.1 M phosphate buffer solution was kept constant as 20.0 mL for all measurement. Solu- tion was prepared with various concentrations of chloro- form in DI water. The ratio of voltage and current (slope of calibration curve) is used as a measure of chloroform sensitivity. Electrometer is used as a voltage sources for I-V measurement in simple two electrode system. 3. Results and Discussion 3.1. UV/Visible Spectroscopy An optical property is one of the most important proper- ties of any material for evaluation of its photo-catalytic activity. The wavelength ( max) of as-grown Sb2O3 was measured by using UV/visible spectrometer and pre- sented in Figure 1. It displays a well-defined and strong absorption peak at 291.0 nm which is a characteristic absorption peak corresponds to the orthorhombic type of Sb2O3. No other peak related with impurities and struc- tural defects were observed in the spectrum which con- firms that the synthesized micro-materials contain only crystalline Sb2O3. Further band gap energy was calcu- lated on the basis of the maximum absorption band of Sb2O3 microstructures and found to be 4.26 eV according to Equation (1). 1240 bg E (eV) (1) where Ebg is the band-gap energy and l is the wavelength (nm) of the photo catalyst. 3.2. FT-IR Spectroscopy The FT-IR spectrum (Figure 2) of as-grown Sb2O3 powder was measured with standard KBr powder by spectrometer. The absorption band at 546 cm–1 and 690 cm–1 represent the stretching frequencies (Sb-O) and ox- ide bridge functional group (O-Sb-O) in Sb2O3 [20]. The absorption band at 3450 cm–1 is mainly due to the stretching vibration of H2O (O-H) on the surface hy- droxyl group or absorbed water. The peak at 1605 cm–1 is observed due to bending vibration of H2O. The lower intensity of the peak revealed the low contents of mois- ture in the as-grown sample. The peak at 1420 cm–1 was assigned to CO2 absorbed from the environment. 3.3. Raman Spectroscopy Raman spectroscopy was used to elucidate the structure of the as-grown product and discriminate from other metal oxides. Figure 3 shows the Raman spectrum taken using Raman microscope objective at low laser power at room conditions. The main features of the wave-number are observed at about 220, 298, 443, 505, 590, and 685 Wave length (nm) Figure 1. UV/visible spectrum of as-grown Sb2O3 synthe- sized compound. Wave number (cm –1 ) Figure 2. FT-IR spectrum of as-grown Sb2O3 microstruc- ture with standard KBr compound. Figure 3. Studies of Raman spectroscopy of as-grown Sb2O3 microstructures. Copyright © 2011 SciRes. MSA  Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using as-Grown Antimony Oxide Microstructures Copyright © 2011 SciRes. MSA 679 cm–1 which are responsible for Sb-O stretching vibration [21]. These large bands may be assigned to a magnetite phase of antimony oxide micro-flowers. other than Sb and O were detected. The atomic percent of Sb and O were determined in the as-grown microstruc- ture as 42.76% and 57.24%, respectively. 3.4. XRD Analysis 4. Applications The crystal phase of the as-grown antimony oxide was investigated by XRD analysis and shown in Figure 4. XRD analysis showed diffraction peaks which could be indexed to (110), (111), (121), (131), (002), (112), (211), (221), (002), (240), (052), (161), (113), (133), (072), (341), and (312) phases, which revealed the existence of well-crystalline orthorhombic type of Sb2O3. The diffrac- tion pattern of the as-prepared sample matches JCPDS # 74-1725. The diffraction peaks showed that the antimony oxide produced different crystalline phase [22,23]. X-ray diffraction thus confirmed that the obtained microstruc- ture is well-crystalline orthorhombic type of Sb2O3. 