Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 509-520 doi:10.4236/msa.2011.26069 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 509 Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, Characterization and Sustained Protein Release Studies Michel Bocourt Povea1, Waldo Argüelles Monal2, Juan Valerio Cauich Rodríguez3, Alejando May Pat3, Nancy Badas Rivero1, Carlos Peniche Covas1 1Department of Macromolecular Chemistry, University of Havana, Havana, Cuba; 2Center for Food and Development Research, Unit Guaymas, Sonora, México; 3Scientific Research Centre of Yucatan, Mérida, México. Email: bocourt@biomat.uh.cu Received March 1st, 2011; revised April 16th, 2011; accepted May 5th, 2011. ABSTRACT Interpenetrated polymer networks of chitosan (CHI), polyacrylic acid (PAA) and polyacrylamide (PAM) were prepared by free radical polymerization. These hydrogels were either washed with double distilled water (CHI/PAA/PAM) A or hydrolyzed with 1M sodium hydroxide (NaOH), (CHI/PAA/PAM) S. Both types of hydrogels were characterized by in- frared spectroscopy, microstructural techniques and compressive mechanical testing. Finally, hydrogels were loaded with bovine serum albumin (BSA) and release followed at different pHs. Infrared spectra analysis showed correspon- dence between hydrogels and monomer feed compositions. Hydrolyzed hydrogels, had increased water content and pH swelling dependence. Compression modulus of swelled hydrolyzed hydrogels decreased with increasing equilibrium water content. Higher BSA loadings were achieved on hydrolyzed hydrogels due to their high water content and poros- ity. Protein release from hydrogels was low ( 20% after 10 hours) at pH 1.2, but sustained release was observed at pH 6.8 and 7.4. The integrity of the protein released at 6.8 and 7.4 by hydrolyzed hydrogels was unaffected. The hydrogles showed no cytotoxic effects on human skin dermal fibroblasts as determined by MTT assay except for two compositions of (CHI/PAA/PAM) A samples, which after seven days presented a viability lower than 80% respect to the control. Keywords: Hydrogels, Controlled Release, Protein, Polyacrylamide, Interpenetrated Polymer 1. Introduction Hydrogels are three dimensional hydrophilic polymer networks that swell, but do not dissolve, when brought into contact with water [1]. Hydrogels have been actively studied, particularly those experiencing reversible vol- ume changes in response to external stimulus, such as pH, temperature and ionic concentration. These “smart” hy- drogels have found applications in biomedicine and bio- technology [2] including soft contact lenses [3], immobi- lization of enzymes and proteins [4], antibodies and an- tigens [5] and matrices for drug delivery systems [6,7]. The ability of these hydrogels to respond to their envi- ronment increase drug loading and provide protection from environmental conditions such as those found in the gastrointestinal tract [8]. In this regard, stimuli responsive hydrogels can be useful for the design of site-specific drug delivery devices; for instance, colon-specific drug delivery systems. Another important advantage of these hydrogels is that the active ingredient remains on the organ or tissue for longer times than conventional ones [9]. Intelligent hydrogels have also been prepared from in- terpenetrated polymer networks (IPNs), and it has been found that they combine high swelling capacity with in- creased mechanical properties [10,11]. Superabsorbent hydrogels of poly(acrylamide-co-acrylic acid) character- ized mainly by fast swelling and pH swelling dependence, ionic strength and composition have been prepared by a number of authors [12-14]. These materials have been proposed for different applications, such as waste water purification from textile industry [15], for the environ- mental analysis of Cu and Cd [16] and as mechanical 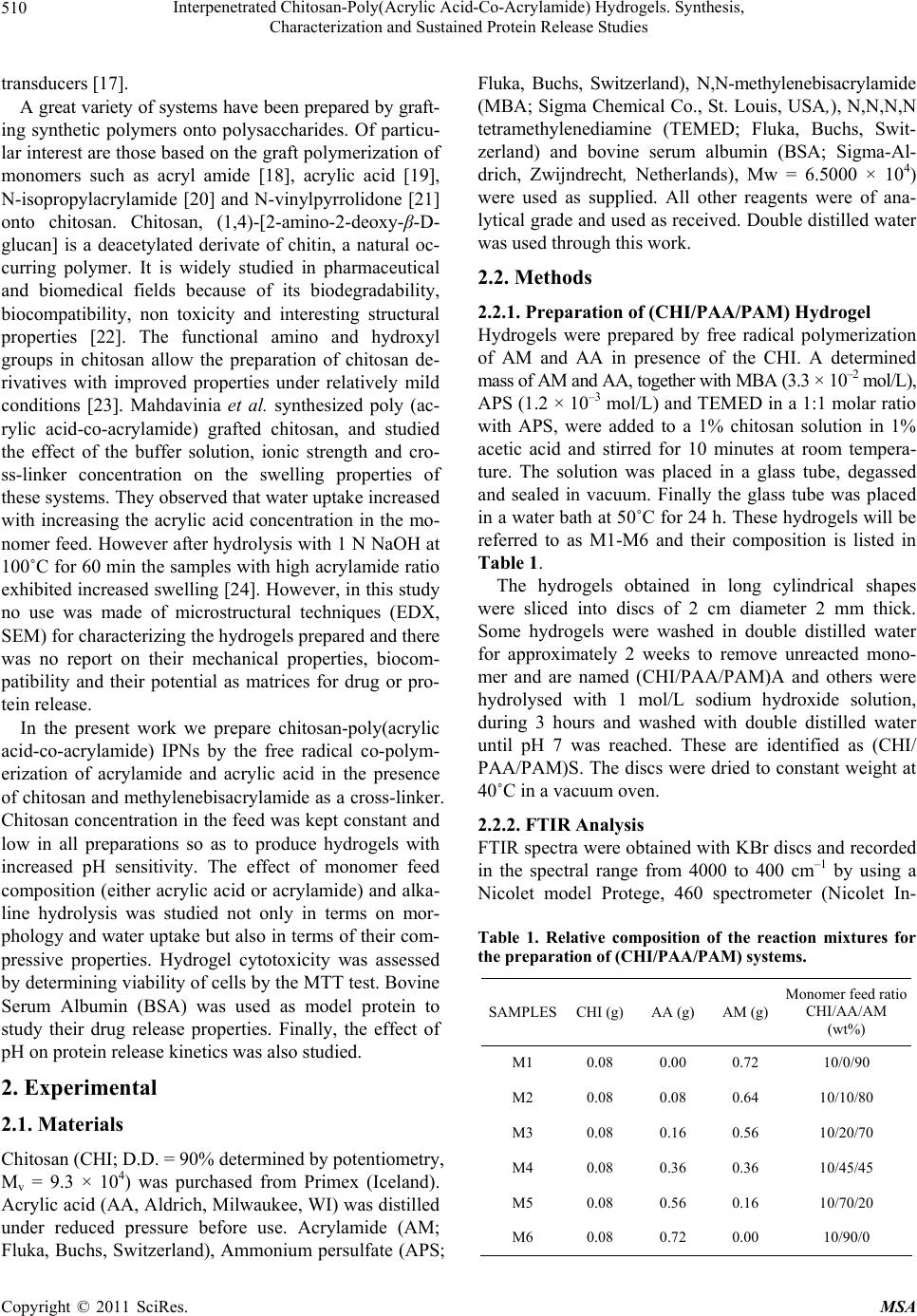 Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 510 Characterization and Sustained Protein Release Studies transducers [17]. A great variety of systems have been prepared by graft- ing synthetic polymers onto polysaccharides. Of particu- lar interest are those based on the graft polymerization of monomers such as acryl amide [18], acrylic acid [19], N-isopropylacrylamide [20] and N-vinylpyrrolidone [21] onto chitosan. Chitosan, (1,4)-[2-amino-2-deoxy-β-D- glucan] is a deacetylated derivate of chitin, a natural oc- curring polymer. It is widely studied in pharmaceutical and biomedical fields because of its biodegradability, biocompatibility, non toxicity and interesting structural properties [22]. The functional amino and hydroxyl groups in chitosan allow the preparation of chitosan de- rivatives with improved properties under relatively mild conditions [23]. Mahdavinia et al. synthesized poly (ac- rylic acid-co-acrylamide) grafted chitosan, and studied the effect of the buffer solution, ionic strength and cro- ss-linker concentration on the swelling properties of these systems. They observed that water uptake increased with increasing the acrylic acid concentration in the mo- nomer feed. However after hydrolysis with 1 N NaOH at 100˚C for 60 min the samples with high acrylamide ratio exhibited increased swelling [24]. However, in this study no use was made of microstructural techniques (EDX, SEM) for characterizing the hydrogels prepared and there was no report on their mechanical properties, biocom- patibility and their potential as matrices for drug or pro- tein release. In the present work we prepare chitosan-poly(acrylic acid-co-acrylamide) IPNs by the free radical co-polym- erization of acrylamide and acrylic acid in the presence of chitosan and methylenebisacrylamide as a cross-linker. Chitosan concentration in the feed was kept constant and low in all preparations so as to produce hydrogels with increased pH sensitivity. The effect of monomer feed composition (either acrylic acid or acrylamide) and alka- line hydrolysis was studied not only in terms on mor- phology and water uptake but also in terms of their com- pressive properties. Hydrogel cytotoxicity was assessed by determining viability of cells by the MTT test. Bovine Serum Albumin (BSA) was used as model protein to study their drug release properties. Finally, the effect of pH on protein release kinetics was also studied. 