 Journal of Biosciences and Medicines, 2014, 2, 1-7 Published Online November 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.29001 How to cite this paper: Minami, M., Takase, H., Sakakibara, R., Imura, T., Morita, H., Kanemaki, N. and Ohta, M. (2014) LicT Modulates Biofilm Formation of Streptococcus pyogenes. Journal of Biosciences and Medicines, 2, 1-7. http://dx.doi.org/10.4236/jbm.2014.29001 LicT Modulates Biofilm Formation of Streptococcus pyogenes Masaaki Minami1*, Hiroshi Takase2, Ryoko Sakakibara3, Taichi Imura3, Hideo Morita3, Naoto Kanemaki4, Michio Ohta5 1Department of Bacteriology, Graduate School of Medical Sciences, Nagoya City University, Nagoya, Japan 2Core Laboratory, Graduate School of Medical Sciences, Nagoya City University, Nagoya, Japan 3Department of Clinical Investigation, Daido Hospital, Nagoya, Japan 4Department of Gastroenterology, Daido Hospital, Nagoya, Japan 5School of Nursing, Sugiyama Jyogakuen University, Nagoya, Japan Email: *mina mi@ med.n ago ya -cu.ac.jp Received Sep te mber 2014 Abstract Streptococcus pyogenes frequently causes purulent infections in humans. Biofilm formation is an important virulence property of S. pyogenes because of decreased susceptibility of bacteria to an- tibiotic treatment. Biofilm is composed of various types of matrix including glycocalyx which is an important exocellular matrix material related to bacterial sugar metabolism. A putative antiter- minator protein, LicT (Spy0571), is one of the components of the glucose-independent β-gluco- side-specific phosphotransferase system (PTS). Although the PTS, a carbohydrate metabolic sys- tem, may play a role in biofilm formation, the relationship between LicT and biofilm formation has not yet been elucidated. Here, we evaluated whether LicT aff ected biofilm formation in modified chemically defined medium (CDMM) supplemented with glucose or β-glucoside:salicin. We created ∆licT- and licT-complemented mutant strains from S. pyogenes 1529. Although the ∆licT mutant strain tended to have higher growth rate than wild-typestrain in CDMM with glucose, it had a sig- nificant lower growth rate than the wild-type strain in CDMM with salicin. In addition, the ∆licT mutant exhibited lower biofilm formation in CDMM containing salicin than the wild-type strain by 96 well plate analysis and confocal laser scanning microscopic analysis. Our results suggest that LicT plays an important role in biofilm formation of S. pyogenes. Keywords Streptococcus pyogenes, LicT, Biofil m 1. Introduction Streptococcus pyogenes is a gram-positive bacterium that infects the upper respiratory tract, including the tonsils and pharynx, and is responsible for pharyngitis, tonsillitis, rheumatic fever, and glomerulonephritis. In addition, *  M. Minami et al. it causes streptococcal toxic shock syndrome [1]. Many pathogenic and nosocomial bacteria with the ability to form biofilms are responsible for acute and chronic infections. Some examples of typical biofilm-associated diseases are periodontitis, endocarditis, and prostatitis [2]. In particular, medical devices implanted in human hosts, such as intravenous catheters, artificial joints, and cardiac pacemakers, become rapidly coated with human extracellular matrix and serum proteins, making them prime targets for bacterial biofilm formation [3]. Because of the sharply decreased susceptibility of biofilm-forming bacteria to host defenses and antibiotic treatments, biofilms on implanted devices constitute a major medical problem [4]. A previous study revealed that S. pyogenes strains recovered from patients with treatment failure form biofilms in vitro with variable efficiency and that compared to planktonic cultures, S. pyogenes organized in biofilms have higher minimum inhibitory concentrations for all standard antibiotics used in a treatment [5]. Basic carbohydrate metabolism is an important factor for bacterial pathogenesis [6]. Bacteria alter the tran- scription of carbohydrate utilization genes and virulence factor production in response to changes in the envi- ronmental conditions encountered during infection in humans [7]. Pathogenic bacteria have developed molecular strategies to directly link the regulation of carbohydrate utilization and virulence factors such as biofilm forma- tion. The LicT (Spy 0571) gene is a putative transcriptional antitermination gene belonging to the BglG family [8]; which comprises a phosphoenolpyruvate-dependent phosphotransferase system (PTS) [8]. Antiterminator pro- teins are involved in the transcriptional regulation of β-glucoside-specific genes from various bacteria [9]. These antiterminator proteins bind to a ribonucleic antiterminator site present in a specific mRNA secondary structure and prevent the formation of a hairpin terminator structure that terminates transcription [10]. The binding of an antiterminator protein to mRNA permits transcription through the disrupted terminator structure into the β-glu- coside-specific genes that are not normally transcribed. Thus, the antitermination mechanism of transcriptional regulation allows for the expression of β-gluco sid e-specific loci in the absence of a metabolically preferred car- bon source [10]. Although the PTS as a carbohydrate metabolic system may play a role in biofilm formation, the relationship between LicT and biofilm formation has not yet been elucidated. Here, we focused on the role of LicT and eva- luated whether LicT affected biofilm formation. 2. Materials and Methods 2.1. Bacterial Strains and Culture Condition One hundred S. pyogenes clinical isolates from Daido hospitals andM1 serotype S. pyogenes strain 1529 were used in this study [11]. S. pyogenes was usually precultured in brain heart infusion medium (BHI) (Eiken Chemical Co., Tokyo, Japan) containing 0.3% yeast extract (Difco Laboratories, MI, USA) for 18 hours at 37 ˚C. We also used modified chemically defined medium (CDMM) [12]. We performed growth analysis, northern blot analysis, and biofilm assays using CDMM supplemented with 0.5% glucose, or 0.5% β-glucoside:salicin (Wako Pure Chemical Industries, Inc., Osaka, Japan). Turbidity (O.D. 600 nm) of the broth culture was measured at adequate points to estimate growth with a plate reader (SPECTRAmax340; Molecular Devices, LLC, CA, USA). Escherichia coli DH5α (Takara Bio, Ohtsu, Japan) was grown in LBbroth or LB agar (Difco Laboratories) with aeration at 37˚C. The following antibiotics were used; ampicillin (100 µg/mL; Sigma Aldrich, MO, USA) for E. coli; spectinomycin (100 µg/mL; Sigma Aldrich) for E. coli and S. pyogenes; and kanamycin (Sigma Aldrich) 100 µg/mL for E. coli or 200 µg/mL for S. pyogenes. 