4.1. Photo-Catalytic Degradation Figure 6(a) exhibits the change in absorption spectra for the photo-catalytic degradation of AO dye as a function of irradiation time. It is found that irradiation of aqueous suspension of AO dye in the presence of Sb2O3 micro- structure leads to decrease in absorption intensity. It can be seen that the maximum absorbance at 491.0 nm gradually decreases with increase in irradiation time. Figure 6(b) shows the change in absorbance as a func- tion of irradiation time for the dye derivative in the ab- sence and presence of Sb2O3 microstructure. Irradiation of an aqueous solution of AO in the presence of synthe- sized microstructure leads to decrease in absorption in- tensity. Figure 6(c) shows a plot for the percent degrada- tion vs irradiation time (min) for the oxygen saturated aqueous suspension of acridine orange (AO) in the pres- ence and absence the synthesized metal oxide micro- structures. It could be seen from the figure that 63.0% (in the presence of Sb2O3 microstructure) of the compound degraded after 150.0 minutes of irradiation time whereas in the absence of photo-catalyst no observable loss of dye 3.5. Measurement of FE-SEM & EDS Morphology of the microstructure was investigated by FE-SEM and shown in Figure 5. High and low magnifi- cation of FESEM images [Figures 5(a-c)] showed flower shape structure in micro-level for the as-grown products. The EDS spectrum corroborated the composition of Sb2O3, which is presented in the Figure 5(d). The EDS spectra indicate that the as-grown microstructure is composed of Sb and O. No other peak related to elements Figure 4. XRD pattern of as-grow n synthesized Sb2O3 microstructures.  Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using 680 as-Grown Antimony Oxide Microstructures (a) (b) (c) (d) Figure 5. The FE-SEM morphology of as-grown Sb2O3. (a) to (c) low to high magnified images and (d) is EDS spectrum of as grown antimony-oxide powder. (a) (c) Figure 6. Photo-catalytic degradation of AO using mf-Sb2O3. (a) Spectrum of AO at different time interval; (b) Comparison of absorbance and (c) % degradation in different time intervals of AO in presence and absence of as-grown mf-Sb2O3. Copyright © 2011 SciRes. MSA  Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using as-Grown Antimony Oxide Microstructures Copyright © 2011 SciRes. MSA 681 generate superoxide radical anion [24-26] as mentioned in the Equation (1)-(3). could be seen. Above results clearly indicate that pre- pared microstructure showing considerable photo cata- lytic activity has very simple synthesis procedure and low cost, so it can also be used as a photo catalyst beside other metal oxide. MO x cb vb heh (1) 2 O CB e2 O (2) Antimony oxide is one of the promising semiconduc- tors due to their various morphological (micro-flower shape) and chemical properties, availability, easy to use, easy to synthesis, less toxic, lower cost, higher surfaces area, and high absorption of light quanta. Mechanism of heterogeneous photo catalysis has been discussed exten- sively in literature. Briefly when metal oxide absorb en- ergy which is more than its band gap energy it results in the generation of electron and hole pairs with free elec- trons produced in the empty conduction band (CB e ) leaving