2. Experimental 2.1. Materials Chitosan (CHI; D.D. = 90% determined by potentiometry, Mv = 9.3 × 104) was purchased from Primex (Iceland). Acrylic acid (AA, Aldrich, Milwaukee, WI) was distilled under reduced pressure before use. Acrylamide (AM; Fluka, Buchs, Switzerland), Ammonium persulfate (APS; Fluka, Buchs, Switzerland), N,N-methylenebisacrylamide (MBA; Sigma Chemical Co., St. Louis, USA,), N,N,N,N tetramethylenediamine (TEMED; Fluka, Buchs, Swit- zerland) and bovine serum albumin (BSA; Sigma-Al- drich, Zwijndrecht, Netherlands), Mw = 6.5000 × 104) were used as supplied. All other reagents were of ana- lytical grade and used as received. Double distilled water was used through this work. 2.2. Methods 2.2.1. Preparation of (CHI/PAA/PAM) Hydrogel Hydrogels were prepared by free radical polymerization of AM and AA in presence of the CHI. A determined mass of AM and AA, together with MBA (3.3 × 10–2 mol/L), APS (1.2 × 10–3 mol/L) and TEMED in a 1:1 molar ratio with APS, were added to a 1% chitosan solution in 1% acetic acid and stirred for 10 minutes at room tempera- ture. The solution was placed in a glass tube, degassed and sealed in vacuum. Finally the glass tube was placed in a water bath at 50˚C for 24 h. These hydrogels will be referred to as M1-M6 and their composition is listed in Table 1. The hydrogels obtained in long cylindrical shapes were sliced into discs of 2 cm diameter 2 mm thick. Some hydrogels were washed in double distilled water for approximately 2 weeks to remove unreacted mono- mer and are named (CHI/PAA/PAM)A and others were hydrolysed with 1 mol/L sodium hydroxide solution, during 3 hours and washed with double distilled water until pH 7 was reached. These are identified as (CHI/ PAA/PAM)S. The discs were dried to constant weight at 40˚C in a vacuum oven. 2.2.2. FTIR Analysis FTIR spectra were obtained with KBr discs and recorded in the spectral range from 4000 to 400 cm–1 by using a Nicolet model Protege, 460 spectrometer (Nicolet In- Table 1. Relative composition of the reaction mixtures for the preparation of (CHI/PAA/PAM) systems. SAMPLESCHI (g)AA (g) AM (g) Monomer feed ratio CHI/AA/AM (wt%) M1 0.08 0.00 0.72 10/0/90 M2 0.08 0.08 0.64 10/10/80 M3 0.08 0.16 0.56 10/20/70 M4 0.08 0.36 0.36 10/45/45 M5 0.08 0.56 0.16 10/70/20 M6 0.08 0.72 0.00 10/90/0 Copyright © 2011 SciRes. MSA  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 511 Characterization and Sustained Protein Release Studies strument Corp., Madison, WI). Spectra were obtained with a resolution of 2 cm–1 and were averaged over 100 scans. 2.2.3. Swelling Studies The discs were cut into fragments of about 30 mg and weighed accurately. These fragments were placed into flasks with 10 mL solution of a given pH and kept in a thermostated bath at 37˚C. Solutions with pH 1.2 (simu- lated gastric fluid), pH 7.4 (phosphate buffered saline), and pH 6.8 (double distilled water) were prepared. The water uptake, W, was calculated by measuring the weight gain of the sample at different times after carefully wip- ing the surface with a filter paper. It was reported as 0 0 t WW WW (1) where W0 is the weight of the dry sample and Wt is that of the sample at time t. 2.2.4. Scanning Electron Microscopy (SEM) The morphology of the hydrogels was determined using a scanning electron microscope (SEM-JEOL JSM- 6360LV, Japan). Small pieces of swelled gels were freeze-dried to avoid the collapse of porous structure. Then they were cut with a knife to expose the inner sur- face. Samples were placed on an aluminum mount, sput- tered with gold palladium, and then scanned at an accel- erating voltage of 15 kV. 2.2.5. Mechanical Properties For the determination of compressive properties, fully swollen hydrogels were cut into cylindrical species of 6 mm diameter and 12 mm length measured with a Mitu- toyo digital caliper. Hydrogels were confined in a cylin- drical metal frame and compressed at 0.5 mm/min, using a Minimat testing machine with a 10 N load cell. The stress (σ) and strain (ε) were obtained according to Equa- tions (2) and (3). P A (2) 0 F l l (3) where P represents the compression force, A the area of the cylinder and l0 and lF are the initial and final length, respectively. From the initial portion of the stress-strain curve, the modulus was obtained and the values reported as the mean ± SD of six determinations. 2.2.6. Elemental Analysis Elemental analysis (C, N, O and Na) was conducted with an INCA 7582 Elemental Microanalyzer (Oxford In- struments, UK) coupled to the Jeol JSM-6360LV SEM. 2.2.7. BSA Release Studies 2.2.7.1. BSA Loading Dried sample discs weighing 30 mg were placed in vials containing 10 mL of aqueous solutions of BSA (1.0 mg/ mL). Then, vials were placed in a temperature–regulated incubator at 4˚C. After 7 days, hydrogels were separated from the solutions and freeze-dried. The concentration of the supernatant solutions were analyzed with the modi- fied Coomassie Blue protein assay (Biorad) using UV spectroscopy at 595 to determine the amount of BSA loaded in each hydrogel disc [25]. The BSA load in hy- drogels was determined from the difference in the solu- tions concentrations before and after incubation. All ex- periments were performed in triplicate. 