Paper Menu >>
Journal Menu >>
 World Journal of Neuroscience, 2011, 1, 1-8 WJNS doi:10.4236/wjns.2011.11001 Published Online May 2011 (http://www.SciRP.org/journal/wjns/). Published Online May 20 11 in SciRes. http://www.scirp.org/journal/wjns Analyses of fear memory in Arc/Arg3.1-deficient mice: intact short-term memory and impaired long-term and remote memory Kaz u yu ki Yama da 1*, Chihiro Homma1, Kentaro Tanemura2, Toshio Ikeda3, Shigeyoshi Itohara3, Yoshi ko Na ga oka 1 1Support Unit for Animal Resources Development, Research Resources Center, Brain Science Institute, RIKEN, Saitama, Japan; 2Division of Cellular & Molecular Toxicology, Biological Safety Research Center, National Institute of Health Sciences, Tokyo, Japan; 3Laboratory for Behavioral Genetics, Brain Science Institute, Saitama, Japan. Email: kaz-yamada@brain.riken.jp Received 25 April 2011; 23 May 2011; 26 May 2011 ABSTRACT Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) was originally identified in patients with seizures. It is densely distributed in the hip- pocampus and amygdala in particular. Because the expression of Arc/Arg3.1 is regulated by nerve in- puts, it is thought to be an immediate early gene. As shown both in vitro and in vivo, Arc/Arg3.1 is in- volved in synaptic consolidation and regulates some forms of learning and memory in rats and mice [1,2]. Furthermore, a recent study suggests that Arc/Arg3.1 may play a significant role in signal transmission via AMPA-type glutamate receptors [3-5]. Therefore, we conducted a detailed analysis of fear memory in Arc/Arg3.1-deficient mice. As previously reported, the knockout animals exhib- ited impaired fear memory in both contextual and cued test situations. Although Arc/Arg3.1-defic ient mice showed almost the same performance as wild-type littermates 4 hr after a conditioning trial, their performance was impaired in the retention test after 24 hr or longer, either with or without reconsolidation. Immunohistochemical analyses showed an abnormal density of GluR1 in the hippocampus of Arc/Arg3.1-deficient mice; however, an applica- tion of AMPA potentiator did not improve memory performance in the mutant mice. Memory impair- ment in Arc/Arg3.1-deficient mice is so robust that the mice provide a useful tool for developing treat- ments for memory impairment. Keywords: Activity-Regulated Cytoskeleton-Associated Protein (Arc/Arg3.1); Knockout (Ko) Mouse; Short-Term Memory; Long-Term Memory; Reconsoli- da tio n; AM PA Rece pto r 1. INTRODUCTION Activity-regulated cytoskeleton-associated protein (Arc/ Arg3.1) is encoded by an effector immediate early gene and is selectively localized in neuronal dendrites [6]. Arc/Arg3.1 and its encoded protein are thought to play a role in activity-dependent plasticity of dendrites [7]. Arc/Arg3.1 mRNA is greatly increased by long-term potentiation (LTP)-inducing electrical stimuli [8,9]; ad- ministration of psycho-stimulant drugs such as cocaine [10], amphetamines/methamphetamines [11-13], and phencyclidine [14]; insulin [15], middle cerebral artery occlusion [16], electroconvulsive shock [17,18], olfac- tory inputs [19], mating [20], stress [21], and other stim- uli that prompt neuronal activity [22,23]. The mRNA is then rapidly delivered to the dendrites [9]. Furthermore, intense synaptic activity induces selective localizatio n of Arc/Arg3.1 mRNA to activated synapses [9,24]. There- fore, Arc/Arg3.1 is believed to be related to synaptic plasticity, and thus many electrophysiological and bio- chemical studies have been conducted to investigate this possibility [7-9,25]. These studies have revealed that Arc/Arg3.1 may be a key molecule involved in late- phase LTP, during which long-term memories (LTM) are thought to be established. Guzowski et al. [1] found that rats in which Arc/ Arg3.1 antisense oligonucleotides were infused into the hippocampus perform relatively poorly in a water-maze probe test. This result strongly suggested that Arc/Arg3.1 plays an important role in the formation of some forms of spatial LTM. Furthermore, Plath et