Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 592-595 doi:10.4236/msa.2011.26079 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Tannins from Rhizophora Racemosa Makanjuola Oki1,2, Ebitei Charles3, Collins Alaka4, Tambari Kayode Oki1 1Department of Technical Services, Greenfield-Oaks Limited, London, UK; 2Department of Petroleum Engineering, Covenant Uni- versity, Ota, Nigeria; 3Department of Mechanical Engineering, Niger Delta University, Yenagoa, Nigeria; 4Department of Pure & Industrial Chemistry, University of Port Harcourt, Port Harcourt, Nigeria. Email: m.oki11@yahoo.com Received December 11th, 2010; revised February 15th, 2011; accepted April 11th, 2011. ABSTRACT Studies on the corrosion behaviour of mild steel electrodes in inhibited hydrochloric acid are described. Conventional weight loss measurements show that a maximum concentration of 140 ppm of tannin from Rhizophora racemosa is re- quired to achieve 72% corrosion inhibition. Similar concentration of tannin: H3PO4 in ratio 1:1 gave 61% inhibition efficiency, whereas efficiency obtained for phosphoric acid as inhibitor in the same environment was 55%. Corrosion rates obtained over six hours of exposure in 1 M HCl solution at inhibitor concentrations of 140 ppm are 2 mA/cm2, 2.4 mA/cm2, 2.6 mA/cm2 and 6 mA/cm2 for tannin, tannin/H3PO4 and H3PO4-inhibited and uninhibited specimens re- spectively. Natural atmospheric exposure studies revealed that specimens treated in H3PO4 resisted corrosion for three weeks, while tannin treated specimens suffered corrosion attack after one week of exposure tests. Keywords: Inhibitor, Tannin s, Corrosion Rate, Rhizophora Racemosa, Phosphoric Acid 1. Introduction Rhizophora racemosa is in abundance in the Mangrove forests of southern Nigeria. The bark of its stem is rich in tannins which can be described as any group of naturally occurring phenolic compounds. Their basic structure consists of garlic acid residues which are linked to glu- cose via glycosidic bonds [1]. Thus tannins have an array of hydroxyl and carboxyl groups through which the mo- lecules can adsorb on corroding metallic surfaces. Fer- rous materials, especially mild steel, on the other hand are largely used in acidic media in most industries in- cluding oil/gas exploration and ancillary activities. Dur- ing such activities, inhibited hydrochloric acid is widely used in pickling , descaling and stimulation of oil wells in order to increase oil and gas flow. The inhibitors em- ployed are varied and some have been found to be haz- ardous to health and the environment at large [2]. Thus efforts are now directed towards formulation of modern environmentally safe inhibitors [3] in which plant ex- tracts have become important as eco-friendly, economi- cal, readily available and renewable sources of effective corrosion inhibitors. Other researchers [4-8] have dem- onstrated corrosion inhibition in the order of 80% and above by extracts from Mentha Pulegium [4], Azadirachta [5], and Zenthoxylum alatum [6], Kidney bean [7] and Occimum viridis [8] amongst many others. The inhibi- tion efficiency has been described as primarily due to their adsorption at corroding metal surfaces [9]. A pro- tective film forms due to adsorption of these inhibitor molecules which restricts either the movement of ions away from the corroding surface or the consumption of electrons; however in