2.2.7.2. In Vitro Protein Release Study Release of BSA was carried out as established by the USP XXIV Standard. Three different release media were tested: simulated gastric fluid (SGF), consisting of NaCl (2.0 g), HCl (7 mL) and pH adjusted to 1.2 ± 0.5; simu- lated intestinal fluid (SIF), consisting of KH2PO4 (6.8 g), 0.2 N NaOH (77 mL), and pH adjusted to 6.8 ± 0.1. Phosphate buffered saline (PBS) 7.4 was prepared with deionized water. For release studies the BSA–loaded hydrogel discs were suspended in 10 mL of these solu- tions at 37˚C in a ZHICHENG ZHWX-200B Incubator with reciprocating motion (100 rpm). At predetermined time intervals, 200 µl aliquots were removed and simul- taneously replenished by 200 µl of fresh solutions to maintain sink conditions. Protein content of each sample was analyzed with the modified Coomassie Blue protein assay (Biorad) using UV spectroscopy at 595 nm. A ca- libration curve was generated at each time interval using a non-loaded gel in order to correct for the intrinsic ab- sorbance of the polymer. All experiments were per- formed in triplicate. 2.2.7.3. Analysis of Released Proteins by Gel Electrophoresis (SDS-PAGE) The structural integrity of BSA released from CHI/PAA/ PAM) hydrogels was analyzed by SDS-PAGE in 10% acrylamide gels according to Laemli [26] using a Mini- Protean II Cell (Bio-Rad Laboratories Hercules, CA, USA). BSA solutions were directly loaded into the test wells with a micropipette, and the electrophoresis was performed at 100 V. The gel was stained with 0.1% AgNO3 to visualize protein bands. The study was con- ducted according to the manufacturer’s protocol. The gel pictures were taken with a scanner after wiping off all the water from the gel membrane. Copyright © 2011 SciRes. MSA  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 512 Characterization and Sustained Protein Release Studies 2.3. Cytotoxicity Test 2.3.1. In Vitro Cell Culture for Biocompatibility Experiment Sterile hydrogel pieces M1-M6 (CHI/PAA/PAM)A and M1-M6 (CHI/PAA/PAM)S of uniform weight (50 mg each) were used for biocompatibility experiments. The negative control was tissue culture plastic, Thermanox (TMX), in discs of 13 mm diameter (Nunc) and the posi- tive control (toxic agent) was Triton-1% (Merck). Ex- periments were carried out using human skin dermal fi- broblasts (Pharmakine DPK-SKDF-H). Cells were cul- tured at 37˚C and 5% CO2. The culture medium was Dulbecco’s modified Eagle’s medium (DMEM), modi- fied with HEPES (Sigma), rich in glucose and supple- mented with 10% (v/v) fetal bovine serum (FBS; Gibco), 200 mM glutamine (Sigma), 1% of penicillin-streptomycin solution (10000 Units/mL penicillin and 10 mg/mL streptomycin, Sigma). The culture medium was changed at selected time intervals with care to cause little distur- bance to culture conditions. 2.3.2. MTT Assay TMX, M1-M6 (CHI/PAA/PAM)A and M1-M6 (CHI/ PAA/PAM)S discs were set up in 5 mL of DMEM. They were placed on a roller mixer at 37˚C and the medium was removed at different time periods (8 h, 1, 4, 7 days) [27] and replaced with other 5 mL of fresh medium. All the extracts were obtained under sterile conditions. Fi- broblasts cells were seeded at a density of 105 cells/mL in complete medium in a sterile 96-well culture plate and incubated to confluence. Then, the medium was replaced with the corresponding eluted extract and incubated at 37˚C for 24 h. A solution of MTT was prepared in warm PBS (0.5 mg/mL; Sigma) and the plates were incubated at 37˚C for 4 h. Excess medium and MTT were removed and 100 μl dimethylsulphoxide (DMSO; Sharlau) was added to all wells in order to dissolve the MTT taken up by the cells. This was mixed for 10 min and the absorb- ance was measured with a Biotek SYNERGY spectro- photometer using a test wave length of 570 nm and a reference wave length of 630 nm. From the values of relative cell viability a statistical analysis was carried out using Student t test in order to detect significant differ- ences between the distribution of measured values for the negative control and those obtained for the experimental groups studied. 3. Results and Discussion 3.1. Synthesis and Spectral Characterization The free radical polymerization of AA and AM in the presence of chitosan results in a hydrogel in which chi- tosan chains become grafted with the copolymer [19,24]. The use of the difunctional monomer MBA in the react- ing mixture promotes the formation of a tridimensional interpenetrated polymer network reinforcing the me- chanical properties of the hydrogel as well as restraining its swelling capacity [28]. During the alkaline treatment, amide groups are hydrolyzed into carboxylate ions. The FTIR spectra of CHI, AM, AA, (CHI/PAA/PAM)A and (CHI/PAA/PAM)S for composition corresponding to M5 are shown in Figure 1(A). The spectra of hydrogels at other compositions were qualitatively similar to that of M5 and are not shown. Chitosan spectrum exhibited the distinctive absorption bands at 1658 cm–1 (Amide I), 1595 cm–1 (–NH2 bending) and 1314 cm–1 (Amide III). The absorption bands at 1154 cm–1 (anti-symmetric stretching of the C–O–C bri- dge), 1082 and 1032 cm–1 (skeletal vibrations involving the C–O stretching) are characteristic of its saccharide structure [29]. The IR spectrum of PAA exhibits the characteristic absorption band at 1728 cm–1 