al. [2] developed Arc/Arg3.1-deficient mice and demonstrated learning and memory impairment in these mice in the Morris 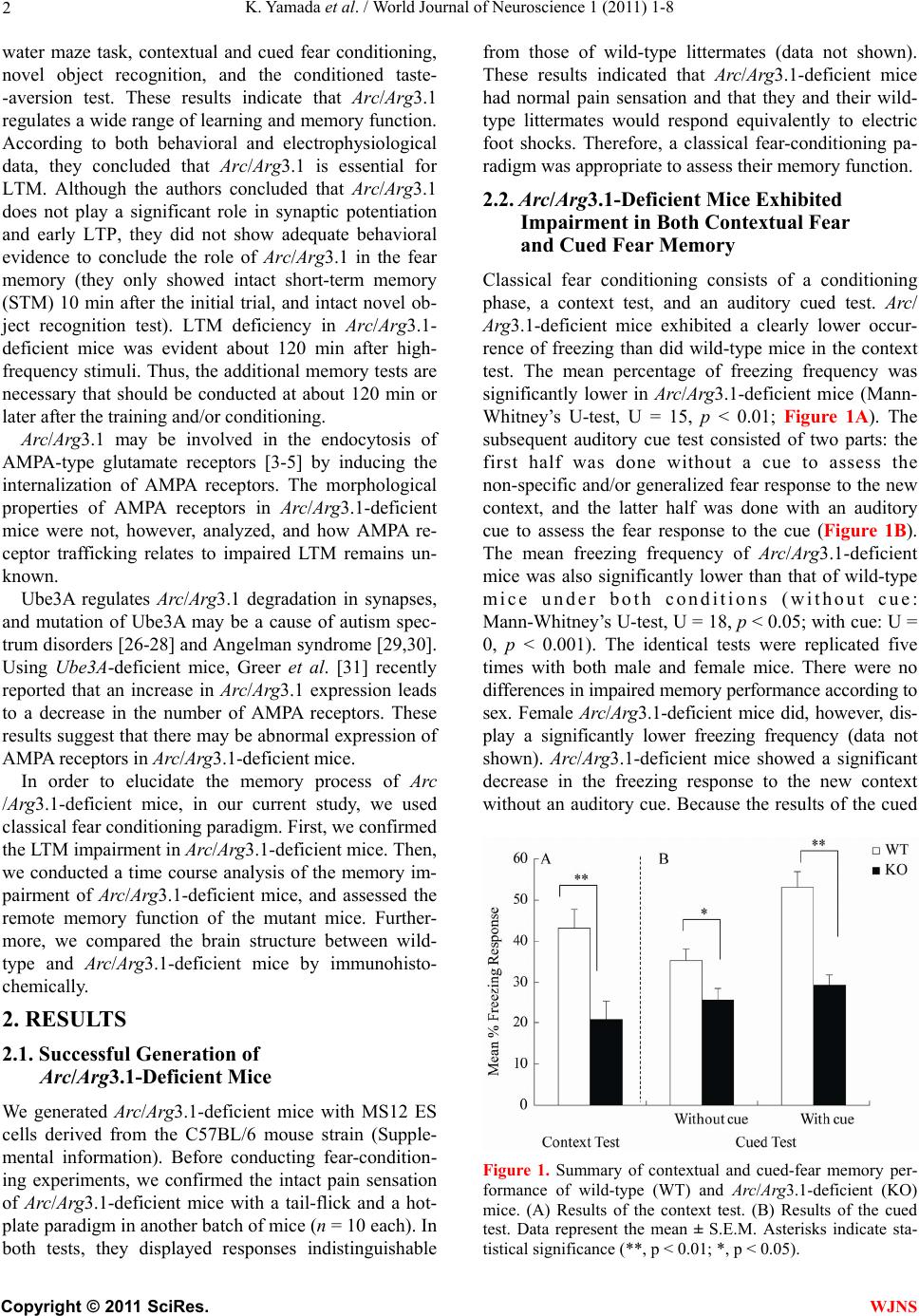 K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 2 water maze task, contextual and cued fear conditioning, novel object recognition, and the conditioned taste- -aversion test. These results indicate that Arc/Arg3.1 regulates a wide range of learning and memory function. According to both behavioral and electrophysiological data, they concluded that Arc/Arg3.1 is essential for LTM. Although the authors concluded that Arc/Arg3.1 does not play a significant role in synaptic potentiation and early LTP, they did not show adequate behavioral evidence to conclude the role of Arc/Arg3.1 in the fear memory (they only showed intact short-term memory (STM) 10 min after the initial trial, and intact novel ob- ject recognition test). LTM deficiency in Arc/Arg3.1- deficient mice was evident about 120 min after high- frequency stimuli. Thus, the addition al memory tests are necessary that should be conducted at about 120 min or later after the training and/or conditioning. Arc/Arg3.1 may be involved in the endocytosis of AMPA-type glutamate receptors [3-5] by inducing the internalization of AMPA receptors. The morphological properties of AMPA receptors in Arc/Arg3.1-deficient mice were not, however, analyzed, and how AMPA re- ceptor trafficking relates to impaired LTM remains un- known. Ube3A regulates Arc/Arg3.1 degradation in synapses, and mutation of Ube3A may be a cause of autism spec- trum disorders [26-28] and Angelman syndrome [29,30]. Using Ube3A-deficient mice, Greer et al. [31] recently reported that an increase in Arc/Arg3.1 expression leads to a decrease in the number of AMPA receptors. These results suggest that there may be abnormal expression of AMPA receptors in Arc/Arg3.1-deficient mice. In order to elucidate the memory process of Arc /Arg3.1-deficient mice, in our current study, we used classical fear conditioning paradigm. First, we confirmed the LTM impairment in Arc/Arg3.1-deficient mice. Then, we conducted a time course analysis of the memory im- pairment of Arc/Arg3.1-deficient mice, and assessed the remote memory function of the mutant mice. Further- more, we compared the brain structure between wild- type and Arc/Arg3.1-deficient mice by immunohisto- chemically. 2. RESULTS 2.1. Successful Generation of Arc/Arg3.1-Deficient Mice We generated Arc/Arg3.1-deficient mice with MS12 ES cells derived from the C57BL/6 mouse strain (Supple- mental information). Before conducting fear-condition- ing experiments, we confirmed the intact pain sensation of Arc/Arg3.1-deficient mice with a tail-flick and a hot- plate paradigm in another batch of mice (n = 10 each). In both tests, they displayed responses indistinguishable from those of wild-type littermates (data not shown). These results indicated that Arc/Arg3.1-deficient mice had normal pain sensation and that they and their wild- type littermates would respond equivalently to electric foot shocks. Therefore, a classical fear-conditioning pa- radigm was appropriate to assess their memory function . 2.2. Ar c/Arg3.1-Deficient Mice Exhibited Impairment in Both Contextual Fear and Cued Fear Memory Classical fear conditioning consists of a conditioning phase, a context test, and an auditory cued test. Arc/ Arg3.1-deficient mice exhibited a clearly lower occur- rence of freezing than did wild-type mice in the context test. The mean percentage of freezing frequency was significantly lower in Arc/Arg3.1-deficient mice (Mann- Whitney’s U-test, U = 15, p < 0.01; Figure 1A). The subsequent auditory cue test consisted of two parts: the first half was done without a cue to assess the non-specific and/or generalized fear response to the new context, and the latter half was done with an auditory cue to assess the fear response to the cue (Figure 1B). The mean freezing frequency of Arc/Arg3.1-deficient mice was also significantly lower than that of wild-type mice under both conditions (without cue: Mann-Whitney’s U-test, U = 18, p < 0.05; with cue: U = 0, p < 0.001). The identical tests were replicated five times with both male and female mice. There were no differences in impaired memory performance according to sex. Female Arc/Arg3.1-deficient mice did, however, dis- play a significantly lower freezing frequency (data not shown). Arc/Arg3.1-deficient mice showed a significant decrease in the freezing response to the new context without an auditory cue. Because the results of the cued Figure 1. Summary of contextual and cued-fear memory per- formance of wild-type (WT) and Arc/Arg3.1-deficient (KO) mice. (A) Results of the context test. (B) Results of the cued test. Data represent the mean ± S.E.M. Asterisks indicate sta- tistical significance (**, p < 0.01; *, p < 0.05).  K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 3 test reflect both generalized contextual fear [32] and cued fear, cued tests may be redundant. Therefore, only the contextual test trial was used in the following ex- periments. 2.3. Arc/Arg3.1-Deficient Mice Exhibited Intact STM but Impaired LTM We conducted fear-conditioning tests to compare STM, LTM, and the retention of LTM in wild-type and Arc/ Arg3.1-deficient mice. In the initial context test (4 hr after conditioning), the mean percentage of freezing of Arc/Arg3.1-deficient mice was indistinguishable from that of wild-type mice (Mann-Whitney’s U-test, U = 42, n.s.; Figure 2). In the second context test conducted 24 hr after cond ition ing , the mean p ercentag e of freezing of Arc/Arg3.1-deficient mice was, however, significantly lower than that of wild-type mice (Mann-Whitney’s U-test, U = 24, p < 0.05; Figure 2). In the 1-week and 4-week tests, the mean percentage of freezing of Arc/ Arg3.1-deficient mice was also significantly lower than that of wild-type mice (1-week test: Mann-Whitney’s U-test, U = 2, p < 0.01; 4-week test: Mann-Whitney’s U-test, U = 0, p < 0.01; Figure 2). The freezing fre- quency of wild-type mice increased significantly during the experiment, contrary to that of Arc/Arg3.1-deficient mice, whose freezing frequency decreased significantly (Two-way ANOVA with repeated measures: genotype, F(79,1) = 58.5, p < 0.001; retention time, F(79,3) = 2.47, p = 0.07, n.s.; genotype × retention time, F(79,3) = 12.6, p < 0.001; wild-type 4 hr vs. 4 week, t = 3.33, p < 0.01; Arc/Arg3.1-deficient mice 4 hr vs. 4 week, t = 4.91, p < 0.01). 2.4. Arc/Arg3.1-Deficient Mice Showed Almost No Remote Memory of Fear As in the STM and LTM test, the same mice were used Figure 2. Summary of the STM and LTM tests of wild-type (WT) and Arc/Arg3.1-deficient (KO) mice. Da- ta represent the mean ± S.E.M. Asterisks indicate statisti- cal significance (**, p < 0.01; *, p < 0.05). for all experiments, because the retention curve could have been affected by the repeated exposure to the con- ditioning context (e.g., reconsolidation, Suzuki et al., [33]). Therefore, we conducted a remote memory test. Mice were conditioned and kept without any treatment except standard daily care for 4 weeks, and then we conducted a context test. Wild-type mice showed a high freezing frequency, but Arc/Arg3.1-deficient mice did not show any freezing response (Mann-Whitney’s U-test: U = 0, p < 0.01; Figure 3). 2.5. AMPA Receptors Were Expressed at Higher Levels in the Hippocampus of Arc/Arg3.1-Deficient Mice Immunohistochemical analyses were conducted to clar- ify the distribution of neuronal processes and synapses. Although increased immunoreactivity for neuronal processes (NF-M, MAP1A) was detected in the cerebral cortex of Arc/Arg3.1-deficient mice, no substantial dif- ferences were detected in the hippocampal region (Fig- ure 4). Nevertheless, immunoreactivity for pre- and post-synaptic proteins (SYP, HOM) was somewhat in- creased in the hippocampus of Arc/Arg3.1-deficient mice (Figure 4). We also found increased immunoreactivity for GluR1 in the CA1 region and dentate gyrus (DG) of the hippocampus and for SYP in the CA3 region of Arc/Arg3.1- deficient mice as compared with that of wild-type mice (Figure 5). In order to confirm the in creased GluR1 reactivity in hippocampus, we calculated the ratio of fluorescence intensity and compared between wild-type and Arc/Arg3.1-deficient mice in a semi- qua- ntitative manner. Arc/Arg3.1-deficient mice exhibited Figure 3. Summary of remote memory test of wild- type (WT) and Arc/Arg3.1-deficient (KO) mice. Data represent the mean ± S.E.M. Asterisks indicate statis- tical significance (**, p < 0.01). 