most cases, they act as bo th anodic and cathodic inhibitors [10]. It is not uncommon to use either tripolyph osphates or phosphoric acid [11] and/or a mixture of either with other inhibitors to inhibit the cor- rosion of mild steel especially in the petrochemical in- dustry. In such cases synergistic effects where improved corrosion inhibitive efficiency is observed, lower costs implications of using either of the two are the primary motives. Thus, the present investigation is directed at the evalu- ation of tannin from Rhizophora racemosa, phosphoric acid and a combination of the two as inhibitors for the corrosion of mild steel in hydrochloric acid. 2. Experimental 2.1. Materials Mild steel specimen which contained, Carbon, 0.16%;  Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Tannins Rom Rhizophora Racemosa593 Magnesium 0.53%; Silicon 0.16% and Iron 99.25% was made out into electrodes. All chemicals used were of laboratory grade by BDH Chemicals, UK, while tannin was obtained from the bark of Rhizophora racemosa. Distilled water was used throughout the ex periment. 2.2. Methods Tannin was extracted from the bark of Rhizophora ra- cemosa by the methods described by Ojinnaka [12]. Mild steel specimens of dimensions 40 × 20 × 4 mm were made into electrodes by fastening each on to Copper wire, 100mm in length through a notch at one end and con- solidated with Araldite, an epoxy resin. The final ex- posed area of each electrode was 20 × 20 × 4 mm respec- tively, giving an exposed total surface area of 10.4 cm2. Prior to exposure to various corrosive media, the speci- mens were abraded with emery paper after which the specimens were individually rinsed in ethanol, dried un- der the fan and stored in desiccators prior to use in the experiments. Weight loss measurements were carried out by immersing each pre-weighed electrode in 100 ml of 1 M of HCl to act as control. Other electrodes were simi- larly exposed in 1 M HCl media containing various con- centrations of tannin. During exposure, the potentials of the various specimens were measured with reference to saturated calomel electrode connected through a high impedance voltmeter. Triplicate experiments were per- formed in each case and the mean values of the weight loss recorded. The specimens were then examined under Olympus optical metallographic microscope. Further, electrodes immersed in concentrated inhibitor solutions for 10 minutes each and dried under the fan for 30 min- utes and untreated specimens were exposed vertically to the outside environment behind the laboratory at Choba, Port Harcourt, Nigeria. These specimens were observed regularly and at the end of 21 days, the electrodes were examined under an Olympus metallographic microscope. Similar tests as described above were performed on mild steel electrodes with phosphoric acid and tannin: phosphoric acid in ratio 1:1 as inhibitors of interests. 