due to the C=O stretching vibration of the carboxylic groups. The intense band at 1670 cm–1 in the spectrum of PAM corresponds to the C=O stretching vibration (Amide I). The main absorption bands of the CHI, AM, AA infrared spectra, appeared also in (CHI/PAA/PAM)A and (CHI/PAA/PAM)S spec- tra. The bands at 1558 cm–1 (asymmetric COO– stretch- ing) and 1406 cm–1 (symmetric COO– stretching) present in the (CHI/PAA/PAM) S spectrum result from the alka- line hydrolysis of the amide groups of AM into carboxy- late ions. Since the strong absorption band at 1082 cm–1 in the chitosan spectrum is absent in the spectrum of PAM, the ratio of the intensity of the absorption band at 1630 cm–1 (A1630) to the intensity of the absorption band at 1082 cm–1 (A1082) was used as indicative of the composition of the sample. As it can be seen in Figure 1(B) the absorp- tion bands ratio A1670/A1082 is very sensitive to the AM composition of the systems i.e. it decreased when the concentration of AA increased in the reaction mixture. This trend indicates that the proportion of AA in the hy- drogels increased from M1 to M6, as expected from the monomer feed composition. Furthermore, the values of the ratio A1670/A1082 for (CHI/PAA/PAM)S hydrogels were smaller than those of the corresponding (CHI/PAA/ PAM)A samples indicating that hydrolysis of the amide groups on PAM to carboxylate ions was effectively achieved by the treatment with NaOH. EDX analysis of hydrogels corroborated the IR spec- troscopy results. Figures 2(a) and (b) show the EDX of the sample M4 (CHI/PAA/PAM)A and M4 (CHI/PAA/ PAM)S, respectively, which are representative of the Copyright © 2011 SciRes. MSA 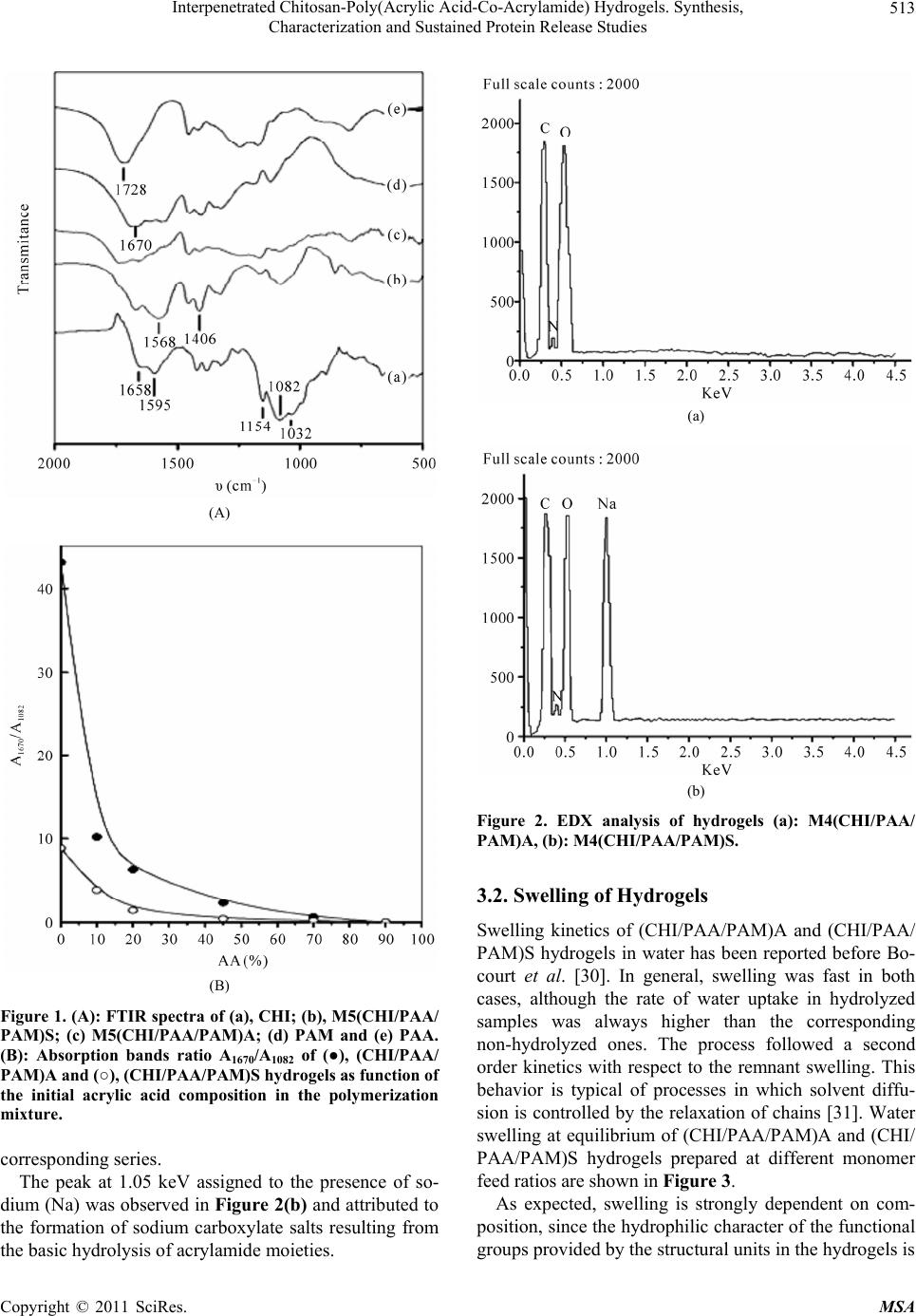 Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 513 Characterization and Sustained Protein Release Studies (A) (B) Figure 1. (A): FTIR spectra of (a), CHI; (b), M5(CHI/PAA/ PAM)S; (c) M5(CHI/PAA/PAM)A; (d) PAM and (e) PAA. (B): Absorption bands ratio A1670/A1082 of (●), (CHI/PAA/ PAM)A and (○), (CHI/PAA/PAM)S hydrogels as function of the initial acrylic acid composition in the polymerization mixture. corresponding series. The peak at 1.05 keV assigned to the presence of so- dium (Na) was observed in Figure 2(b) and attributed to the formation of sodium carboxylate salts resulting from the basic hydrolysis of acrylamide moieties. (a) (b) Figure 2. EDX analysis of hydrogels (a): M4(CHI/PAA/ PAM)A, (b): M4(CHI/PAA/PAM)S. 3.2. Swelling of Hydrogels Swelling kinetics of (CHI/PAA/PAM)A and (CHI/PAA/ PAM)S hydrogels in water has been reported before Bo- court et al. [30]. In general, swelling was fast in both cases, although the rate of water uptake in hydrolyzed samples was always higher than the corresponding non-hydrolyzed ones. The process followed a second order kinetics with respect to the remnant swelling. This behavior is typical of processes in which solvent diffu- sion is controlled by the relaxation of chains [31]. Water swelling at equilibrium of (CHI/PAA/PAM)A and (CHI/ PAA/PAM)S hydrogels prepared at different monomer feed ratios are shown in Figure 3. As expected, swelling is strongly dependent on com- position, since the hydrophilic character of the functional groups provided by the structural units in the hydrogels is Copyright © 2011 SciRes. MSA 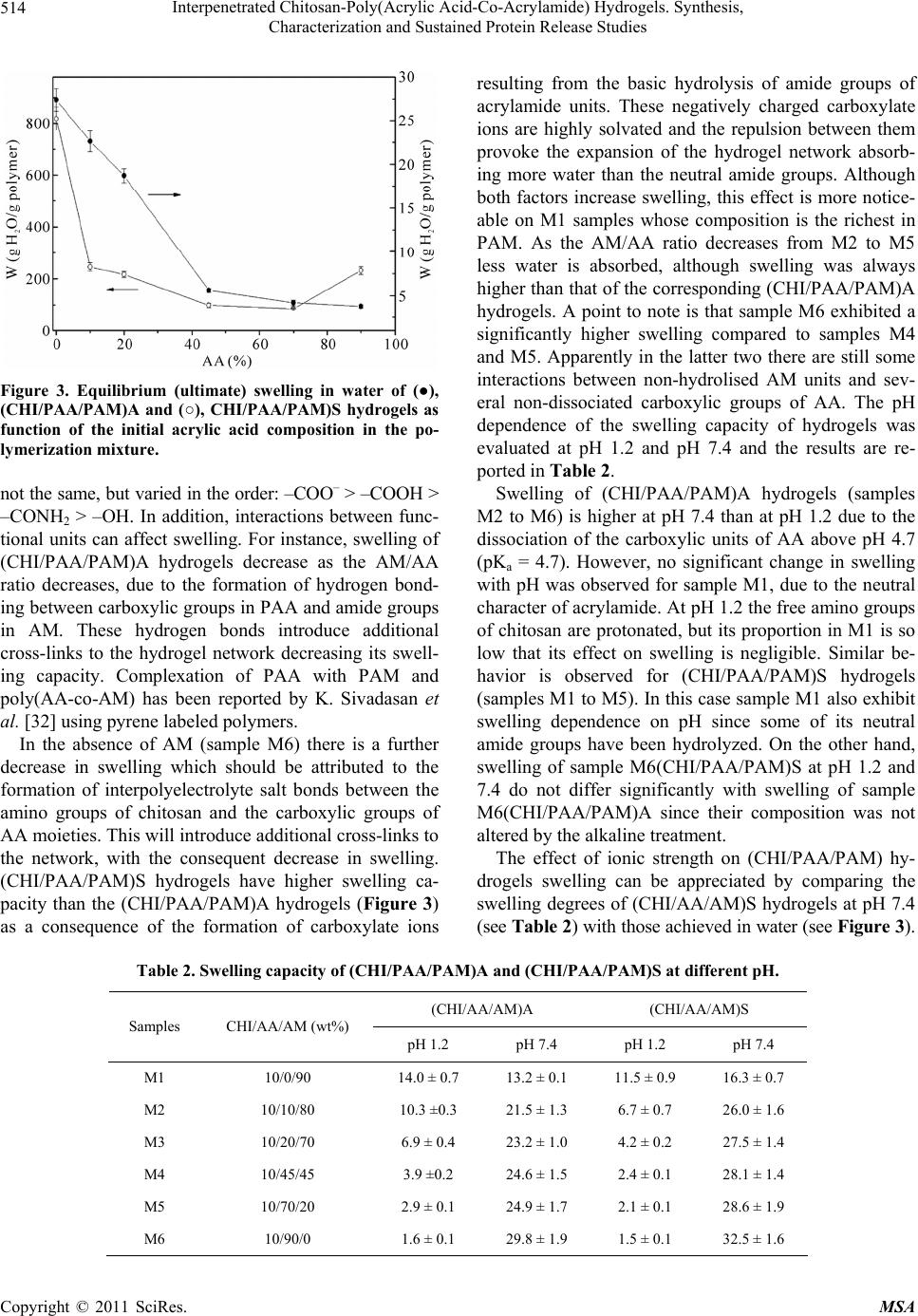 Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, Characterization and Sustained Protein Release Studies Copyright © 2011 SciRes. MSA 514 resulting from the basic hydrolysis of amide groups of acrylamide units. These negatively charged carboxylate ions are highly solvated and the repulsion between them provoke the expansion of the hydrogel network absorb- ing more water than the neutral amide groups. Although both factors increase swelling, this effect is more notice- able on M1 samples whose composition is the richest in PAM. As the AM/AA ratio decreases from M2 to M5 less water is absorbed, although swelling was always higher than that of the corresponding (CHI/PAA/PAM)A hydrogels. A point to note is that sample M6 exhibited a significantly higher swelling compared to samples M4 and M5. Apparently in the latter two there are still some interactions between non-hydrolised AM units and sev- eral non-dissociated carboxylic groups of AA. The pH dependence of the swelling capacity of hydrogels was evaluated at pH 1.2 and pH 7.4 and the results are re- ported in Table 2. Figure 3. Equilibrium (ultimate) swelling in water of (●), (CHI/PAA/PAM)A and (○), CHI/PAA/PAM)S hydrogels as function of the initial acrylic acid composition in the po- lymerization mixture. not the same, but varied in the order: –COO– > –COOH > –CONH2 > –OH. In addition, interactions between func- tional units can affect swelling. For instance, swelling of (CHI/PAA/PAM)A hydrogels decrease as the AM/AA ratio decreases, due to the formation of hydrogen bond- ing between carboxylic groups in PAA and amide groups in AM. These hydrogen bonds introduce additional cross-links to the hydrogel network decreasing its swell- ing capacity. Complexation of PAA with PAM and poly(AA-co-AM) has been reported by K. Sivadasan et al. [32] using pyrene labeled polymers. Swelling of (CHI/PAA/PAM)A hydrogels (samples M2 to M6) is higher at pH 7.4 than at pH 1.2 due to the dissociation of the carboxylic units of AA above pH 4.7 (pKa = 4.7). However, no significant change in swelling with pH was observed for sample M1, due to the neutral character of acrylamide. At pH 1.2 the free amino groups of chitosan are protonated, but its proportion in M1 is so low that its effect on swelling is negligible. Similar be- havior is