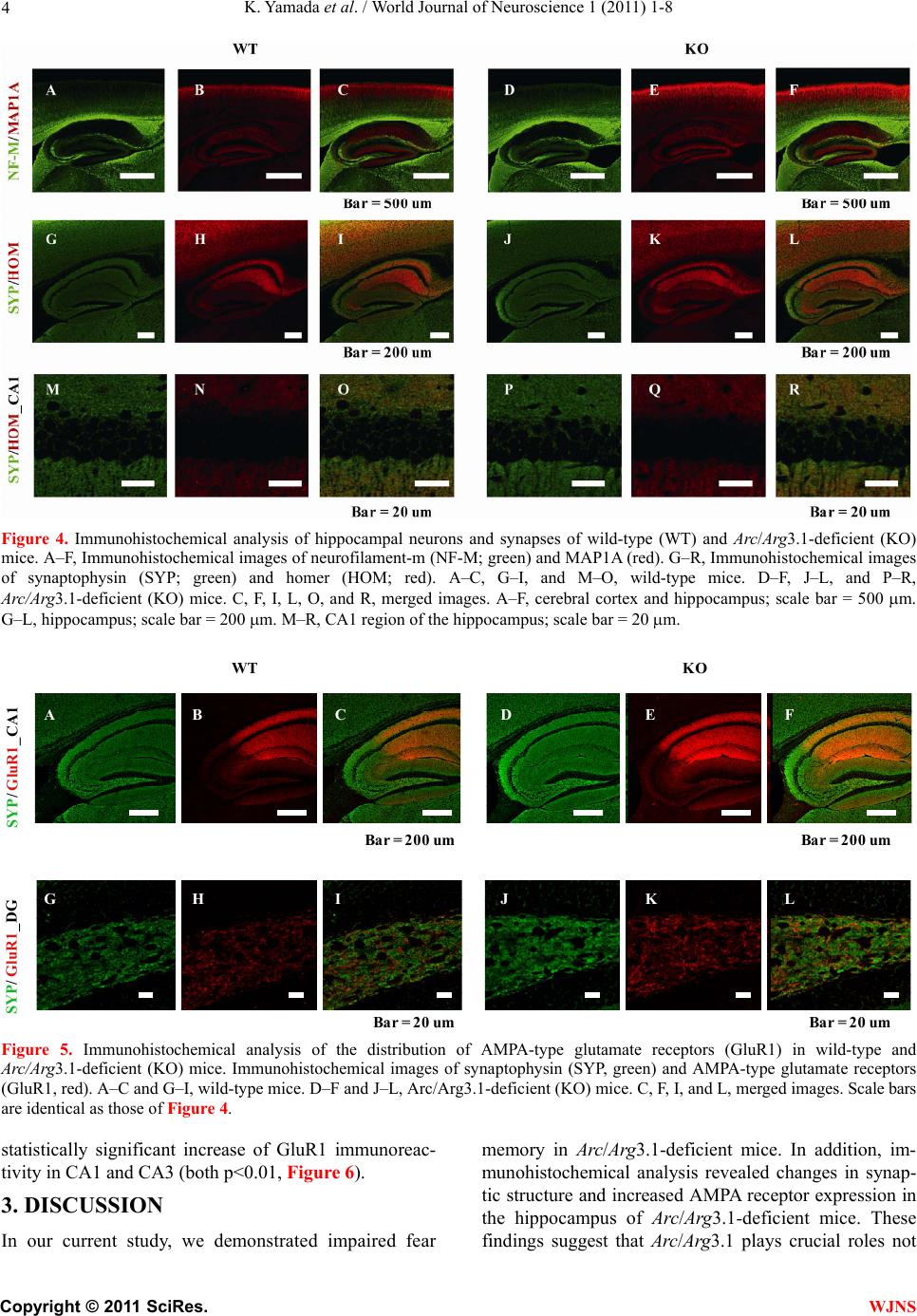 K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 4 Figure 4. Immunohistochemical analysis of hippocampal neurons and synapses of wild-type (WT) and Arc/Arg3.1-deficient (KO) mice. A–F, Immunohistochemical images of neurofilament-m (NF-M; green) and MAP1A (red). G–R, Immunohistochemical images of synaptophysin (SYP; green) and homer (HOM; red). A–C, G–I, and M–O, wild-type mice. D–F, J–L, and P–R, Arc/Arg3.1-deficient (KO) mice. C, F, I, L, O, and R, merged images. A–F, cerebral cortex and hippocampus; scale bar = 500 m. G–L, hippocampus; scale bar = 200 m. M–R, CA1 region of the hippocampus; scale bar = 20 m. KOWT SYP/ GluR1_CA1 SYP/ GluR1_DG ABC DEF GHI JKL B ar = 200 u m B ar = 20 um B ar = 200 u m B ar = 20 u m Figure 5. Immunohistochemical analysis of the distribution of AMPA-type glutamate receptors (GluR1) in wild-type and Arc/Arg3.1-deficient (KO) mice. Immunohistochemical images of synaptophysin (SYP, green) and AMPA-type glutamate receptors (GluR1, red). A–C and G–I, wild-type mice. D–F and J–L, Arc/Arg3.1-deficient (KO) mice. C, F, I, and L, merged images. Scale bars are identical as those of Figure 4. statistically significant increase of GluR1 immunoreac- tivity in CA1 and CA3 (both p<0.01, Figure 6). 3. DISCUSSION In our current study, we demonstrated impaired fear memory in Arc/Arg3.1-deficient mice. In addition, im- munohistochemical analysis revealed changes in synap- tic structure and increased AMPA receptor expression in the hippocampus of Arc/Arg3.1-deficient mice. These findings suggest that Arc/Arg3.1 plays crucial roles not  K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 5 0 1 2 3 R a ti o of i nte nsit y WT-CA1 KO-CA1 WT-CA3 KO-CA3 * * GluR1 WT-CA1 KO-CA1 WT-CA3 KO-CA3 SYP Figure 6. Ratio of fluorescence intensity. SYP: synaptophy sin, GluR1: AMPA-type glutamate receptors, WT: wild-type mice, KO: Arc/Arg3.1-deficient mice. Data represent mean + SEM. *: p<0.01 (compared to wild-type mice). only in LTM formation but also in th e memory retention process. Although an AMPA potentiator did not lead to recovery of the impaired memory in Arc/Arg3.1-defi- cient mice (Supplemental information and Supplemental Figure 2), this is the first study that used Arc/Arg3.1- deficient mice to assess the effect of drugs on memory impairment. Arc/Arg3.1-deficient mice exhibited a lower percent- age of freezing than did wild-type mice in the context and cued-memory test, indicating that Arc/Arg3.1-de- ficient mice were impaired in both contextual and cued memory. Many studies have reported that contextual fear reflects hippocampus-dependent memory function, and cued fear reflects amygdala-dependent memory function (for review, see LeDoux, [34]). Therefore, Arc/Arg3.1- deficient mice may be impaired in both hippocampus- and amygdala-dependent memory function. These re- sults suggest that Arc/Arg3.1 may function in the amyg- dala for auditory fear memory formation as well as in the hippocampus for spatial memory formation. Arc/Arg3.1- deficient mice did, however, show a significant decrease in the freezing response to the new context without an auditory cue in the cued test. This result indicates that the freezing response in the cued test may reflect both generalized contextual fear and cued fear. Therefore, new experimental tasks or protocols should be devel- oped to clarify the role of Arc/Arg3.1 in amygdala-de- pendent memory processes. In this study, we examined STM and LTM in Arc/ Arg3.1-deficient mice. Arc/Arg3.1-deficient mice exhib- ited unimpaired memory performance 4 hr after