3. Results and Discussions 3.1. Atmospheric Exposure General Observations To the naked eye, untreated mild steel specimens showed some red-brown patches of rust on the first day of expo- sure to the atmosphere. This is expected as the corrosion product of iron exposed to moist air, ferrous hydroxide is further oxidised to the hydrated oxide during exposure. However, those specimens treated with tannin, tan- nin/phosphoric acid and phosphoric acid, retained the colourations imparted on th em by the respective inhibito r solutions. For those specimens treated with phosphoric acid and tannin/phosphoric acid, there was no rust exhibited over three weeks of exposure as the phosphate formed a tena- cious film which is integral with the substrate and pro- tected the surface from the inclement atmosphere. How- ever the specimens treated with tannin solution showed some signs of rust after one week of exposure to the at- mosphere. Researchers [5,6,13] agree that inhibitors of organic origin perform by adsorbing on substrates through weak bonds. The bonds formed by tannin with the mild steel substrates were compromised thus expos- ing the substrate to the atmosphere and corrosion reac- tions with formation of rust occurred. These observations were further confirmed with opti- cal microscopy examination of the various specimens. 3.2. Corrosion Rates and Inhibition Efficiencies During this investigation corrosion rates, CR an d perc ent efficiency, E% were derived from Equations (1) and (2) respectively as demonstrated by other researchers [6,8, 13]. o CRww At (1) where wo and w are, respectively, the weights of the spe- cimens before and after exposure to 1 M hydrochloric acid; A is the total surface area, 10.4 cm2 in this investi- gation and t is the time of exposure. % 100 oi ECRCR CR o (2) where, CRo and CRi are the corrosion rates of mild steel in 1 M HCl without and in the presence of various con- centrations of different inhibitors respectively and E% are the inhibition efficien cy. The weight loss of mild steel in 1 M HCl solution ini- tially increased rapidly with a rate that decreased with time, Figure 1, as a result of formation of corrosion products which may stifle corrosion reactions when de- posited on the subst rate [1 4] . After 6 hours of exposure, the we ight loss was 413 mg, which translates to a corrosion rate, CR of 6.619 mg/cm2/h. At that instance and beyond, the electrode potential, as measured with respect to saturated calomel electrode through a high impedance voltmeter, was –1.490V. For iron corroding fr eely as Fe Fe2+ + 2e–, 2.51 mdd is equivalent to 1 × 10–6 A/cm2 [15]. Thus, 6.619 mg/cm2/h is equivalent to about 6 mA/cm2. Figure 2 describes the efficiency of tannin, tannin/ phosphoric acid and phosphoric acid respectively in 1 M HCl, where it is observed that tannin showed a maximum efficiency of about 72% at a concentration of 140 ppm whereas, at the same concentration, efficiencies of about 61% and 55% were achieved by tannin/H3PO4 and H3PO4 Copyright © 2011 SciRes. MSA 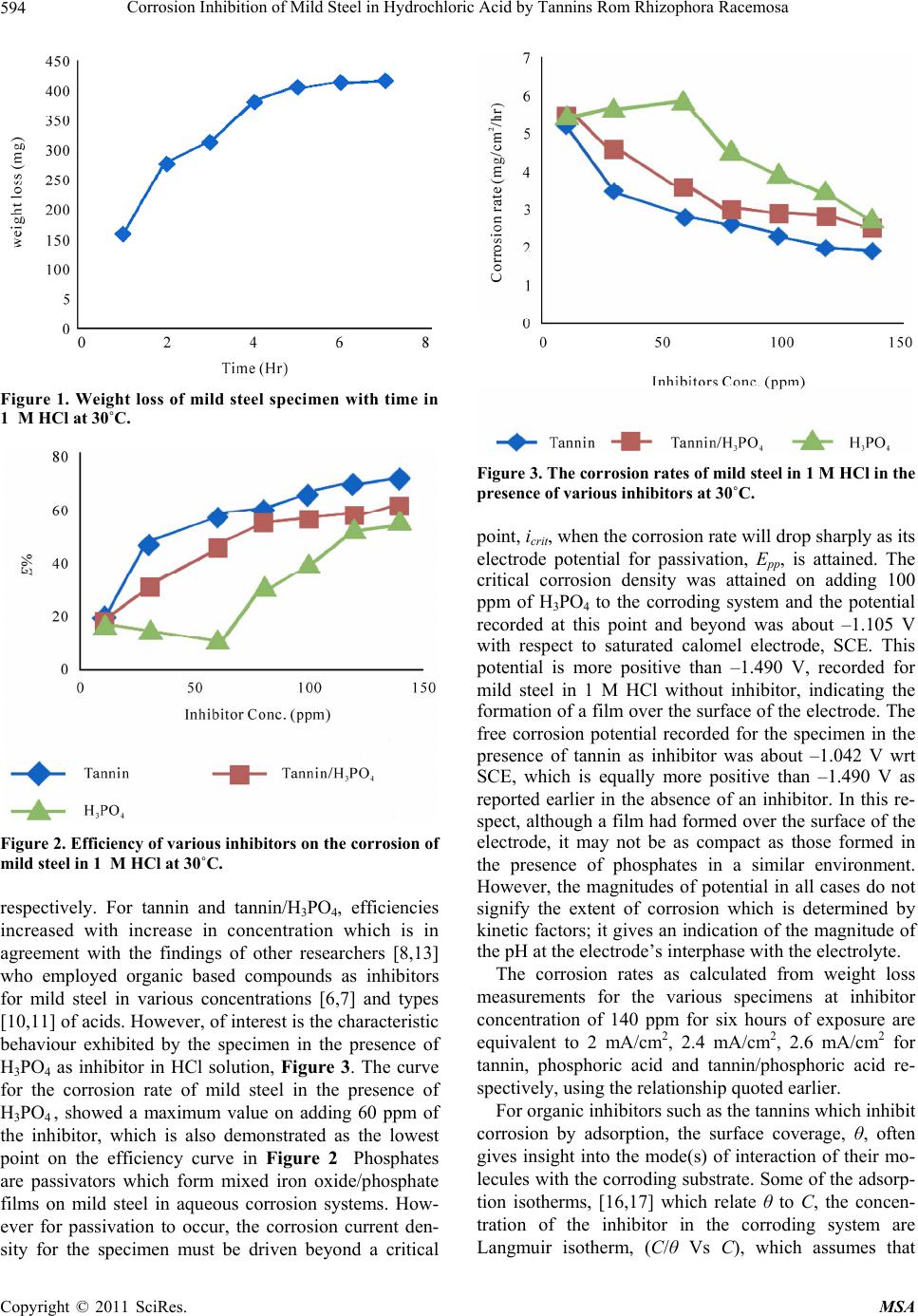 Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Tannins Rom Rhizophora Racemosa 594 Figure 1. Weight loss of mild steel specimen with time in 1 M HCl at 30˚C. Figure 2. Efficiency of various inhibitors on the corrosion of mild steel in 1 M HCl at 30˚C. respectively. For tannin and tannin/H3PO4, efficiencies increased with increase in concentration which is in agreement with the findings of other researchers [8,13] who employed organic based compounds as inhibitors for mild steel in various concentrations [6,7] and types [10,11] of acids. However, of interest is the characteristic behaviour exhibited by the specimen in the presence of H3PO4 as inhibitor in HCl solution, Figure 3. The curve for the corrosion rate of mild steel in the presence of H3PO4 , showed a maximum value on