2.2. Detection of licT Gene in S. pyogenes Clinical Isolates The presence of the licT gene was assessed by PCR. The Spy571 gene encoding LicT was identified from DNA sequence data obtained from the University of Oklahoma S. pyogenes genome databases [13]. The LicT ORF consists of 840 nucleotides that encode a putative protein of 280 amino acids. Genomic DNA was extracted us- ing the Promega DNA extraction kit (Promega, MA, USA) according to the manufacturer’s instructions. The licT gene was amplified by PCR using a thermal cycler (GeneAmp PCR 9700; Applied Biosystems, Foster City, CA, USA) and primersSpy0571-F1 (5’-ATGA ATCGCTT CACAGAGTT -3’) and Spy0571-R1 (5’-AAGG AGTGTCCAAACT AGCA-3’). The PCR protocol for amplifying the licT gene was as follows: de-  M. Minami et al. naturation over 2 min at 94˚C; 30 cycles of 60 seconds at 94˚C, 60 seconds at 52˚C and 60 seconds at 72˚C; and a final extension at 72˚C for 5 minutes. Amplified products were analyzed by 1.5 % agarose gel electrophoresis in 1× TBE buffer. The gel was stained with ethidium bromide and then exposed to UV light to visualize the am- plified products. When the bands were not clear, we repeated the experiment a few times to confirm the repro- ducibility. 2.3. Creation of lic T-Inactivated and Complemented Mutants The general methods of constructing mutant and complemented strains are described elsewhere [11]. Briefly, a licT DNA fragment was amplified by PCR using two sets of primers, licT-F1 (5’-AAAAGGGATT ACCTT TGG AA-3’) and licT-R1 (5’-GTTCATGTTAAACGTTTAGTGA-3’)and subcloned into the NheI-BamHI site of the pFW12 vector (pFW12-licT). Next, inverse PCR with primers licT-F2 (5’-AGATGATAACATTAAAGCGTTC-3’) and licT-R2(5’ -AGTACC AAATTATCT-3’) was performed (pFW12- licT2) as described elsewhere [14]. The plasmid (licT::aad9/pFW12) was asuicide vector for S. pyogenes after subcloning the sp c3 cassette of pSL60-3DNA into pFW12-licT2. To construct a plasmid for licT complementa- tion, the DNA fragment of licT was amplified using oligonucleotide primers licT-F1 and licT-R1 with PrimeS- TAR HS DNA Polymerase (Takara Bio). The protocol for transformation is the same as that mentioned above. This experimental procedure was approved by the Institutional Transgenic Committee at Nagoya City Universi- t y. 2.4. RNA Isolation and Northern Blot Analysis Total RNA was extracted and purified as described previously [11]. The DNA for probe was amplified with oligonucleotide primers as follows: Spy0571-F1 and Spy0571-R1. This probe was 32P-labeled using the random primer DNA labeling kit version 2 (Takara Bio). The membranes were then autoradiographed and analyzed with a bioimaging analyzer (BAS-1800II; Fujifilm, Tokyo, Japan). 2.5. Quantification of Biofilm Formation Quantification of the biofilm formation assay was described elsewhere [15]. Briefly, bacteria were precultured on brain heart infusion agar (Eiken Chemical Co.) plates containing 0.3% yeast extract at 37˚C for 24 hours. A single colony was inoculated on CDMM supplemented with glucose or salicin and transferred into a 96-well round -bottom polystyrene microtiter plate (Thermo Fisher Scientific, Inc., MA, USA). The microtiter plate was incubated statically at 37˚C for 24 hours. The supernatant was discarded, and the wells were washed three times with 200 μL of phosphate-buffered saline. The wells were allowed to dry, then 200 μL of 0.1% crystal violet was added, and the wells were stained at room temperature for 1 hour. The wells were then washed three times with distilled water to remove the excess dye and dried. The dye staining the biofilm in each well was extracted with 200 μL of methanol, and the absorbance was measured at O.D. 570 nm with a plate reader. 