behind an electron vacancy or “hole” in the va- lence band (VB h). During this photo catalytic activity, the electron and hole may migrate to the catalyst surface where they participate in redox reactions. Specially, h+ VB may react with surface-bound H2O or OH– to produce the hydroxyl radical and is picked up by oxygen to CB e 2 HOOH H VB h (3) The hydroxyl radicals () and superoxide radical OH anions (2 O ) are shows as a primary oxidizing species in the photo catalytic processes and contribute to the oxida- tion process by attacking the dye molecules and would results in the bleaching of the AO dye. 4.2. Chloroform Detection The as-grown flower-shape Sb2O3 was employed for the detection of chloroform in solution phase. Pd and gold electrodes are used as counter electrode and working electrode respectively. I-V technique is followed to measure the changing of current in each injection of chemical solution in the 20.0 mL phosphate buffer solu- (a) (b) (c) (d) Figure 7. I-V characterization of as-grown Sb2O3 microstructures. (a) Comparison of with and without coating surface; (b) Comparison of with and without chloroform sample injection; (c) Concentration variation of chloroform and (d) calibration plot.  Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using 682 as-Grown Antimony Oxide Microstructures chloroform was used as a detecting chemical in ons haped Sb2O3 has been successfully roform, the performance of the developed chloroform nancial support to the dean- University, Najran, REFERENCES [1] K. Ozawa, Y, “Preparation and Electrical Conpes of Antimonic ion. Thet the liquid phase as a chemical sensor [25,26]. The sens- ing characteristics of I-V sensors (two electrodes system) having Sb2O3 thin film has been studied, which is pre- sented in the Figure 7. I-V responses sensor having Sb2O3 thin film as a function of time for the chloroform is shown in Figures 7(a) and (b). The time delaying for electrometer was kept 1.0 sec. The concentration of chloroform was varied from 0.122 mM to 12.2 M by adding de-ionized water in different proportions. A sig- nificant increase in the current value with applied poten- tial is clearly demonstrated. The gray-solid and dark- solid dotted curves indicate the response of the film be- fore and after injecting 100.0 µL chemicals in bulk solu- tion. Significant increase in the sample current is meas- ured after injection of target component. 0.122 mM con- centration of chloroform was initially taken in the cell and added the higher concentration (each step, 10 times) is in each injection from the stock concentration of chlo- roform, which was added to the 20 mL bulk buffer solu- tion. Each I-V response to varying concentration of chemicals from 0.122 mM to 12.2 M on thin micro-flower Sb2O3 coatings for 10s (delay of time) was presented in the Figure 7(c). It shows current of Sb2O3 as a function of target concentration at room temperature. It is ob- served that at lower to higher concentration of target compound, the current increases gradually. A wide range of chloroform concentration was chosen to study the pos- sible detection limit, which is examined in 0.122 mM to 1.22 M. The calibration curve was plotted from the varia- tion of chloroform concentration, which is shown in the Figure 7(d). The sensitivity is calculated from the calibra- tion curve, which is closed to 0.1154 µA·cm–2·mM–1. The linear dynamic range of this sensor exhibits from 0.122 mM to 1.22 M with linearity (R = 0.7898) in short re- sponse time (10.0 s) and the detection limit was found 0.1 mM (3N/S). 