adding 60 ppm of the inhibitor, which is also demonstrated as the lowest point on the efficiency curve in Figure 2 Phosphates are passivators which form mixed iron oxide/phosphate films on mild steel in aqueous corrosion systems. How- ever for passivation to occur, the corrosion current den- sity for the specimen must be driven beyond a critical Figure 3. The corrosion rates of mild steel in 1 M HCl in the presence of various inhibitors at 30˚C. point, icrit, when the corrosion rate will drop sharply as its electrode potential for passivation, Epp, is attained. The critical corrosion density was attained on adding 100 ppm of H3PO4 to the corroding system and the potential recorded at this point and beyond was about –1.105 V with respect to saturated calomel electrode, SCE. This potential is more positive than –1.490 V, recorded for mild steel in 1 M HCl without inhibitor, indicating the formation of a film over the surface of the electrode. The free corrosion potential recorded for the specimen in the presence of tannin as inhibitor was about –1.042 V wrt SCE, which is equally more positive than –1.490 V as reported earlier in the absence of an inhibitor. In this re- spect, although a film had formed over the surface of the electrode, it may not be as compact as those formed in the presence of phosphates in a similar environment. However, the magnitudes of potential in all cases do not signify the extent of corrosion which is determined by kinetic factors; it gives an indication of the magnitude of the pH at the electrode’s interphase with the electrolyte. The corrosion rates as calculated from weight loss measurements for the various specimens at inhibitor concentration of 140 ppm for six hours of exposure are equivalent to 2 mA/cm2, 2.4 mA/cm2, 2.6 mA/cm2 for tannin, phosphoric acid and tannin/phosphoric acid re- spectively, using the relationship quoted earlier. For organic inh ibitors su ch as th e tann ins which inh ib it corrosion by adsorption, the surface coverage, θ, often gives insight into the mode(s) of interaction of their mo- lecules with the corroding substrate. Some of the adsorp- tion isotherms, [16,17] which relate θ to C, the concen- tration of the inhibitor in the corroding system are Langmuir isotherm, (C/θ Vs C), which assumes that Copyright © 2011 SciRes. MSA  Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Tannins Rom Rhizophora Racemosa Copyright © 2011 SciRes. MSA 595 there is no interaction between adsorbed molecules on the surface. Others are Temkin (θ Vs log C) and Frumkin (θ Vs C); these assume the effect of multiple layer and some interactions between molecules on the surface re- spectively. For tannin and phosphoric acid as inhibitors, the data derived from the gravimetric studies obeyed the Langmuir adsorption isotherm. However for tannin/ H3PO4, as inhibitor, none of the isotherms clearly defined its mode of interactions on the substrates. 4. Conclusions At a concentration of 140ppm, tannin has efficiency of 72%, tannin: H3PO4 61% and H3PO4 showed 55% effi- ciency in 1 M HCl. Mild steel specimens treated in H3PO4 were protected against atmospheric attack for three weeks while those treated in tannin resisted corrosion for one week. 5. Acknowledgements C. O Alaka acknowledges the Head of Department, De- partment of Pure and Industrial Chemistry, University of Port Harcourt for the use of laboratory facilities. REFERENCES [1] G.