condi- tioning, indicating that their STM or early stage of LTM was intact. In contrast, 24 hr after the conditioning trial, the memory performance of Arc/Arg3.1-deficient mice was greatly impaired. After 4 weeks, their memory was almost completely absent, whereas that of wild-type mice slightly but significantly increased. This differen- tial retention process may be partly due to repeated ex- posure to the conditioning context. Short exposure to conditioned stimuli may enhance fear memory (reconso- lidation: [33,35]). Decreased fear memory by repeated exposure indicates that the reconsolidation process may also be impaired in Arc/Arg3.1-deficient mice. Thus, we assessed remote memory (ability to retrieve distant epi- sodes/events) directly and confirmed complete impair- ment of remote memory in Arc/Arg3.1-deficient mice. Immunohistochemical analyses revealed increased expression of AMPA-type glutamate receptors in hippo- campal regions. This result is consistent with previous studies reporting that Arc/Arg3.1 may be involved in endocytosis of AMPA-type glutamate receptors and may prompt the internalization of AMPA receptors [3-5]. Al- though LTP, especially early-phase LTP, depends on NMDA receptors ([36] for review), activation of AMPA receptors may improve memory function in rats and mice [37,38]. Therefore, this mutant mouse strain will be useful for developing treatments including drugs for memory impairment. 4. EXPERIMENTAL PROCEDURES 4.1. The Behavioral Laboratory Environment and Housing Conditions of Mice Mice were housed individually before transfer to the behavioral laboratory. They were kept in the laboratory during the behavioral analysis under a light/dark cycle of 12 hr/12 hr (lights on at 8:00). The laboratory was air conditioned, and the temperature and humidity were maintained at ~22˚C – 23˚C and 50% – 55%, respec- tively. Food and water were freely available except dur- ing experimentation unless otherwise indicated. We used large tweezers with soft vinyl tips to handle the mice to avoid potential differences in the handling technique of the different researchers involved in the study. Animal experiments in this study were conducted in strict accordance with the guidelines of the Institute of Physical and Chemical Research (RIKEN) and were approved by the Animal Investigation Committee of the Institute. 4.2. Classical Fear Conditioning 4.2.1 Contextual and Cued Test Twenty mice (wild type, n = 10; Arc/Arg3.1 deficient, n = 10; 9 weeks of age) were used. This test consisted of  K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 6 three parts: a conditioning trial, a contex t test trial, and a cued test trial. Fear conditioning was carried out on a clear plastic chamber equipped with a stainless-steel grid floor (34 26 30 [H] cm). The luminance of the floor was 225 Lux in the conditioning and context test trial and 0–1 Lux in the cued test trial. A CCD camera was installed on the ceiling of the chamber and was con- nected to a video monitor and a Windows PC. The grid floor was wired to a shock generator. White noise (65 dB) was supplied from a loudspeaker as an auditory cue (conditioned stimulus, CS). The conditioning trial con- sisted of a 2-min exploration period followed by two CS–unconditioned sti mulus (US) pairings sep arated by 1 min each. A US (foot-shock: 0.5 mA, 2 sec) was admin- istered at the end of the 30-sec CS period. A context test was performed in the same conditioning chamber for 3 min in the absence of the white noise 24 hr after the conditioning trial. In addition, a cued test was performed in an alternative context with distinct cues: the test chamber was different from the conditioning chamber in brightness (dark environment, almost 0–1 Lux), color (white), floor structure (no grid), and shape (triangular). The cued test was conducted 24 hr after the contextual test was finished, and consisted of a 2-min exploration period (no CS) to evaluate the nonspecific contextual fear followed by a 2-min CS period (no foot shock) to evaluate the acquired cued fear. 4.2.2. STM