2.6. Confocal Laser Scanning Microscopy Analysis of Biofilm The protocol of confocal laser scanning microscopy analysis of biofilm has been described elsewhere [15]. Briefly, freshly prepared bacterial solutions were inoculated in 10 mL of CDMM containing salicin in glass- based dishes. A sterile glass coverslip (22 mm × 22 mm) was placed at the bottom of 20-mL dishes containing 10 mL of medium. The dishes were incubated statically at 37˚C for 24 hours. The culture supernatant was dis- carded, and the dishes were carefully rinsed three times with 0.85% NaCl. The bacterial biofilm attached to the glass surfaces was observed at 630× magnification using a confocal laser scanning microscope (LSM5 Pascal; Carl Zeiss Co., Ltd., Germany). The images were obtained from at least two independent experiments. 2.7. Statistical Analy sis Statistical significance between the mean values was determined by one-way analysis of variance. A confidence interval with a p value of <0.05 was considered to be significant. The compared experiments were repeated a minimum of three times to improve the resulting data. 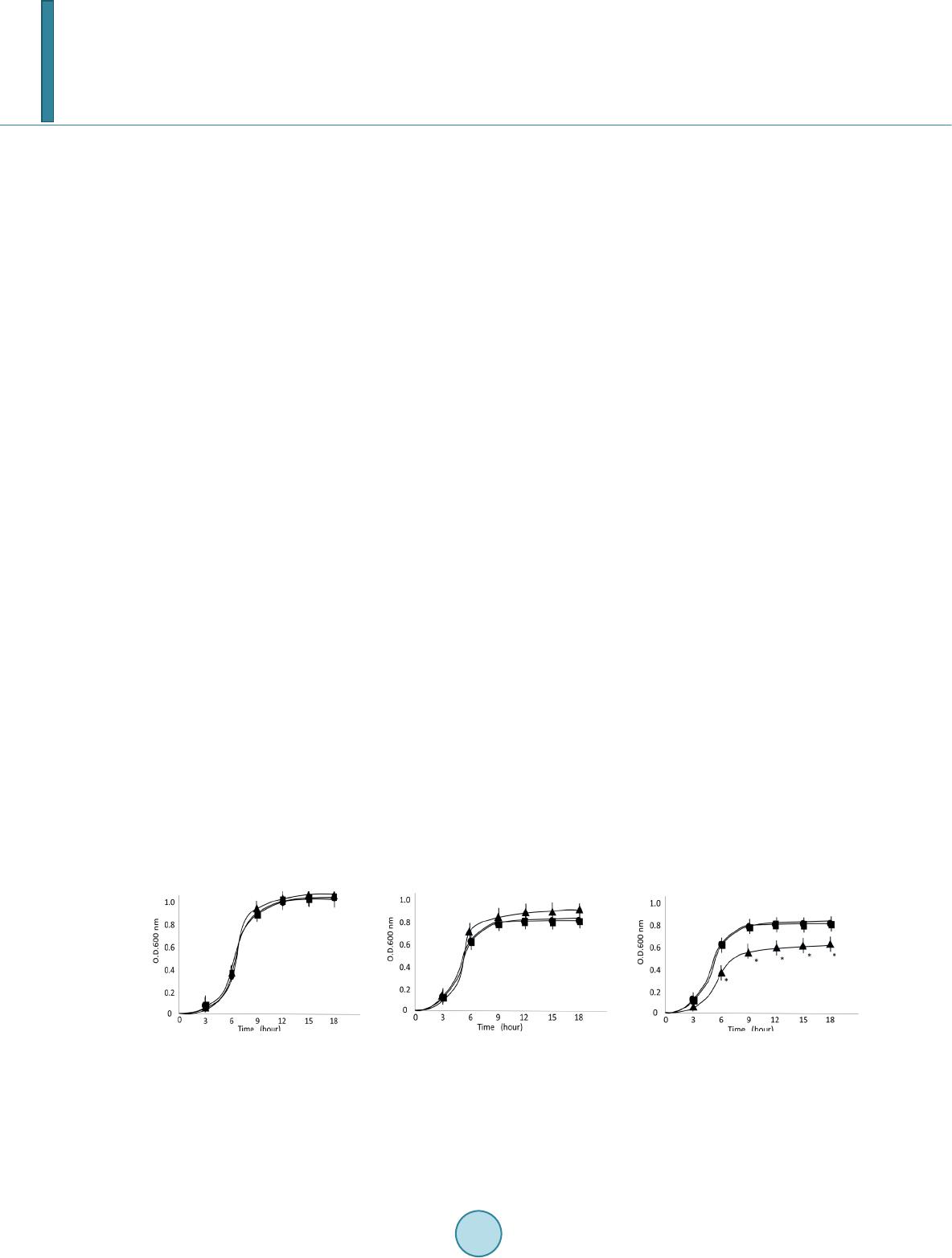 M. Minami et al. 3. Result 3.1. The Growth Rate of ∆licT Mutant in CDMM with Salicin Was Lower than the Wild-Type Strain by Measuring Turbidity At first, we screened 101 S. pyogenes for the licT gene and detected positive PCR products in all strains. From these results, we confirmed that S. pyogenes generally possessed licT gene. Thus, we constructed ∆licT mutant and lic T-complemented strains from S. pyogenes 1529. We confirmed the expression of licT mRNA in both wild-type and licT-complemented strains by northern blot analysis. But we could not find in ∆licT mutant, confirming that the ∆licT mutant strain were successfully created. Next we evaluated the growth rates of S. pyogenes 1529 wild-type, isogenic ∆licT mutant, and licT-comple- mented strains in BHI containing 0.3% yeast extract (Figure 1(a) ). However, no significant differences in growth were observed among the 1529 licT-derivatives. Because a rich medium may mask the role of specific β-glucoside metabolism, we tried to evaluate the growth rate in CDMM with glucose or specific β-glucosides: salicin. The optical turbidity of the ∆licT mutant strain tended to be higher than those of the wild-type and licT-complemented strains in CDMM with glucose at 24 hours (Figure 1(b)). However, the optical turbidity of the ∆licT mutant strain was significant lower than that of the wild-type and licT -complemented strains in CDMM with salicin at 24 hours (Figure 1(c)). 