observed for (CHI/PAA/PAM)S hydrogels (samples M1 to M5). In this case sample M1 also exhibit swelling dependence on pH since some of its neutral amide groups have been hydrolyzed. On the other hand, swelling of sample M6(CHI/PAA/PAM)S at pH 1.2 and 7.4 do not differ significantly with swelling of sample M6(CHI/PAA/PAM)A since their composition was not altered by the alkaline treatment. In the absence of AM (sample M6) there is a further decrease in swelling which should be attributed to the formation of interpolyelectrolyte salt bonds between the amino groups of chitosan and the carboxylic groups of AA moieties. This will introduce additional cross-links to the network, with the consequent decrease in swelling. (CHI/PAA/PAM)S hydrogels have higher swelling ca- pacity than the (CHI/PAA/PAM)A hydrogels (Figure 3) as a consequence of the formation of carboxylate ions The effect of ionic strength on (CHI/PAA/PAM) hy- drogels swelling can be appreciated by comparing the swelling degrees of (CHI/AA/AM)S hydrogels at pH 7.4 (see Table 2) with those achieved in water (see Figure 3). Table 2. Swelling capacity of (CHI/PAA/PAM)A and (CHI/PAA/PAM)S at different pH. (CHI/AA/AM)A (CHI/AA/AM)S Samples CHI/AA/AM (wt%) pH 1.2 pH 7.4 pH 1.2 pH 7.4 M1 10/0/90 14.0 ± 0.7 13.2 ± 0.1 11.5 ± 0.9 16.3 ± 0.7 M2 10/10/80 10.3 ±0.3 21.5 ± 1.3 6.7 ± 0.7 26.0 ± 1.6 M3 10/20/70 6.9 ± 0.4 23.2 ± 1.0 4.2 ± 0.2 27.5 ± 1.4 M4 10/45/45 3.9 ±0.2 24.6 ± 1.5 2.4 ± 0.1 28.1 ± 1.4 M5 10/70/20 2.9 ± 0.1 24.9 ± 1.7 2.1 ± 0.1 28.6 ± 1.9 M6 10/90/0 1.6 ± 0.1 29.8 ± 1.9 1.5 ± 0.1 32.5 ± 1.6  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 515 Characterization and Sustained Protein Release Studies This occurs because the ionic strength provided by the phosphate buffered saline (I = 0.2) hampers swelling in spite of the higher dissociation degree expected for the carboxylic groups of AA at pH 7.4. In this case, charge screening of the counterions on the fixed charges of the polymer network, leads to a decrease in the osmotic pressure [33]. 3.3. Morphologic Studies Figure 4 shows the SEM pictures of M4(CHI/PAA/ PAM)A and M4(CHI/PAA/PAM)S hydrogels. From these pictures, the higher porosity of the hydrolyzed sample is evident. The same morphologies were ob- served for hydrogels prepared at other compositions. This increased porosity allows faster water diffusion through the hydrogel network which in turn is another factor that contributes to the higher rate of water uptake observed in hydrolyzed samples. It is important to men- tion that even when elimination of water by different means leads to different structures, in this case the same method was used for all hydrogels. (a) (b) Figure 4. SEM pictures of M4 hydrogels: (a) M4(CHI/ PAA/PAM)A and (b) (CHI/PAA/PAM)S. 3.4. Mechanical Properties Hydrogels can swell to several hundred times within a few minutes. However, the modulus of hydrogels will decrease when decreasing polymer fraction, as predicted by rubber elasticity theory. Therefore, highly swelled hydrogels are usually mechanically poor and difficult to handle without breaking. This is an obvious limitation for applications requiring mechanical performance like soft tissue replacement and addition to drug release matrices. In relation to this, the preparation of semi-interpenetrated or interpenetrated polymer networks is a suitable proce- dure to achieve superabsorbent hydrogels with better mechanical properties. The improvement of the me- chanical properties of IPN hydrogels is due to an increase in polymer mass as well as inter- and intra-molecular interactions from the incorporation of the second network into the normal hydrogel network. The initial compres- sion modulus of (CHI/PAA/PAM)S systems with differ- ent composition at equilibrium swelling are shown in Table 3. As expected, the compression modulus decreases in the sequence M5 M4 M3 M6 M2 M1, i.e. by increasing hydrogel water content. The values of the compression moduli found for (CHI/PAA/PAM)S hy- drogels are of the same order of magnitude as those re- ported by Tang et al. [34] for high mechanical strength (PAA/PAM) IPN hydrogels. 3.5. BSA Incorporation and in Vitro Protein Release Therapeutic protein administration usually faces the need of frequent doses due to the small residence time in blood of the protein. A promising strategy to overcome this drawback is the development of sustained drug re- lease systems based on hydrogel matrices loaded with the therapeutic drug [35]. Loading capacities for BSA of (QUI/PAA/PAM)A and (QUI/PAA/PAM)S are reported in Table 4, together with Table 3. Compression modulus and swelling capacity in water of (CHI/PAA/PAM)S hydrogels. SAMPLES COMPRESSION MODULUS (kPa) SWELLING (g H2O/g polymer) M1 45 ± 2 819 ± 45 M2 68 ± 2 246 ± 16 M3 84 ± 3 216 ±11 M4 110 ± 7 99 ± 8 M5 122 ± 9 86 ± 2 M6 76 ± 4 231 ± 16 Copyright © 2011 SciRes. MSA  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 516 Characterization and Sustained Protein Release Studies Table 4. Protein load and swelling of (CHI/PAA/PAM)A and (CHI/PAA/PAM)S hydrogels in 1 mg/mL BSA solution. (CHI/PAA/PAM)A (CHI/PAA/PAM)S Samples Load (mg/g) Swelling (g H2O/g polymer) Load (mg/g) Swelling (g H2O/g polymer) M1 232.2 25.7 511.3 677.8 M2 202.2 20.8 618.3 210.7 M3 141.9 15.3 730.4 166.5 M4 0 4.2 323.6 72.5 M5 0 3.1 200.6 63.8 M6 0 1.9 172.7 185.6 the water uptake of hydrogels in these experimental con- ditions. In the case of (QUI/PAA/PAM)A hydrogels only samples M1 to M3 were able to incorporate the protein. Apparently, both low swelling and small pore sizes on samples M4 to M6 did not allow BSA incorporation. On the other hand, highly