-I. Nonaka, “The Isolation and Structure Elucidation of Tannins,” Pure and Applied Chemistry, Vol. 6, No. 3, 1989, pp. 357-360. doi:10.1351/pac198961030357 [2] A. Veawab, P. Totinwachwuthikul and A. Chakma, “In- vestigation of Low-Toxic Corrosion Inhibitors for Carbon Dioxide Separation Process Using Aqueous MEA Sol- vent,” Industrial & Engineering Chemistry Research, Vol. 40, No. 22, 2001, pp. 4771-4777. doi:10.1021/ie010248c [3] B. Raja and M. G. Sethuraman, “Inhibitive Effect of Black Pepper Extract on the Sulphuric Acid Corrosion of Mild Steel,” Materials Letter, Vol. 62, No. 17-18, 2008, pp. 113-116. doi:10.1016/j.matlet.2007.04.079 [4] A. Bouyanzer, B. Hammouti and L. Majidi, “Pennyroyal Oil from Menthapulegium as Corrosion Inhibitor for Steel in 1 M HCl,” Material Letters, Vol. 60, No. 23, 2006, pp. 2840-2843. doi:10.1016/j.matlet.2006.01.103 [5] P. C. Okafor, E. E. Ebenso and U. J. Ekpe, “Äzadirachta Indica Extracts as Corrosion Inhibitor for Mild Steel in Acid Medium,” International Journal Electrochemical Science, Vol. 5, No. 7, 2010, pp. 978-984. [6] L. R. Chaulhan and G. Gunasekaran, “Corrosion Inhibi- tion of Mild Steel by Plant Extract in Dilute HCl Me- dium,” Corrosion Science, Vol. 49, No. 3, 2007, pp. 1143-1161. doi:10.1016/j.corsci.2006.08.012 [7] Y. Abed, B. Hammouti, F. Touhami, A. Aouniti, S. Kertit, A. Mansri and K. Elkacemi, “Poly (4-Vinylpyrindine) (P4VP) as Corrosion Inhibitors of Armco Iron in Molar Sulphuric Acid Solution,” Bulletin of Electrochemistry, Vol. 17, No. 3, 2001, pp. 105-108. [8] E. E. Oguzie, “Studies on the Inhibitive Effects of Oc- cimum Viridis Extracts on the Acid Corrosion Ofmild Steel,” Materials Chemistry and Physics, Vol. 99, No. 2-3, 2006, pp. 441-445. doi:10.1016/j.electacta.2004.04.030 [9] G. Gunasekaran and L. R. Chaulhan, “Ecofiendly Inhibi- tors for the Inhibition of Mild Steel in Phosphoric Acid Medium,” Electrochimica Acta, Vol. 49, No. 25, 2004, pp. 4387-4395. [10] M. Abdallah, E. E. Helal and A. S. Fouda, “Amino- Pyrimidine Derivatives as Inhibitors for Corrosion of 1018 Carbon Steel in Nitric Acid Solution,” Corrosion Science, Vol. 48, 2006, pp. 639-643. doi:10.1016/j.corsci.2005.06.020 [11] L. S. Moiseeva and Y. Y. Tur, “The Role of Combined Corrosion Inhibitor Contact Time in Neutral Aqueous Media,” Chemical and Petroleum Engineering, Vol. 59, No. 5-6, 2003, pp. 298-302. doi:10.1023/A:1025627502442 [12] C. M. Ojinnaka, “Tanner’s Guide to Nigerian Plants,” Leather Research Institute of Nigeria, Zaria, 1988. [13] E. Chaieb, A. Bouyanzer, B. Hammouti and M. Berrabh, “Limonene as Green Inhibitor for Steel Corrosion in HCl Solutions,” Acta Physico-Chimica Sinica, Vol. 25, No 7, 2009, pp. 254-258. [14] U. Rammelt, S. Kohler and G. Reinhard, “EIS Charac- terization of the Inhibition of Mild Steel Corrosion with Carboxylates in Neutral Aqueous Solution,” Electro- chimica Acta, Vol. 53, No. 23, 2008, pp. 6968-6972. doi:10.1016/j.electacta.2008.01.004 [15] E. Bardal, “Corrosion and Protection,” Springer Verlag London Ltd., London, 2004. [16] I. Langmuir, “The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum,” Journal of American Chemical Society, Vol. 40, No. 9, 1918, pp. 1361-1403. [17] A. N. Frumkin, “Electrocapillary Curve of Higher Ali- phatic Acids and the State Equation of the Surface Layer,” Physical Chemistry, Vol. 116, 1925, pp. 466-470. |