5. Conclusi The micro-flower s synthesized using SbCl3 and HMDA by direct thermal stirrer technique in the alkaline medium. The composi- tion and detail structural characterization have been stud- ied by UV, FT-IR, Raman spectroscopy, XRD, FE-SEM, and EDS which revealed that the synthesized micro- structures are well-crystalline, possessing orthorhombic type of Sb2O3. The potential applications on catalytic behavior and chemical sensing were carried out with as-grown micro-flower Sb2O3 materials. The photo cata- lytic performance of Sb2O3 materials were evaluated by degradation of AO which efficiently degraded the dye. By applying to the detection and quantification of chlo- sensor is excellent in terms of sensitivity, detection limit, linear dynamic ranges, and response time. 6. Acknowledgements Authors are thanks full for fi ship of scientific research, Najran Saudi Arabia. . Sakka and A. Amamo ductivity of Three Ty Acid Films,” Journal of Materials Research, Vol. 13. No. 4, 1998, pp. 830-833. doi:10.1557/JMR.1998.0107 [2] K. S. Liu, J. Zhai and L. Jiang, “Fabrication and Charac- terization of Superhydrophobic Sb2O3 Films,” Nanotech- nology, Vol. 19, No. 16, 2008, p. 165604. doi:10.1088/0957-4484/19/16/165604 [3] Z. T. Deng, D. Chen, F. Q. Tang, X. W. M L. Zhang, “Orientated Attachment eng, J. Ren and Assisted Self-As- ant-Assisted Sol- sembly of Sb2O3 Nanorods and Nanowires: End-to-End Versus Side-by-Side,” Journal of Physical Chemistry, Vol. 111, No. 14, 2007, pp. 5325-5330. [4] Y. X. Zhang, G. H. Li and L. D. Zhang, “Growth of Sb2O3 Nanotubes via a Simple Surfact vothermal Process,” Chemistry Letters, Vol. 33, No. 3, 2004, pp. 334-335. doi:10.1246/cl.2004.334 [5] S. B. Khan, M. Faisal, M. M. Rahman and A. Jamal, “Low-temperature Growth of ZnO Nanoparticles: Photo- al, catalyst and Acetone Sensors,” Talanta, March 2011. [6] M. Faisal, S. B. Khan, M. M. Rahman, A. Jamal and A. Umar, “Ethanol Chemi-Sensor: Evaluation of Structur Optical and Sensing Properties of CuO Nanosheets,” Ma- terials Letters, Vol. 65, No. 9, 2001, pp. 1400-1403. doi:10.1016/j.matlet.2011.02.013 [7] B. J. Li, Y. B. Zhao, X. M. Xu, C. L. Zhang, Z. S. and Z. J. Zhang, “Fabrication of Hollow Wu Sb2O3 Micro- spheres by PEG Coil Template,” Chemistry Letters, Vol. 35, No. 9, 2006, p. 1026. doi:10.1246/cl.2006.1026 [8] C. Ye, G. Meng, L. Zhang, G. Wang and Y. Wang, “A Facile Vapor-Solid Synthetic Route to Sb2O3 Fibrils and Tubules,” Chemical Physics Letters, Vol. 363, No. 1-2, 2002, pp. 34-38. doi:10.1016/S0009-2614(02)01018-7 [9] A. Umar, M. M. Rahman and Y. B. Hahn. “MgO Polyhe- dral Nanocages and Nanocrystals Based Glucose Biosen- sor,” Electrochemistry Communications, Vol. 11, No. 7, 2009, pp. 1353-1357. doi:10.1016/j.elecom.2009.04.033 [10] M. M. Rahman, A. Jamal, S. B. Khan and M. Faisal, “Characterization and Applications of as Grown β-Fe2O3 Nanoparticles Prepared by Hydrothermal Method,” Journal of Nanoparticle Research, 2011. doi:10.1007/s11051-011-0301-7 [11] R. W. Siegel, “Nanostructured Ma Matter,” Nanostructured Materia terials—Mind over ls, Vol. 4, No.1, 1994, Copyright © 2011 SciRes. MSA  Studies on Photocatalytic Degradation of Acridine Orange and Chloroform Sensing Using 683 as-Grown Antimony Oxide Microstructures pp. 121-138. doi:10.1016/0965-9773(94)90134-1 [12] A. Umar, M. M. Rahman, A. Al-Hajry and Y. B. Hahn. “Highly-Sensitive Cholesterol Biosensor Based on