swollen (QUI/PAA/PAM)S hydrogels incorporated BSA at all compositions. Higher loadings were achieved for samples with lower AA con- tent (M1 to M3) while samples M4 and M5 which are richer in AA content, exhibited lower swelling. The cause of this should be the higher density of carboxylate groups these samples should posses in the surface, which could hamper BSA adsorption by anionic electrostatic repulsion between the protein and the hydrogel surface. This explains why sample M6 (which is essentially a CHI/PAA hydrogel) having a similar swelling degree as sample M3 exhibits only 24% its BSA loading capacity. A similar effect was reported by Karadag et al. [36] for BSA adsorption on acrylamide-itaconic acid hydrogels. BSA release from (CHI/PAA/PAM) hydrogels was studied at three different pHs: pH 1.2 (SGF); pH 6.8, (SIF), and pH 7.4 (PBS). Figure 5 shows the in vitro cumulative release profiles of the BSA loaded (CHI/ PAA/PAM)A hydrogels. At pH 1.2 protein release is favored in AM richer samples (M1 > M2 > M3), since in acid medium the hydrogels containing AA units become more compact due to hydrogen bonding of carboxylic groups of AA with the amide groups of AM units. However, a considerable amount of BSA remains trapped in the gels at pH 1.2, presumably due to interac- tions between the protein and the polymer. At pH 6.8 and 7.4 the carboxylic groups of AA are dissociated (pKa = 4.7), and the consequent expansion of the gels together with the electrostatic repulsive force between the (COO–) charged sites of AA and BSA (pK = 4.8) favours protein release, reaching 80 per cent at pH 6.8 and almost 100 per cent at H 7.4. It is worth pointing out that BSA re- lease form (CHI/PAA/PAM) hydrogels was relatively fast at pH 6.8 and 7.4 because after 10 hours it reached almost 100 per cent. On the other hand, BSA release from (QUI/PAA/ PAM)S hydrogels (Figure 6) at pH 1.2 was even lower than that of (CHI/PAA/PAM)A samples (about 20 per cent for all compositions). This is to be expected since at this pH they should be more compact due to poorer swelling (see Table 2) than (CHI/PAA/PAM)A hydrogels. At pH 6.8 and 7.4 protein release resulted slower and more composition dependent for (QUI/PAA/PAM)S than for (QUI/PAA/PAM)A hy- drogels. BSA release increased with increasing AA con- tent (M1 < M2 < M3 < M4 < M5 < M6). Best release profiles were obtained at pH 7.4 for samples M4 to M6, which after liberating approximately 20% of the protein during the first hour exhibited sustained release for the 48 hours of the experiment. 3.6. BSA Structural Integrity The effect of the loading-release process on the integrity of BSA was studied, since it may have affected the pro- tein structure and stability. Possible detrimental effects include protein denaturation, aggregation and hydrolysis. Experiments were carried out on the BSA released from (QUI/PAA/PAM)A and (QUI/PAA/PAM)S hydrogels after 2 days and compared with the original BSA in solu- tion (a BSA standard). Figure 7 shows the SDS-PAGE results of the released and commercial BSA. Molecular weight markers are shown in lane I. The SDS-PAGE gel banding patterns of the BSA released from the (QUI/PAA/PAM)A hydrogels at pH 1.2, 6.8 and 7.4 are shown in lanes II, III and IV respectively. Similarly the protein released from the (QUI/PAA/PAM)S hydrogels at pH 1.2, 6.7 and 7.4 ap- pear in lanes V, VI and VII, respectively and the com- mercial BSA, lane VIII, exhibited clear bands at 66 KDa. The presence of bands of lower molecular weight species in lanes II, III and V indicates appreciable modification Copyright © 2011 SciRes. MSA 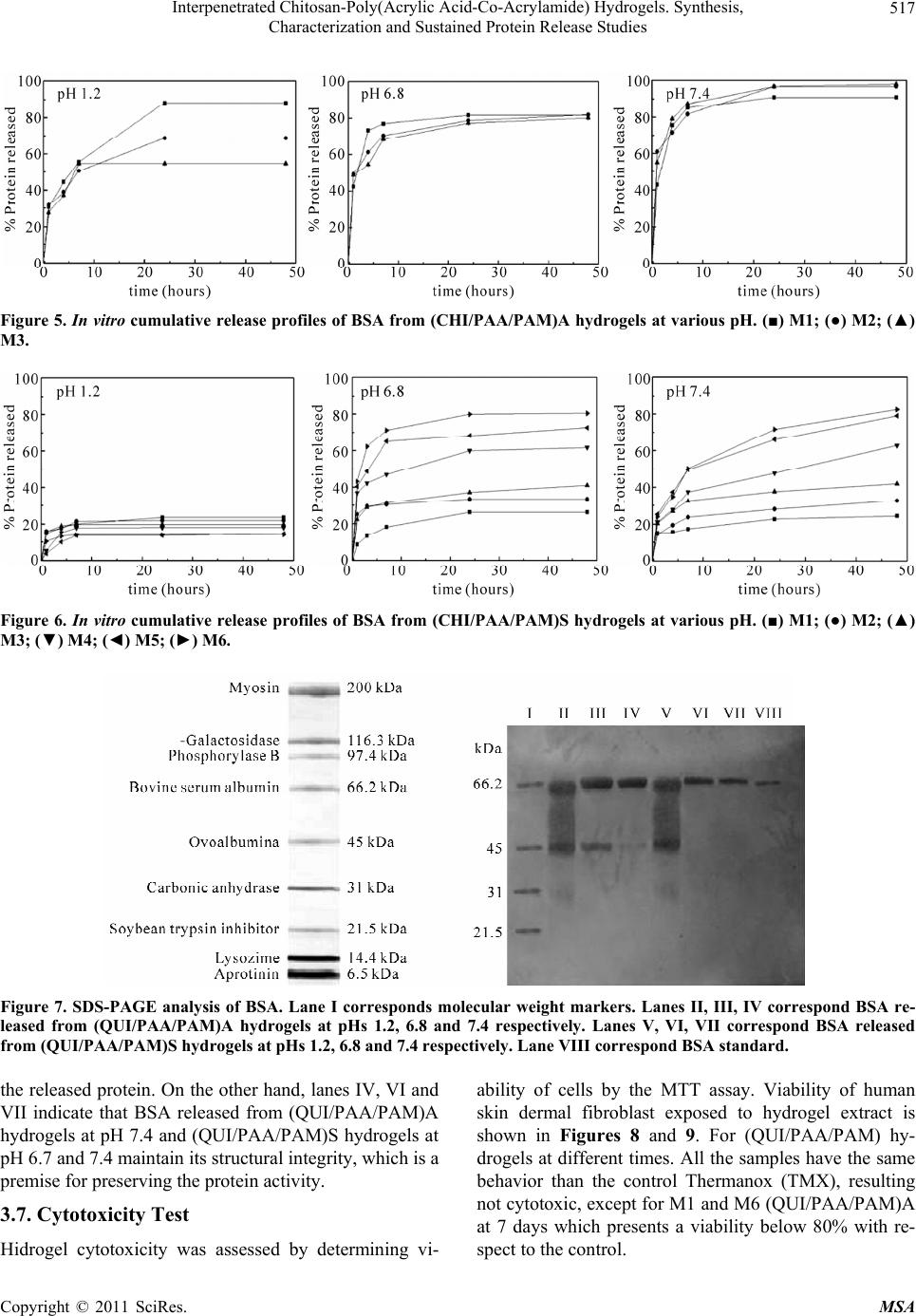 Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 517 Characterization and Sustained Protein Release Studies Figure 5. In vitro cumulative release profiles of BSA from (CHI/PAA/PAM)A hydrogels at various pH. (■) M1; (●) M2; (▲) M3. Figure 6. In vitro cumulative release profiles of BSA from (CHI/PAA/PAM)S hydrogels at various pH. (■) M1; (●) M2; (▲) M3; (▼) M4; (◄) M5; (►) M6. Figure 7. SDS-PAGE analysis of BSA. Lane I corresponds molecular weight markers. Lanes II, III, IV correspond BSA re- leased from (QUI/PAA/PAM)A hydrogels at pHs 1.2, 6.8 and 7.4 respectively. Lanes V, VI, VII correspond BSA released from (QUI/PAA/PAM)S hydrogels at pHs 1.2, 6.8 and 7.4 respectively. Lane VIII correspond BSA standard. the released protein. On the other hand, lanes IV, VI and VII indicate that BSA released from (QUI/PAA/PAM)A hydrogels at pH 7.4 and (QUI/PAA/PAM)S hydrogels at pH 6.7 and 7.4 maintain its structural integrity, which is a premise for preserving the protein activity. 3.7. Cytotoxicity Test Hidrogel cytotoxicity was assessed by determining vi- ability of cells by the MTT assay. Viability of human skin dermal fibroblast exposed to hydrogel extract is shown in Figures 8 and 9. For (QUI/PAA/PAM) hy- drogels at different times. All the samples have the same behavior than the control Thermanox (TMX), resulting not cytotoxic, except for M1 and M6 (QUI/PAA/PAM)A at 7 days which presents a viability below 80% with re- pect to the control. s Copyright © 2011 SciRes. MSA  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 518 Characterization and Sustained Protein Release Studies Figure 8. Results of MTT test for M1-M6 (CHI/PAA/PAM)A discs. Values are average ± standard deviation for n = 8. *p < 0.05 and ***p < 0.001 with respect to TMX. Figure 9. Results of MTT test for M1-M6 (CHI/PAA/PAM)S discs. Values are average ± standard deviation for n = 8. *p < 0.05 and ***p < 0.001 with respect to TMX. This could be related with the possible presence of re- sidual monomer in these systems, M1 richest in AM monomer and M6 richest in AA monomer that inde- pendently of the exhaustive washings with double dis- tilled water could have remained present. 