and LTM Test A second set of mice (wild type, n = 10; Arc/Arg3.1 de- ficient, n = 10; 9 weeks of age) was used for these tests. The conditioning trial was the same as in the above con- textual and cued test (see 4.2.1). After 4 hr, an STM test was conducted, and LTM tests were conducted 24 hr, 1 week, and 4 weeks later. STM and LTM tests were the same as the context test of 4.2.1. In this test, the cued test was not conducted to exclude any sensitization ef- fect caused by the auditory cue. 4.2.3. Remote Memory Test A third set of mice (wild type, n = 10; Arc/Arg3.1 defi- cient, n = 10; 9 weeks of age) was used. The condition- ing trial was the same as in the above STM and LTM test (see 4.2.2). In this test, a context test was conducted 4 weeks after the conditioning trial. Both the contextual and cued tests and the STM and LTM tests were replicated five times to confirm their reproducibility. The rate of the freezing response (im- mobility excluding respiration and heartbeat) of mice was measured as an index of fear memory. Data were collected and analyzed with Image J FZ2 (O’Hara, To- kyo, Japan; Image J XX is modified software based on the public domain Image J progr am developed at the U.S. National Institutes of Health and is available at http://rsb.info.nih.gov/ij). 4.3. Immunohistochemical Analysis Brains (n = 4 male mice per group) were fixed with me- thacarn fixative (methanol/chloroform/acetic acid, 60:30: 10 [v/v]), and paraffin-embedded sections were prepared [39]. Mouse monoclonal anti-neurofilament (NF-M, sc-20013; Santa Cruz Biotechnology, CA, USA; 1:1000; a marker for axons), rabbit polyclonal anti- micro- tubule-associated protein 1A (MAP1A, sc-25728; Santa Cruz Biotechnology; 1:1000; a marker for dendrites), mouse monoclonal anti-synaptophysin (SYP, sc-17750; Santa Cruz Biotechnology; 1:1000; a pre- synaptic mar- ker), rabbit polyclonal anti-homer (HOM, sc-15321; Santa Cruz Biotechnology; 1:500; a post- synaptic marker), and anti-GluR1(GluR1, sc-28779; Santa Cruz Biotechnology; 1:500) were used for immunohisto- chemistry. Sections were pretreated with HistoVT-One (Na- calai Tesque, Japan) and incubated with primary antibodies. Signals were visualized with Alexa 568-conjugated anti-mouse IgG and Alexa 488-conjugated anti-rabbit IgG (Molecular Probes, OR). Images were obtained with an FV-300 confocal la- ser-scanning microscope (Olympus, Japan). For semi-quantitative analysis of image, the ratio of fluorescence intensity was calculated and compared Arc/Arg3.1-deficient mice to wild-type by using IMAGE J program (http://rsb.info.nih.gov/ij/index.html. National Institute of Health, Bethesda), after adjusting back- ground noise (n = 4 images per mouse). 5. ACKNOWLEDGEMENTS We thank Dr. Yoshitake Sano for his comments and suggestions re- garding this study. We also thank Dr. Mariko Katayama for her assis- tance in maintaining the Arc/Arg3.1-deficient mouse line. REFERENCES [1] Guzowski, J., Lyford, G., Stevenson, G., et al. (2000) In- hibition of activity-dependent arc protein expressio n in th e rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. The Journal of Neuroscience, 20, 3993-4001. [2] Plath, N., Ohana, O., Dammermann, B., et al. (2006) Arc/ Arg3.1 is essential for the consolidation of synaptic plas- ticity and memories. Neuron, 52, 437-444. doi:10.1016/j.neuron.2006.08.024 [3] Chowdhury, S., Shepherd, J. D., Okuno, H., et al. (2006) Arc/Arg3.1 interacts with the endocytic machinery to re- gulate AMPA receptor trafficking. Neuron, 52, 445- 459. doi:10.1016/j.neuron.2006.08.033 [4] Rial Verde, E.M., Lee-Osbourne, J., Worley, P.F., et al. (2006) Increased expression of the immediate-early gene Arc/Arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron, 52, 461-474.  