3.2. Biofilm Assays Revealed That the ∆licT Mutant Had Lower Biofilm Formation than the Wild-Type Strain We measured the biofilm formation by 1529 licT-derivatives by a 96-well plating assay (Figure 2). We did not find significant difference of biofilm formations among 1529 licT-derivative strains in CDMM containing glu- cose. However, the biofilm formation of ∆licT mutant strain was lower in CDMM supplemented with salicin. We also confirmed biofilm formation by confocal laser scanning microscopy. Confocal laser scanning micro- scopy analysis revealed the same results as the 96-well plating assay (Figure 3). 4. Discussion In this study, we clarified the role of LicT as a regulator of biofilm formation. The ∆licT mutant strain had lower ability to form biofilm than the wild-type strain. Many factors influence biofilm formation, and each factor is subjected to complex cross-talk regulation. Our results revealed part of the catabolite pathway of biofilm net- work formation. Further elucidation of biofilm formation via catabolite pathways is desired. β-Glucosides including salicin are carbohydrates that are largely derived from plant sources. β-Glucoside uti- lization systems have been described in several bacteria [16]. These organisms rely on the PTS for the transport and subsequent utilization of various β-glucosides [8]. The structural features of the genes encoding these β-glucoside utilization systems are markedly similar. In most bacteria, these genes are organized in a simple operon structure [16]. In S. pyogenes, these genes are organized as a regulon as described for S. mutans [16]. The S. pyogenesbgl regulon encodes a β-gluco sid e-specific enzyme II of the PTS (bglP) and a phospho-β-glu - cosidase (bglA). (a) (b) (c) Figure 1. Growth rate of S.pyogenes strain determined by measuring turbidity. Turbidity (O.D.600 nm) for growth in broth culture was measured at adequate time points. Panels (a) (b) and (c) present the findings for brain heart infusion medium containing 0.3% yeast extract, modified CDMM containing 0.5% glucose, and CDMM containing 0.5% salicin respectively. Closed circles, closed triangles, closed box represent wild-type, ∆licT mutant, and licT-complemented strains, respectively. The experiments were performed at least three times. The results are presented as means and standard deviations. Asterisks indicate p < 0.05.  M. Minami et al. Figure 2. Biofilm formation analysisusing a 96-well plating assay. S.pyogenes-produced biofilm formed on the wells of a polystyrene microplate. CDMM for biofilm formation was supplemented with glucose or salicin. After 24 hours incu- bation, biofilm that formed on the surface of the wells was stained with crystal violet. The dye staining the biofilm was then extracted, and absorbance was measured at O.D.570 nm. The letters A, B, and C represent the wild-type, ∆licT mutant, and licT-complemented strains CDMM containing 0.5% glucose, respectively. The letter D, E and F represent the wild-type, ∆licT mutant, andlicT-complemented strains in CDMM containing 0.5% salicin, respectively. The expe- riments were performed at least three times. The results are presented as means and standard deviations. Asterisks indi- cate p < 0.05. wild-type ∆licT mutant licT-c o mp lem ented (a) wild-type ∆li c T mutant licT-c o mp lem ented (b) Figure 3. Biofilm formation analysis by confocal laser scanning microscopy. S.pyogenes was incubated in CDMM sup- plemented with glucose or salicin in glass-based dishes at 37˚C for 24 hours. Panels (a) and (b) present the findings for CDMM containing 0.5% glucose, CDMM containing 