4. Conclusions Interpenetrated chitosan-poly(acrylic acid-co-acrylamide) hydrogels with different compositions were synthesized by the free radical polymerization of AM and AA in presence of the CHI and MBA. Hydrogels swelling was controlled by the interaction between the amino groups of AM and the carboxylic groups of AA. Therefore, wa- ter uptake decreased with increasing AA concentration in the polymer and was also higher at pH 7.4. After treat- ment with 0.1 N NaOH swelling capacity increased con- Copyright © 2011 SciRes. MSA  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, 519 Characterization and Sustained Protein Release Studies siderably due to AM hydrolysis but decreased considera- bly with increasing ionic strength. Mechanical properties of these systems can be tailored by adjusting hydrogel composition i.e. for high mechanical strength hydrolysed hydrogels prepared with 0.56 g AA, 0.16 g Am and 0.08 g CHI should be preferred. BSA loading capacity was higher in (CHI/PAA/PAM)S hydrogels due to their high water content and greater porosity. Hydrogels were not cytotoxic for both systems except for samples M1(QUI/PAA/PAM)A and M6(QUI/PAA/ PAM)A. BSA release experiments showed that these hydrogels (hydrolysed and non hydrolysed) are capable of sustained release at pH 6.8 and 7.4 after 10 h. BSA released from (QUI/PAA/PAM)A and (QUI/PAA/PAM)S hydrogels maintained its structural integrity at 6.7 and 7.4, which is a desirable requirement for preserving pro- tein activity. The fact that (CHI/PAA/PAM)S hydrogels exhibited limited BSA release in simulated gastric fluid and sustained release in simulated intestinal fluids sug- gests that they are good candidates as matrices for co- lon-specific sustained protein release formulations. 5. Acknowledgements M. Bocourt thanks the financial support for fellowship program for doctoral training in the Cuba-Mexico Minis- try for Foreign Affairs of Mexico. We like to thank Dr. Ileana Echevarria Machado at the Biochemistry and Mo- lecular Biology of Plants Department for UV spectros- copy and Dr. Francis Avilés Cetina for mechanical test- ing at the materials Department. This project is funding from SEP-CONACYT. Project 2006-C01-61252, México. REFERENCES [1] A. Singh, S. S. Narvi, P. K. Dutta and N. D. Pandey, “External Stimuli Response on a Novel Chitosan Hy- drogel Crosslinked with Formaldehyde,” Bulletin of Ma- terials Science, Vol. 29, No. 3, June 2006, pp. 233-238. doi:10.1007/BF02706490 [2] C. C. Lin and K. Anseth, “PEG Hydrogels for the Con- trolled Release of Biomolecules in Regenerative Medi- cine,” Pharmaceutical Research, Vol. 26, No. 3, March 2009, pp. 631-643. doi:10.1007/s11095-008-9801-2 [3] J. Kopeček, “Hydrogel from Soft Contact Lenses and Implants to Self-Assembled Nanomaterials,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 47, No. 22, November 2009, pp. 5929-5946. doi:10.1002/pola.23607 [4] K. Murota, S. Sakamoto and K. Kudo, “Reversible Im- mobilization of Protein into Hydrogel Using Designed Coiled-Coil Peptides,” Chemistry Letters, Vol. 36, No. 11, September 2007, pp. 132-137. doi:10.1246/cl.2007.1320 [5] E. Fonseca, F. Campos, A. Pereira, R. Cerqueira, L. Guilherme, W. L. Vasconcelos, Z. I. Portela and H. Sander, “Synthesis and Characterization of Poly(Vinyl Alcohol) Hydrogels and Hybrids for rMPB70 Protein Adsorption,” Journal of Materials Research, Vol. 9, No. 2, June 2006, pp. 185-190. doi:10.1590/S1516-14392006000200014 [6] P. M. Torre, Y. Enobakhare, G. Torrado and S. Torrados, “Release of Amoxicillin From Polyionic Complexs of Chitosan and Poly(Acrylic Acid), Study of Polymer/Po- lymer and Polymer/Drug Interactions within the Network Structure,” Biomaterials, Vol. 24, No. 8, April 2003, pp. 1499-1509. doi:10.1016/S0142-9612(02)00512-4 [7] L. I. Yang, J. S. Chu and J. A. Fix, “Colon-Specific Drug Delivery: New Approaches and in Vitro/in Vivo Evalua- tion,” International Journal of Pharmaceutics, Vol. 235, No. 1, March 2000, pp. 1-15. doi:10.1016/S0378-5173(02)00004-2 [8] Y. Qui and K. Park, “Environment-Sensitive Hydrogels for Drug Delivery,” Advanced Drug Delivery Reviews, Vol. 53, No. 3, December 2001, pp. 321-339. doi:10.1016/S0169-409X(01)00203-4 [9] S. Torrado, P. Prada, M. Paloma and S. Torrado, “Chito- san-Poly(Acrylic) Acid Polyionic Complex: In Vivo Stu- dy to Demostrate Prolonged Gastric Retention,” Biomate- rials, Vol. 25, No. 5, July 2003, pp. 917-923. doi:10.1016/S0142-9612(03)00579-9 [10] S. Jeong, S. Jun and S. I. Kim, “Swelling Behavior of Interpenetrating Polymer Network Hydrogels Composed of Poly(Vinyl Alcohol) and Chitosan,” Reactive and Fun- ctional Polymers, Vol. 55, No. 1, February 2003, pp. 53- 59. doi:10.1016/S1381-5148(02)00214-6 [11] A. Borzacchielo, L. Ambrosio, P. A. Netti, L. Nicolais, C. Peniche, A. Gallardo and J. S. Román, “Chitosan-Based Hydrogels: Synthesis and Characterization,” Journal of Materials Science: Materials in Medicine, Vol. 12, No. 10, May 2001, pp. 861-864. doi:10.1023/A:1012851402759 [12] T. R. Singh, G. Indu, S. Reena and A. K. Nagpal, “Syn- thesis of Poly(Acrylamide-co-Acrylic Acid) Based Su- perabsorbent Hydrogels: Study of Network Parameters and Swelling Behaviour,” Polymer-Plastics Technology and Engineering, Vol. 46, No. 5, May 2007, pp. 481-488. doi:10.1080/03602550701297095 [13] A. Gemeinhart, A. Richard, et al., “pH-Sensitivity of Fast Responsive Superporous Hydrogels,” Journal of Bioma- terials Science, Polymer Edition, Vol. 11, No. 