K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 7 doi:10.1016/j.neuron.2006.09.031 [5] Shepherd, J.D., Rumbaugh, G., Wu, J., Chowdhury, S., et al. (2006) Arc/Arg3.1 mediates homeostatic synaptic sca- ling of AMPA receptors. Neuron, 52, 475-484. doi:10.1016/j.neuron.2006.08.034 [6] Steward, O. and Worley, P. (2002) Local synthesis of pro- teins at synaptic sites on dendrites: role in synaptic plas- ticity and memory consolidation? Neurobiology of Lear- ning and Memory, 78, 508-527. doi:10.1006/nlme.2002.4102 [7] Lyford, G., Yamagata, K., Kaufmann, W., et al. (1995) Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron, 14, 433-445. doi:10.1016/0896-6273(95)90299-6 [8] Rodríguez, J.J., Davies, H.A., Silva, A.T., et al. (2005) Long-term potentiation in the rat dentate gyrus is associ- ated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. European Journal of Neuro- science, 21, 2384-2396. doi: 10.1111/ j.1460 -9568.2005.04068.x [9] Steward, O. and Worley, P. (2001) A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proceedings of the National Academy of Sciences of the United States of America, 98, 7062-7068. doi:10.1073/pnas.131146398 [10] Fosnaugh, J., Bhat, R., Yamagata, K., et al. (1995) Acti- vation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. Journal of Neurochemistry, 64, 2377-2380. doi:10.1046/j.1471-4159.1995.64052377.x [11] Kodama, M., Akiyama, K., Ujike, H., et al. (1998) A robust increase in expression of arc gene, an effector immediate early gene, in the rat brain after acute and chronic methamphetamine administration. Brain Re- search, 796, 273-283. doi:10.1016/S0006-8993(98)00349-7 [12] Moro, H., Sato, H., Ida, I., et al. (2007) Effects of SKF- -38393, a dopamine D1 receptor agonist on expression of amphetamine-induced behavioral sensitization and ex- pression of immediate early gene arc in prefrontal cortex of rats. Pharmacology Biochemistry and Behavior, 87, 56-64. doi:10.1016/j.pbb.2007.03.020 [13] Yamagata, K., Suzuki, K., Sugiura, H., et al. (2000) Ac- tivation of an effector immediate-early gene arc by me- thamphetamine. Annals of the New York Academy of Sci- ences, 914, 22-32. doi: 10.1111/ j.1749 -6632.2000.tb05180.x [14] Nakahara, T., Kuroki, T., Hashimoto, K., et al. (2000) Effect of atypical antipsychotics on phencyclidine-in- du- ced expression of arc in rat brain. NeuroReport, 11, 551-555. doi:10.1097/00001756-200002280-00025 [15] Kremerskothen, J., Wendholt, D., Teber, I., et al. (2002) Insulin-inducedexpression of the activity-regulated cyto- skeleton-associated gene (ARC) in human neuroblastoma cells requires p21(ras), mitogen-activated protein kinase/ extracellular regulated kinase and src tyrosine kinases but is protein kinase C-inde pendent. Neuroscience Letters, 22, 153-156. [16] Kunizuka, H., Kinouchi, H., Arai, S., et al. (1999) Acti- vation of arc gene, a dendritic immediate early gene, by middle cerebral artery occlusion in rat brain. NeuroRe- port, 10, 1717-1722. doi:10.1097/00001756-199906030-00017 [17] Larsen, M.H., Olesen, M., Woldbye, D.P., et al. (2005) Regulation of activity-regulated cytoskeleton protein (arc) mRNA after acute and chronic electroconvulsive stimu- lation in the rat. Brain Research, 1064, 161-165. doi:10.1016/j.brainres.2005.09.039 [18] Mikkelsen, J.D. and Larsen, M.H. (2006) Effects of stre- ss and adrenalectomy on activity-regulated cytoskeleton protein (arc) gene expression. Neuroscience Letters, 403, 239-243. doi:10.1016/j.neulet.2006.04.040 [19] Guthrie, K., Rayhanabad, J., Kuhl, D., et al. (2000) Odors regulate Arc expression in neuronal ensembles en- gaged in odor processing. NeuroReport, 11, 1809-1813. doi:10.1097/00001756-200006260-00003 [20] Matsuoka, M., Yamagata, K., Sugiura, H., et al. (2002) Expression and regulation of the immediate-early gene product Arc in the accessory olfactory bulb after mating in male rat. Neuroscience, 11, 251-258. [21] Montag-Sallaz, M. and Montag, D. (2003) Learning- induced arg 3.1/arc mRNA expression in the mouse brain. Learning and Memory, 10, 99-107. doi:10.1101/lm.53403 [22] Kelly, M.P. and Deadwyler, S.A. (2003) Experience- dependent regulation of the immediate-early gene arc differs across brain regions. The Journal of Neuroscience, 23, 6443-6451. [23] Taishi, P., Sanchez, C., Wang, Y., et al. (2001) Conditions that affect sleep alter the expression of molecules associ- ated