Well-Crystallized Flower-Shaped ZnO Nanostructures,” Talanta, Vol. 78, No. 1, 2009, pp.284-289. doi:10.1016/j.talanta.2008.11.018 [13] P. Liu, Y.H. Zhang, M. W. Zhang, W. H. Zh Qian, “Preparation of Nanocrysta ang and Y. T. lline Antimony Oxide Powders by Use of γ-Ray Radiation—Oxidization Route,” Materials Science and Engineering B, Vol. 49, No. 1 , 1997, pp. 42-45. doi:10.1016/S0921-5107(97)00065-2 [14] C. G. Granqvist and R. A. Buhrmann, “Ultrafine Metal Particles,” Journal of Applied Physics, Vol. 47, No. 5, 1976, pp. 2200-2219. doi:10.1063/1.322870 [15] Z. L. Zhang, L. Guo and W. D. Wang, “Synthesis and Characterization of Antimony Oxide Nanoparticles,” Journal of materials Research, Vol. 16, No. 3, 2001, pp. 803-805. doi:10.1557/JMR.2001.0096 [16] M. Faisal, M. A. Tariq and M. Muneer, “Photocatalysed Degradation of Two Selected Dyes in UV-Irradiated Aqueous Suspensions of Titania,” Dyes Pigments, Vol. 72, No. 2, 2007, pp. 233-239. doi:10.1016/j.dyepig.2005.08.020 [17] S. B. Khan, M. Faisal, M. M. R “Exploration of CeO Nanoparticl ahman and A. Jamal, es as a Chemi-Sensor 2 and Photo-Catalyst for Environmental Applications,” Science of the Total Environment, Vol. 409, No. 15, 2011, pp. 2987-2992. doi:10.1016/j.scitotenv.2011.04.019 [18] A. Umar, M. M. Rahman, A. Al-Hajry and Y.-B. Hahn. “Enzymatic Glucose Biosensor Based on Flower-Shaped Copper Oxide Nanostructures Composed of Thin Nanosheets,” Electrochemistry Communications, Vol. 11, No. 2, 2009, pp. 278-281. doi:10.1016/j.elecom.2008.11.027 [19] M. M. Rahman, A. Umar and K. Sawada. “Development of Amperometric Glucose Biosensor Based on Glucose Oxidase Co-Immobilized with Multi-Walled Carbon Nanotubes at Low Potential,” Sensors and Actuators B: Chemical, Vol. 137, No. 1, 2009, pp. 327-333. doi:10.1016/j.snb.2008.10.060 [20] I. O. Mazali, W. C. Las and M. Cilense, “Synthesis and Characterization of Antimony Tartrate for Ceramic Pre- cursors,” Journal of Materials Synthesis and Processing, Vol. 7, No. 6, 1999, pp. 387-391. doi:10.1023/A:1021822115116 [21] Z. Deng, D. Chen, F. Tang, J. Ren and A. J. Muscat, “Synthesis and Purple-Blue Emission of Antimony Tri- oxide Single-Crystalline Nanobelts with Elliptical Cross Section,” Nano Research, Vol. 2, No. 2, 2009, pp. 151- 160. doi:10.1007/s12274-009-9014-y [22] D. Wang, Y. Zhou, C. Song and M. Shao, “Phase and Morphology Controllable Synthesis of Sb2O3 Microcrys- tals,” Journal of Crystal Growth, Vol. 311, No. 15, 2009, pp. 3948-3953. doi:10.1016/j.jcrysgro.2009.06.020 [23] Y. Hu, H. Zhang and H. Yang, “Direct Synthesis of Sb2O3 Nanoparticles via Hydrolysis-Precipitation Method,” Journal of Alloys and Compounds, Vol. 428, No. 1-2, 2007, pp. 327-331. doi:10.1016/j.jallcom.2006.03.057 [24] B. S. Naidu, M. Pandey, V. Sudarsan, R. K. Vatsa and R. Tewari, “Photoluminescence and Raman Spectroscopic Investigations of Morphology Assisted Effects in Sb2O3,” Chemical Physics Letters, Vol. 474, No. 1-3, 2009, pp. 180-184. doi:10.1016/j.cplett.2009.04.050 [25] M. A. Tariq, M. Faisal and M. Muneer, “Semiconduc- tor-Mediated Photocatalysed Degradation of Two Se- lected Azo Dye Derivatives, Amaranth and Bismarck Brown in Aqueous Suspension,” Journal of Hazardous Materials, Vol. 127, No. 1-3, 2005, pp.172-179. doi:10.1016/j.jhazmat.2005.07.001 [26] M. Faisal, S.B. Khan, M.M. Rahman and A. Jamal, “Role of ZnO-CeO2 Nanostructures as a Photocatalyst and Chemi-Sensor,” Journal of Materials Sciences & Tech- nology, 2011 (In press). Copyright © 2011 SciRes. MSA |