12, De- cember 2000, pp. 1371-1380. doi:10.1163/156856200744390 [14] Y. Shen, X. Zhang, J. Lu, A. Zhang, K. Chen and A. Li, “Effect of Chemical Composition on Properties of pH- -Responsive Poly(Acrylamide-co-Acrylic Acid) Mi- crogels Prepared by Inverse Microemulsion Polymeriza- tion,” Colloids and Surfaces A: Physicochemical and En- gineering Aspects, Vol. 350, No. 1, October 2009, pp. 87-90. doi:10.1016/j.colsurfa.2009.09.009 [15] S. Duran, D. Olpan and O. Güven, “Synthesis and Char- acterization of Acrylamide-Acrylic Acid Hydrogels and Copyright © 2011 SciRes. MSA  Interpenetrated Chitosan-Poly(Acrylic Acid-Co-Acrylamide) Hydrogels. Synthesis, Characterization and Sustained Protein Release Studies Copyright © 2011 SciRes. MSA 520 Adsorption of Some Textile Dyes,” Nuclear Instruments and Methods in Physics Research Section B, Vol. 151, No. 1, May 1999, pp. 196-199. doi:10.1016/S0168-583X(99)00151-2 [16] A. Li and A. Wang, “Synthesis and Properties of Clay- -Based Superabsorbent Composite,” European Polymer Journal, Vol. 41, No. 7, May 2005, pp. 1630-1637. doi:10.1016/j.eurpolymj.2005.01.028 [17] A. Fiumefreddo and M. Utz, “Bulk Streaming Potential in Poly(Acrylic Acid)/Poly(Acrylamide) Hydrogels,” Mac- romolecules, Vol. 43, No. 13, January 2010, pp. 5814- 5819. doi:10.1021/ma100565s [18] M. V. Risbud and R. R. Bhonde, “Polyacrylamide-Chi- tosan Hydrogel: In Vitro Biocompatibility and Sustained Antibiotic Release Studies,” Drug Delivery, Vol. 7, No. 2, January 2000, pp. 69-75. doi:10.1080/107175400266623 [19] C. Peniche, W. Arguelles-Monal, N. Davidenko, R. Sas- tre, A. Gallardo and J. S. Roman, “Self-Curing Mem- branes of Chitosan/PAA IPNs Obtained by Radical Po- lymerization: Preparation, Characterization and Inter- polymer Complexation,” Biomaterials, Vol. 20, No. 20, October 1999, pp. 1869-1878. doi:10.1016/S0142-9612(99)00048-4 [20] M. Recillas, L. L. Silva, C. Peniche, F. M. Goycoolea, M. Rinaudo and W. M. Argüelles-Monal, “Thermo-Respon- sive Behavior of Chitosan-g-Nisopropylacrylamide Co- polymer Solutions,” Biomacromolecules, Vol. 10, No. 6, April 2009, pp. 1633-1641. doi:10.1021/bm9002317 [21] A. Srivastava, D. K. Mishra and K. Behari, “Graft Co- polymerization of N-Vinyl-2-Pyrrolidone onto Chitosan: Synthesis, Characterization and Study of Physicochemi- cal Properties,” Carbohydrate Polymers, Vol. 80, No. 3, May 2010, pp. 790-798. doi:10.1016/j.carbpol.2009.12.031 [22] F. Long, Y. Chiun, H. Fa and H. Wen, “In Vivo Biocom- patibility and Degradability of a Novel Injectable Chito- san-Bead Implant,” Biomaterials, Vol. 23, No. 1, January 2002, pp. 181-191. doi:10.1016/S0142-9612(01)00094-1 [23] M. Rinaudo, “Chitin and Chitosan: Chemistry, Properties and Applications,” Progress in Polymer Science, Vol. 31, No. 7, January 2006, pp. 603-632. doi:10.1016/j.progpolymsci.2006.06.001 [24] G. R. Mahdavinia, A. Pourjavadi, H. Hosseinzadeh and M. J. Zohuriaan, “Modified Chitosan 4. Superabsorbent Hydrogels from Poly(Acrylic Acid-Co-Acrylamide) Gra- fted Chitosan with Salt- and pH- Responsiveness Proper- ties,” European Polymer Journal, Vol. 40, No. 7, July 2004, pp. 1399-1407. doi:10.1016/j.eurpolymj.2004.01.039 [25] DC Protein Assay, “Bio-Rad Protein Assay Kit Manual,” Bio-Rad Laboratories, Hercules, 1998. [26] U. K. Laemmli, “Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4,” Nature, Vol. 227, No. 5259, August 1970, pp. 680-685. [27] H. Wan, R. L. Williams, P. J. Doherty and D. F. Williams, “The Cytotoxicity Evaluation of Kevlar and Silicon Car- bide by MTT Assay,” Journal of Materials Science: Ma- terials in Medicine, Vol. 5, No. 6, December 1994, pp. 441-445. doi:10.1007/BF00058980 [28] X. Z. Zhang, D. Q. Wu and C. Chu, “Synthesis, Charac- terization and Controlled Drug Release of Thermosensi- tive IPN-PNIPAM Hydrogels,” Biomaterials, Vol. 25, No. 17, August 2004, pp. 3793-3805. doi:10.1016/j.biomaterials.2003.10.065 [29] W. Argüelles-Monal and C. Peniche-Covas, “Study of the Interpolyelectrolyte Reaction between Chitosan and Car- boxymethyl Cellulose,” Die Makromolekulare Chemie, Rapid Communications, Vol. 9, No. 10, June 1988, pp. 693-697. doi:10.1002/marc.1988.030091004 [30] M. Bocourt, N. Bada, W. Argüelles-Monal and C. Pen- iche, “Síntesis y Caracterización de Redes Poliméricas Interpenetradas de Quitosana-Poli(Ácido Acrílico-co-Ac- rilamida),” Revista CENIC Ciencias Químicas, Vol. 40, No. 2, February 2009, pp. 81-88. [31] H. Schott, “Swelling Kinetics of Polymers,” Journal of Macromolecular Science, Vol. 31, No. 1, March 1992, pp. 1-9. doi:10.1080/00222349208215453 [32] K. Sivadasan, P. Somasundaran and N. J. Turro, “Fluo- rescence and Viscometry Study of Complexation of Poly (Acrylic Acid) with Poly(Acrylamide) and Hydrolysed Poly(Acrylamide),” Colloid & Polymer Science, Vol. 269, No. 2, April 1991, pp. 131-113. doi:10.1007/BF00660302 [33] C. L. Bell and N. A. Peppas, “Biomedical Membranes from Hydrogels and Interpolymer Complexes,” In: N. A. Peppas and R. S. Langer, Eds., Advances in Polymer Sci- ence 122, Biopolymer II, Springer-Verlag, Berlin, 1995, pp. 125-175. [34] Q. Tang, S. X, Q. Li, J. Wu and J. Lin, “A Simple Route to Interpenetrating Network Hydrogel with High Me- chanical Strength,” Journal of Colloid Science, Vol. 339, No. 1, July 2009, pp. 45-52. [35] W. Gombotz and A. S. Hoffman, “Medicine and Phar- macy,” In: N. A. Peppas, Ed., Hydrogels in Medicine and Pharmacy, CRC Press, Boca Raton, 1986, pp. 95-126. [36] E. Karadag, D. Saraydin, H. Nursevin and O. Guven, “Adsorption of Bovine Serum Albumin to Acrylamide- Itaconic Acid Hydrogels,” Polymers for Advanced Tech- nologies, Vol. 5, No. 10, April 1994, pp. 664-668. |