0.5% salicin, respectively. Biofilm formed on the glass surface was observed at 630× magnification with a confocal laser scanning microscopy. In this study, we used confocal laser scanning microscope instead of electron microscopy (EM) to assess the biofilm formation. EM techniques require that biofilm specimens be dehydrated, a process known to signifi- cantly reduce the total volume of extracellular matrix material and lead to collapse of the matrix, compression of the cells, and distortion of the architecture [17]. The structure of bacterial glycocalyx is highly hydrated (>99% wate r), it was difficult to visualize this structure using a light microscope, and the use of EM produced only fur- ther confusion concerning the glycocalyx, as EM involves dehydration of the specimen [18]. To eliminate this  M. Minami et al. problem, a confocal laser scanning microscopic technique was used to study the hydrated biofilm. Although previous results documented that BHI best supported adherence to uncoated plastic for all tested S. pyogenes strains, those also indicated that the M1 serotype S. pyogenes did not display significant primary adhe- sion to any of the uncoated or matrix protein-coated plastic surfaces tested throughout the investigated sampling time points. This finding suggested that these strains are unable to form biofilms [19]. In our study, we changed the culture medium from BHI to CDMM, after which we demonstrated that M1 serotype S. pyogenes can form biofilm successfully. 5. Conclusion In summary, we clarified that LicT modulates biofilm formation of S. pyogenes. In particular, biofilm could play an important role in recurrent and chronic streptococcal infections. Further investigations are needed to elucidate the role of the PTS in biofilm formation. Acknowledgements We thank Mr. Shoji Ishihara and Ms. Miwako Fujimura for special encouragement. We also thank the members of the department of bacteriology for their technical and material supports throughout this investigation. This study was supported by a Grant-in-Aid for Research from the Nagoya City University, Japan and by grants (numbers 21590485 and 23590889) from the Ministry of Education, Science, and Culture of Japan. References [1] Cunningham, M.W. (2000) Pathogenesis of Group A Streptococcal Infections. Clinical Microbiology Review, 13, 470- 511. http://dx.doi.org/10.1128/CMR.13.3.470-511. 2000 [2] Parsek, M.R. and Singh, P.K. (2003) Bacterial Biofilms: An Emerging Link to Disease Pathogenesis. Annual Review of Microbiology, 57, 677-701. http://dx.doi.org/10.1146/annurev.micro.57.030502.090720 [3] Hall -Stoodley, L.J., Costerton, W. and Stoodley, P. (2004) Bacterial Biofilms: From the Natural Environment to In fe c- tious Diseases. Nature Reviews Microbiology, 2, 95-108. http://dx.doi.org/10.1038/nrmicro821 [4] Pajkos, A., Vickery, K. and Cossart, Y. (2004) Is Biofilm Accumulation on Endoscope Tubing a Contributor to the Failure of Cleaning and Decontamination? Journal of Hospital Infection, 58, 224-229. http://dx.doi.org/10.1016/j.jhin.2004.06.023 [5] Conley, J., Olson, M.E., Cook, L.S., Ceri, H., Phan, V. and Davies, H.D. (2003) Biofilm Formation by Group A Strep- tococci: Is There a Relationship with Treatment Failure? Journal of Clinical Microbiology, 41, 4043-40 48 . http://dx.doi.org/10.1128/JCM.41.9.4043-4048.2003 [6] Shelburne, S.A., Davenport, M.T., Keith, D.B. and Musser, J.M. (2008) The Role of Complex Carbohydrate Catabol- ism in the Pathogenesis of Invasive Streptococci. Trend s in Microbiology, 16, 318-325. http://dx.doi.org/10.1016/j.tim.2008.04.002 [7] Schnetz, K. and Rak, B. (1988) Regulation of the bgl Operon of Escherichia coli by Transcriptional Antitermination. The EMBO Journal, 7, 3271-32 77. [8] Postma, P.W., Lengeler, J.W. and Jacobson, G.R. (1993) Phosphoenolpyruvate: Carbohydrate Phosphotransferase Systems of Bacteria. Microbiological Review, 57, 543-594. [9] Cote, C.K. and Honeyman, A.L. (2003) The LicT Protein Acts as Both a Positive and a Negative Regulator of Loci the bgl Regulon of Streptococcus mutans. Microbiology, 149, 1333-1340 . http://dx.doi.org/10.1099/mic.0.26067-0 [10] Rutberg, B. (1997) Antitermination of Transcription of Catabolitc Operons. Molecular Microbiology, 23, 413-421. http://dx.doi.org/10.1046/j.1365-2958.1997.d01-1867.x [11] Minami, M., Kamimura, T., Isaka, M., Tatsuno, I., Ohta, M. and Hasegawa, T. (2010) Clindamycin-Induced CovS - Mediated Regulation of the Production of Virulent Exoproteins Streptolysin O, NAD Glycohydrolase, and Streptoki- nase in Streptococcus pyogenes. Antimicrobial Agents and Chemotherapy, 54, 98-102. http://dx.doi.org/10.1128/AAC.00804-09 [12] van de Rijn, I. and Kessler, R.E. (1980) Growth Characteristics of Group A Streptococci in a New Chemically Defined Medium. Infection and Immunity, 27, 444-448. [13] Ferretti, J.J., McShan, W.M., Ajdic, D., Savic, D.J., Savic, G., Lyon, K., et al. (2001) Complete Genome Sequence of an M1 Strain of Streptococcus pyogenes. Proceedings of the National Academy of Sciences of the United States of America, 98, 4658-46 63. http://dx.doi.org/10.1073/pnas.071559398  M. Minami et al. [14] Tatsuno, I., Sawai, J., Okamoto, A., Matsumoto, M., Minami, M., Isaka, M., et al. (2007) Characterization of the NAD-Gl y- cohydrolase in Streptococcal Strains. Microbiology, 153, 4253-4260. http://dx.doi.org/10.1099/mic.0.2007/009555-0 [15] Ishihara, Y., Hyodo, M., Hayakawa, Y., Kamegaya, T., Yamada, K., Okamoto, A., et al. (2009) Effect of Cyclic Bis(3'-5') Diguanylic Acid and Its Analogs on Bacterial Biofilm Formation. FEMS Microbiology Letters, 301, 193-200. http://dx.doi.org/10.1111/j.1574-6968.2009.01825.x [16] Cote, C.K., Cvitkovitch, D., Bleiweis, A.S. and Honeyman, A.L. (2000) A Novel Beta-Glucoside-Specific PTS Locus from Streptococcus mutans That Is Not Inhibited by Glucose. Microbiology, 146, 1555-1563. [17] Akiyama, H., Morizane, S., Yamasaki, O., Oono, T. and Iwatsuki, K. (2003) Assessment of Streptococcus pyogenes Microcolony Formation in Infected Skin by Confocal Laser Scanning Microscopy. Journal of Dermatological Science, 32, 193-199. http://dx.doi.org/10.1016/S0923-1811(03)00096-3 [18] Costerton, J.W., Lappin-Scott, H.M. and Cheng, K.J. (1992) Glycocalyx. In: Lederberg, L., Ed., Bacteri al, Encyclo pe- dia of Microbiology, Vol. 2, Academic Press, San Diego, 311-317. [19] Lembke, C., Podbielski, A., Hidalgo-Grass, C., Jonas, L., Hanski, E. and Kreikemeyer, B. (2006) Characterization of Biofilm Formation by Clinically Relevant Serotypes of Group A Streptococci. Applied and Environmental Microbiol- ogy, 72, 2864-2875 . http://dx.doi.org/10.1128/AEM.72.4.2864-2875. 2006
|