with synaptic plasticity. American Journal of Physi- ology: Regulatory Integrative and Comparative Physiol- ogy, 281, R839-R845. [24] Kelly, M.P. and Deadwyler, S.A. (2002) Acquisition of a novel behavior induces higher levels of arc mRNA than does overtrained performance. Neuroscience, 11 0, 617- 626. doi:10.1016/S0306-4522(01)00605-4 [25] Ons, S., Martí, O. and Armario, A. (2004) Stress-induced activation of the immediate early gene Arc (activity-re- gulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. Journal of Neurochemistry, 89, 1111-1118. doi: 10.1111/ j.1471 -4159.2004.02396.x [26] Cook, E.H., Jr., Lindgren, V., Leventhal, B.L., et al. (1997) Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. The Ameri- can Journal of Human Genetics, 60, 928-934. [27] Glessner, J.T., Wang, K., Cai, G., Korvatsuka, O., et al. (2009) Autism genome-wide copy number variation re- veals ubiquitin and neuronal genes. Nature, 459, 569- 573. doi:10.1038/nature07953 [28] Suteliffe, J.S., Nurmi, E.L. and Lombroso, P.J. (2003) Genetics of childhood disorders: XLVII. Autism, part 6: duplication and inherited susceptibility of chromosome 15q11-q13 genes in autism. Journal of the American Ac- ademy of Child and Adolescent Psychiatry, 42, 253-256. doi:10.1097/00004583-200302000-00021 [29] Kishino, T., Lalande, M. and Wagstaff, J. (1997) UBE3A/ E6-AP mutations cause Angeleman syndrome. Nature Ge - netics, 15, 70-73. doi:10.1038/ng0197-70 [30] Matsuura, T., Sutcliffe, J. S., Fang, P., et al. (1997) De novo truncating mutations in E6-AP ubiquitin-protein li- gase gene (UBE3A) in Angelman syndrome. Nature Ge- netics, 15, 74-77. doi:10.1038/ng0197-74  K. Yamad a et al . / World Journal of Neuroscience 1 (2011) 1-8 Copyright © 2011 SciRes. WJNS 8 [31] Greer, P.L., Hanayama, R., Bloodgood, B.L., et al. (2010) The Angelman Syndrome protein Ube3A regulates syn- apse development by ubiquitinating arc. Cell, 140, 704- 716. doi:10.1016/j.cell.2010.01.026 [32] Radulovic, J., Kammermeir, J. and Spiess, J. (1998) Ge- neralization of fear responses in C57BL/6J mice subjec- ted to one-trial foreground contextual fear conditioning. Behavioral Brain Research, 95, 179-189. doi:10.1016/S0166-4328(98)00039-4 [33] Suzuki, A., Josselyn, S. A., Frankland, P.W., et al. (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. The Journal of Neuroscience, 24, 4787-4795. doi:10.1523/JNEUROSCI.5491-03.2004 [34] LeDoux, J. (1993) Emotional memory: in search of sys- tems and synapses. Annals of the New York Academy of Sciences, 702, 149-157. doi: 10.1111/ j.1749 -6632.1993.tb17246.x [35] von Hertzen, L.S.J. and Giese, K.P. (2005) Memory re- consolidation engages only a subset of immediate-early genes induced during consolidation. The Journal of Neur o- science, 25, 1935-1942. [36] MacDonald, J.F., Jackson, M.F. and Beazely, M.A. (2006) Hippocampal long-term synaptic plasticity and signal am- plification of NMDA receptors. Critical Reviews in Neu- robiology, 18, 71-84. [37] Sekiguchi, M., Yamada, K., Jin, J., et al. (2001) The AMPA receptor allosteric potentiator, PEPA ameliorates post-ischemic memory impairment. NeuroReport, 12, 2947- 2950. doi:10.1097/00001756-200109170-00038 [38] Yamada, D., Zushida, K., Wada, K., et al. (2009) Phar- macological discrimination of extinction and reconsoli- dation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology, 34, 2574- 2584. doi:10.1038/npp.2009.86 [39] Tanemura, K., Igarashi, K., Matsugami, T.-R., et al. (2009) Intraurine environment-genome interaction and chil- dren’s development (2): EBrain structure impairment and behavioral disturbance induced in male mice offspring by a single intraperitoneal administration of domoic acid (DA) to their dams. The Journal of Toxicological Sci- ences, 34, 279-286. doi:10.2131/jts.34.SP279 Abbreviations Arc/Arg3.1: activity-regulated cytoskeleton-associated protein; LTP: long-term potentiation; LTM: long-term memory; STM: short-term memory; NF-M: neuro- filament-M; MAP1A: microtubule-associated protein 1A; SYP: synaptophysin; HOM: homer; DG: dentate gyrus. |

